A. Finkelshteyn (Andrey Finkelshteyn) dentist, specialist (Israel)
Dentists are increasingly encountering elderly patients who are taking anticoagulants or antiplatelet agents. The use of blood thinning drugs is an important part of the primary and secondary prevention of cardiovascular complications such as myocardial infarction, ischemic stroke and acute coronary syndrome. Most studies and meta-analyses confirm an increased risk of bleeding during surgery while taking anticoagulants/antiplatelet agents.
When planning surgical procedures, operating physicians have to weigh the individual risk of perioperative bleeding and thrombotic complications in such patients. On the one hand, cessation of anticoagulant and antiplatelet therapy leads to an increased risk of thromboembolic complications; on the other hand, while taking anticoagulants and antiplatelet agents, the likelihood of perioperative hemorrhagic complications is high.
It will be useful to remember the physiological mechanisms of hemostasis in order to understand the mechanism of action of antiplatelet or anticoagulant therapy prescribed to such patients.
Physiology of hemostasis
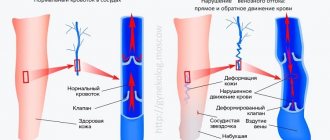
There are cellular and plasma coagulation factors. Cellular factors include platelets, and plasma factors include proteins and enzymes involved in the process of blood coagulation. Plasma coagulation factors are synthesized in the liver and circulate in the blood in an inactive form.
Stopping bleeding occurs in 3 stages and involves both blood vessels and platelets, and plasma coagulation factors:
- Temporary (primary) vasospasm (VascularPhase).
- Formation of an unstable, loose platelet plug due to platelet adhesion and aggregation (PlateletPhase).
- Retraction (contraction and compaction) of the platelet plug due to fibrin threads (CoagulationPhase).
Secondary hemostasis (coagulation)
The formed platelet plug (without subsequent formation of fibrin) can only temporarily stop bleeding, and if the platelet thrombus does not thicken with insoluble fibrin, then such a thrombus will disintegrate after a few hours. Simultaneously with platelet activation, plasma coagulation factors are activated (secondary hemostasis). Goal: stabilization of the primary platelet plug due to the formation of fibrin networks and the formation of a dense and stable fibrin clot.
Clinical significance:
- if disturbances are present at the stages of primary hemostasis (vascular-platelet), for example, thrombocytopenia (< 50,000 cells/mm3) or taking antiplatelet drugs (Aspirin, Plavix), then prolonged and continuous bleeding should be expected during and immediately after surgery (due to disturbances in the formation of the primary platelet plug);
- if disturbances are present at the stages of secondary hemostasis (coagulation), and primary hemostasis is not impaired (for example, taking anticoagulants), bleeding problems will be detected only a few hours, or even the next day, after surgery (when the patient is no longer in the clinic) .
Antiplatelet drugs (drugs that disrupt primary hemostasis)
Acetylsalicylic acid (ASA)
Egyptian papyri that date back to approximately 1550 BC. e., mention the use of a decoction of white willow leaves for many diseases. Hippocrates prescribed willow bark extract for headaches and fever. Willow is the first source of aspirin. Acetylsalicylic acid, the active ingredient in Aspirin, was synthesized from willow bark by Edward Stone in 1897. The age of medicine is shorter than the age of man and many of them quickly become obsolete. But Aspirin not only has not become obsolete over the last hundred years, it has also demonstrated new qualities. It seems to cure everything from colds to strokes.
Acetylsalicylic acid (ASA) has been the gold standard of antiplatelet therapy for many years. Aspirin in low doses (40-100 mg) irreversibly blocks the action of the enzyme cyclooxygenase-1 in platelets with a subsequent decrease in the formation of thromboxane A2, which is a powerful vasoconstrictor and proplatelet agent. Since ASA blocks COX-1 irreversibly, the antiplatelet effect persists throughout the life cycle of the platelet (7–10 days). The ability to irreversibly block platelet COX-1 distinguishes ASA from other non-steroidal anti-inflammatory drugs, the antiplatelet effect of which is short-term [1]. Considering the above, I would like to draw attention to the following clinical point.
What happens when a dentist prescribes an NSAID to a patient taking Aspirin? Data from epidemiological studies indicate that taking drugs from the NSAID group can cancel or significantly reduce the cardioprotective effect of aspirin.
Why? Aspirin and NSAIDs compete for the same substrate - COX 1. Therefore, with parallel administration, part of the platelets will be reversibly associated with NSAIDs, and the other part will be irreversibly associated with acetylsalicylic acid. After a few hours, platelets that were temporarily inactivated by NSAID drugs will restore their function and the patient will be left without cardioprotective action.
Which analgesics are contraindicated and which are the drug of choice in such patients can be learned in detail from my lectures.
In the RISK (Research on Instability in Coronary Artery Disease) study, when ASA was prescribed at a dose of 75 mg/day to patients with unstable angina, the risk of developing myocardial infarction decreased by 50% [2]. In another study in patients with acute MI, the effectiveness of ASA (160 mg/day) was comparable to the effectiveness of thrombolytic therapy [3].
Many patients receive dual antiplatelet therapy (Aspirin + Clopidogrel). In acute coronary syndrome, as well as after percutaneous coronary intervention (PCI) or stent placement, ASA is usually prescribed in combination with Clopidogrel. According to numerous studies, discontinuation of antiplatelet therapy in patients with an active stent is correlated with a 90-fold increase in the risk of stent thrombosis [4]. The literature has also documented thromboembolic complications with a fatal outcome due to unauthorized discontinuation of anticoagulant/antiplatelet drugs by the dentist before surgery. The risk of bleeding increases dramatically with dual antiplatelet therapy during surgery.
Modern antiplatelet therapy: place of ticagrelor in clinical guidelines
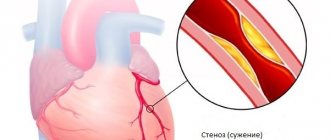
The leading cause of mortality worldwide is still cardiovascular pathology, despite the high level of development of cardiology over the past decades [1, 2]. The various clinical manifestations of vascular pathology are based on a common anatomical substrate in the form of dysfunction of the arterial endothelium, chronic inflammation and damage to the cap of the atherosclerotic plaque, slowing of blood flow, and the formation of an intravascular thrombus [3]. In this regard, reducing the risk of developing thrombotic complications is the main task that a doctor who wants to increase the duration and improve the quality of life of patients with cardiovascular diseases should set.
The pathogenesis of thrombosis includes three main points for drug action: platelet link - the action of antiplatelet agents, the coagulation system - the zone of action of anticoagulants, fibrin - the action of fibrinolytics. Platelets are the first to respond to the rupture of an atherosclerotic plaque, launching a coagulation cascade; they are a source of active synthesis of humoral factors that simultaneously stimulate the processes of thrombus formation and inflammation. According to a meta-analysis of 287 randomized studies on secondary prevention and 6 studies on primary prevention, the use of antiplatelet therapy can reduce the risk of non-fatal myocardial infarction and non-fatal cerebral infarction by 23% [4]. This meta-analysis confirms that the leading role in the prevention of complications of atherosclerosis should be given to antiplatelet agents.
Antiplatelet agents are medications that prevent thrombosis by reducing the functional activity of platelets. To date, more than 20 different drugs are known that can inhibit platelet function through various mechanisms of action. However, during many years of practice and clinical studies, effectiveness was confirmed only for cyclooxygenase inhibitors (acetylsalicylic acid), adenosine diphosphate (ADP) receptor blockers - P2Y12 (clopidogrel, prasugrel, ticagrelor), phosphodiesterase inhibitors (dipyridamole) and glycoprotein IIb-IIIa antagonists for intravenous use (abciximab, tirofiban, eptifibatide). Activation of platelets and their subsequent aggregation occur under the influence of various mediators, the most important of which are thromboxane A2 and ADP, therefore acetylsalicylic acid (ASA) and ADP inhibitors (clopidogrel, prasugrel, ticagrelor) are most widely used.
The history of the creation of the class began with the discovery of the antiplatelet properties of ASA. In 1987, the first randomized Canadian trial was published involving 585 stroke patients treated with ASA for 26 months. The study demonstrated the effectiveness of ASA against recurrent stroke [5]. This was the reason that in 1980, the US Food and Drug Administration (FDA) approved ASA for the treatment of patients after stroke. Subsequently, the effectiveness of ASA in reducing the risk of death and recurrent myocardial infarction in patients with unstable angina and non-ST segment elevation myocardial infarction was proven [4]. Thus began the era of antiplatelet therapy and its first worthy representative - acetylsalicylic acid.
Acetylsalicylic acid blocks platelet activation by inhibiting cyclooxygenase (COX), preventing the formation of thromboxane A2. Platelets are anucleate cells, so they lack the ability to synthesize proteins. Irreversible inhibition of COX-1, the impossibility of its resynthesis due to the absence of a nucleus, as well as the daily renewal of the platelet pool by only 10% lead to the fact that the blockade of thromboxane synthesis during ASA therapy persists throughout the platelet life span, up to 10 days. Complete suppression of thromboxane production is achieved with constant long-term administration of ASA in doses ≥ 75 mg/day. In most patients with stable coronary heart disease (CHD), low-dose ASA is preferable due to its favorable benefit-risk ratio. ASA for this category of patients remains the basis of drug prevention of arterial thrombosis [6]. The damaging effect of ASA on the gastrointestinal tract (GIT) increases as the dose increases. The drug is recommended for all patients with an established diagnosis of coronary artery disease without any restrictions on the duration of use. The optimal benefit-risk ratio is achieved when using ASA in a dose range from 75 to 150 mg/day; when used as part of dual antiplatelet therapy, the dose is 75–100 mg.
However, in recent years, the problem of resistance to ASA therapy has been actively discussed, which is understood as the inability of the drug in some patients to adequately suppress platelet function, reduce the synthesis of thromboxane A2 and/or prolong bleeding time. The prevalence of resistance to ASA therapy, according to various studies, ranges from 10% to 45% [7]. Among the possible reasons for this phenomenon are the following:
- pharmacodynamic interactions of ASA with non-steroidal anti-inflammatory drugs (NSAIDs);
- the presence of non-platelet sources of thromboxane A2 synthesis;
- expression of COX-2 in newly formed platelets;
- hydrolysis of ASA by esterases of the gastrointestinal mucosa;
- increased synthesis of thromboxane A2;
- hyperlipidemia;
- genetic features.
A number of independent studies have found that in patients with acute coronary syndrome (ACS) without ST segment elevation (ESSENCE, PRISM PLUS), the immediate prognosis depends on previous use of ASA before the development of exacerbation of coronary artery disease. Thus, in the PRISM PLUS study, when using ASA for ACS, the incidence of myocardial infarction, refractory angina and sudden death by the 7th day of observation was 12.1% among patients who had not previously taken ASA, and 23.5% among those taking ASA before the development of an exacerbation. This fact was called the “aspirin paradox,” which led DL Bhatt and EJ Topol (2004) to classify ASA as “suboptimal antiplatelet drugs” [8, 9]. All this contributed to the development and study of new antiplatelet drugs - ADP P2Y12 receptor inhibitors and the identification of approaches to dual antiplatelet therapy.
The group of ADP-P2Y12 receptor blockers includes the drugs ticlopidine, clopidogrel, prasugrel, and ticaglerol. These drugs inhibit platelet aggregation induced by adenosine diphosphate by causing changes in the platelet ADP receptor, which is called P2Y12 [9]. There are significant differences between the drugs listed above, for example, irreversible inhibitors of P2Y12 receptors include thienopyridines (ticlopidine, clopidogrel and prasugrel), and reversible inhibitors include triazolopyridines (ticagrelor). Comparative characteristics of the drugs are presented in table. 1.
Clopidogrel is the most well-known and actively used antiplatelet agent after ASA in domestic medicine today [10]. The results of large clinical trials have proven the effectiveness of reducing the incidence of complications in a wide range of patients with coronary artery disease when adding clopidogrel to ASA [11, 12], which served as the basis for the development of indications for dual antiplatelet therapy in patients with ACS without ST elevation, as well as after coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI) for the prevention of thrombosis [13].
Clopidogrel, as can be seen from the table, is a prodrug; the drug has a complex metabolism. The absorption of clopidogrel in the intestine is controlled by a special protein (P-glycoprotein), encoded by the ABCB1 gene, in this regard, only about 15% of the absorbed clopidogrel in the liver is converted into an active metabolite. The process is two-step (oxidation and hydrolysis), depending on several isoenzymes of the cytochrome P450 system, the most important of which are CYP2C19 and CYP3A4 [14]. Despite the wide evidence base for the effectiveness of this drug, clopidogrel has a number of disadvantages, which include a delayed antiplatelet effect, since it is a prodrug and requires time to activate; maximum suppression of ADP receptors occurs only on the 4th–5th day of regular use. In addition, there is variability in the antithrombotic effect of clopidogrel in different patients, which may be due to a number of pharmacokinetic factors, including insufficient loading and maintenance doses of the drug, impaired absorption and formation of the active metabolite, drug interactions, in particular with proton pump inhibitors, which are often prescribed for the prevention of bleeding from the upper gastrointestinal tract [15, 16].
Due to the existing shortcomings of clopidogrel and the inability to solve this problem, the need to create a new drug from the group of ADP receptor blockers - P2Y12 - became obvious to the world community.
A new antiplatelet drug is ticagrelor, a direct-acting reversible antagonist of P2Y12 receptors. The drug is an active substance that is metabolized via the CYP3A4 isoenzyme to form an active metabolite. The degree of inhibition of P2Y12 receptors is determined primarily by the plasma levels of ticagrelor and, to a lesser extent, its active metabolite. The half-life is about 12 hours, and therefore the drug is prescribed twice a day. Ticagrelor has a faster onset of therapeutic action and provides a more pronounced and persistent inhibition of platelet activation compared to clopidogrel. At the same time, recovery of platelet function after discontinuation of ticagrelor occurs faster compared to clopidogrel. The presence of more attractive pharmacological properties, as well as existing problems associated with taking clopidogrel, were the main reasons for organizing the large-scale PLATO (Platelet inhibition and patient outcomes) study, which compared the effectiveness and safety of ticagrelor compared with clopidogrel in patients with ACS [17] . According to a study published on August 30, 2009 at the Congress of the European Society of Cardiology (ESC), the new antithrombotic drug ticagrelor is more effective than clopidogrel in treating patients with acute coronary syndrome and does not increase the risk of bleeding.
Researchers led by Lars Wallentin randomized 18,624 patients with ACS hospitalized between 2006 and 2008 at 862 hospitals included in the PLATO trial. Patients were divided into 2 groups: in the first group, patients received ticagrelor (180 mg loading dose and 90 mg twice a day), in the other - clopidogrel (300 or 600 mg loading dose and 75 mg daily). All patients also took ASA at a dose of 75–100 mg. The groups were carefully balanced taking into account baseline clinical parameters, concomitant diseases and treatment tactics. 37.5% of patients had acute myocardial infarction with ST segment elevation, 42.9% had acute myocardial infarction without ST segment elevation, and 16.6% had unstable angina. The duration of medication use ranged from 6 to 12 months, with an average of 277 days. The results showed that with ticagrelor compared with clopidogrel there was a significant reduction in the total number of primary endpoints (cardiovascular death, myocardial infarction or stroke): 9.8% versus 11.7%, a risk reduction of 16%, p < 0.001. In those receiving ticagrelor, compared with those treated with clopidogrel, there was a significant decrease in the incidence of myocardial infarction: from 6.9% to 5.8%, and cardiovascular death - from 5.1% to 4%. At the same time, the total number of strokes was the same in both subgroups: 1.5% and 1.3%. The incidence of the combined secondary endpoint (death from vascular causes, myocardial infarction, stroke, recurrent myocardial ischemia, transient ischemic attack or other types of arterial thrombosis), as well as death from all causes, was significantly lower in the ticagrelor group compared with clopidogrel: 14.6 % versus 16.7% and 4.5% versus 5.9%, respectively. There were no significant differences between the groups in the incidence of major bleeding or fatal or life-threatening bleeding. It is interesting to note that the risk of major bleeding, including fatal intracranial bleeding not related to the CABG procedure, was slightly higher in the ticagrelor group compared with clopidogrel (4.5% vs. 3.8%, p = 0.03). However, the incidence of CABG-related bleeding was lower among those receiving ticagrelor (7.4% vs. 7.9%) [18].
The results of 13,408 (72%) patients with an invasive treatment strategy planned at the randomization stage were analyzed separately [18]. 49.1% of patients were diagnosed with acute coronary syndrome with ST segment elevation on electrocardiography (ECG) and 50.9% with acute coronary syndrome without ST segment elevation on ECG. During the first hospitalization, PCI was performed in 10,298 (72%) patients and CABG in 782 (5.8%). The average time to PCI was 2.4 (0.8–20.1) hours after randomization in patients with ACS without ST segment elevation on the ECG and 0.5 (0.2–1) hours in patients with ACS with ST segment elevation on the ECG . The median time to CABG was 6 (3–10) days. The total number of myocardial infarctions, strokes and cardiovascular deaths during therapy with ticagrelor decreased to 9% (clopidogrel - by 10.7%), i.e., the risk reduction was 16%, p < 0.0025.
It is important to emphasize that the benefit of ticagrelor on the primary endpoint was observed in different subgroups and was independent of the loading dose of clopidogrel. Major bleeding was equally common in those treated with ticagrelor and those treated with clopidogrel (11.6% vs. 11.5%). The incidence of stent thrombosis was significantly lower in the ticagrelor group, both with and without drug-eluting stents. The incidence of certain stent thrombosis in patients receiving ticagrelor was significantly lower at both 30 days and 360 days of follow-up compared with those treated with clopidogrel, including those patients taking a loading dose of 600 mg or more.
In a substudy analysis of 1,261 patients undergoing CABG within 7 days of the last study drug dose, there was no significant difference in the reduction in primary endpoints (10.6% in the ticagrelor group and 13.1% in the clopidogrel group). Moreover, among those taking ticagrelor, there was a significant decrease in overall mortality by 51%, and cardiovascular mortality by 48%, both in the early and late periods after surgery [19].
Thus, PLATO was the first large-scale study to demonstrate the clinical effectiveness of ticagrelor in reducing the incidence of major vascular events in patients with ACS, without a significant increase in the risk of bleeding. A more significant reduction in the risk of thrombotic episodes during therapy with ticagrelor appears to be due to more rapid and intense inhibition of platelet P2Y12 receptors. When a loading dose of 600 mg of clopidogrel is administered, it takes 2–4 hours to achieve 50% inhibition of platelet aggregation, and the same effect is achieved after 30 minutes when taking 180 mg of ticagrelor. In addition, there is a fairly large group of patients with the presence of defective variants of alleles of the cytochrome P450 system, which is associated with a slowdown in the formation of the active metabolite of clopidogrel, insufficient suppression of platelet function when taking it, as well as with a higher risk of cardiovascular complications after acute coronary syndrome and during PCI. The advantages of ticagrelor also include the reversible nature of inhibition of platelet P2Y12 receptors, which means a more rapid cessation of the antiplatelet effect after discontinuation of the drug. This circumstance seems important during invasive interventions, as well as before the upcoming CABG procedure. Although the incidence of major bleeding was no lower with ticagrelor than with clopidogrel, it should be noted that the greater inhibition of platelet function was not associated with an increase in the incidence of major bleeding. This distinguishes ticagrelor from prasugrel, whose more pronounced antiplatelet effect is accompanied by an increased risk of major bleeding.
The European Society of Cardiology recommended the use of ticagrelor (at a loading dose of 180 mg and 90 mg 2 times a day as a maintenance dose) to all patients with ACS, regardless of the planned treatment strategy (invasive or conservative) as first-line therapy. If patients received clopidogrel at the very beginning of the disease, it should be replaced with ticagrelor. Taking clopidogrel in patients with ACS with invasive or conservative strategies is possible only in cases of absence or intolerance to ticagrelor or prasugrel. The duration of therapy with P2Y12 receptor inhibitors in patients who have suffered acute coronary syndrome is 12 months. In patients on therapy with P2Y12 receptor inhibitors, in cases of planned surgery (including CABG), ticagrelor and clopidogrel are discontinued 5 days before, and prasugrel 7 days before. Dual antiplatelet therapy is mandatory while taking ASA at a dose of 75–100 mg/day [13]. The use of dual antiplatelet therapy in stable coronary artery disease could provide more effective prevention of coronary thrombosis. However, in the CHARISMA study, which included stable patients with atherosclerotic lesions of various vascular territories or multiple cardiovascular risk factors, the addition of clopidogrel to ASA did not bring additional benefit [20]. The 2013 European Society of Cardiology guidelines indicate that dual antiplatelet therapy is beneficial only in certain categories of patients at high risk of ischemic events. Routine administration of this therapy to patients with stable coronary artery disease is not recommended [21].
Thus, atherothrombosis is the cause of high mortality in patients with cardiovascular diseases throughout the world. One of the key points of therapy is the competent prescription of antiplatelet drugs. The main effective oral drugs prescribed in clinical practice are ASA, clopidogrel, ticagrelor, prasugrel. In table Figure 2 presents an algorithm for selecting antiplatelet agents. Modern cardiology is actively developing, and one can hope that new facets of known drugs and the development of new ones will help doctors in the daily fight against cardiovascular diseases.
Literature
- Singh, VV, Toskes PP Small Bowel Bacterial Overgrowth: Presentation, Diagnosis, and Treatment // Curr Treat Options Gastroenterol. 2004. Vol. 7(1). R. 19–28.
- McMurray JJ, Adamopoulos S, Anker SD et al. Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology // Eur. Heart J. 2012. Vol. 33 (14). P. 1787–1847.
- Uster V., Fallon JT, Badimon JJ et al. The unstable atherosclerotic plaque: clinical significance and therapeutic intervention // Thrombosis and Hemostasis. 1997. Vol. 78(1). P. 247–255.
- Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction? And stroke in high-risk patients // BMJ. 2002. Vol. 324. P. 71–86.
- A randomized trial of aspirin and sulfinpyrazone in threatened stroke. The Canadian Cooperative Study Group // N. Engl. J. Med. 1978. Vol. 299(2). P. 53–59.
- ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines // Circulation. 2012. Vol. 126. P. 354–471.
- Ushkalova E. A. Aspirin resistance: mechanisms of development, methods of determination and clinical significance // Farmateka. 2006. No. 13 (128). pp. 35–41.
- Ainetdinova D. Kh., Udovichenko A. E., Sulimov V. A. The role of antiplatelet therapy in the primary and secondary prevention of cardiovascular diseases // Effective pharmacotherapy in cardiology and angiology. 2007. No. 2. pp. 36–41.
- Shalaev S.V. Antiplatelet agents in the treatment of acute coronary syndromes // Pharmateka. 2003. No. 312. pp. 94–97.
- Kei A.A., Florentin M. et al. Antiplatelet Drugs: What comes next? // Clin. Applied Thrombosis // Hemostasis. 2011. Vol. 17(1). P. 9–26.
- Patrono C., Baigent C., Hirsh J. On behalf of the American College of Chest Physicians. Antiplatelet drugs: American College of Chest Physicians evidence-based clinical practice guidelines (8 th edition) // Chest 2008. Vol. 133(6). P. 1995–2335.
- Steg G., James SK, Atar D. et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation The Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC) // European Heart Journal. 2012. Vol. 33. R. 2569–2619.
- ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST segment elevation. The Task Force for the management of acute coronary syndromes in patients presenting without persistent ST segment elevation of the European Society of Cardiology // Eur. Heart J. 2011. Vol. 32. P. 2999–3054.
- Cattaneo M. ADP receptors antagonists. In Michelson AD, ed Platelets. San Diego, Calif.: Academic Press. 2006. P. 1127–1144.
- Snoep JD, Hovens MM Clopidogrel nonresponsiveness in patients under-going percutaneous coronary intervention with stenting: a systematic review and meta-analysis // Am. Heart J. 2007. Vol. 154. P. 221–231.
- Norgard NB, Mathews KD, Wall GC Drug-drug interaction between clopidogrel and the proton pump inhibitors // Ann. Pharmacother. 2009. Vol. 43. P. 1266–1274.
- Wallentin L., Becker RC, Budaj A. et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes // N. Engl. J. Med. 2009. Vol. 361. P. 1045–1057.
- Cannon CP, Harrington RA, James S. et al. Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomized double–blind study // Lancet. 2010. Vol. 375 (9711). P. 283–293.
- Held C., Asenblad N., Bassand JP et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes undergoing coronary artery bypass surgery, results from the PLATO // J. Amer. Coll. Cardiol. 2011. Vol 57. P. 672–684.
- Bhatt DL, Flather MD, Hacke W. et al. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial // J. Am. Coll. Cardiol. 2007. Vol. 49. P. 1982–1988.
- ESC guidelines on the management of stable coronary artery disease. The Task Force on the management of stable coronary artery disease of the European Society of Cardiology // Eur. Heart J. 2013. Vol. 38. P. 2949–3003.
G. I. Nechaeva1, Doctor of Medical Sciences, Professor O. V. Drokina, Candidate of Medical Sciences N. I. Fisun, Candidate of Medical Sciences
State Budgetary Educational Institution of Higher Professional Education Omsk State Medical Academy of the Ministry of Health of the Russian Federation, Omsk
1 Contact information
* The drug is not registered in the Russian Federation.
Clopidogrel (Plavix)
Platelet aggregation is a process that consumes energy. Platelets receive this energy from a special cell energy carrier, adenosine diphosphate (ADP). To do this, adenosine diphosphate needs to attach to a special binding site (receptor).
Clopidogrel irreversibly changes platelet ADP receptors, and therefore platelets remain nonfunctional throughout life, and restoration of normal function occurs as platelets are renewed (after approximately 7 days).
ADP receptor blockers are used if ASA is intolerant or if Aspirin does not work sufficiently. Compared with ASA, patients taking clopidogrel are more prone to bleeding and bruising.
results
The average age of the patients was 69 years, 63% were men, 11% had an indication of a previous stroke, and 19% had diabetes mellitus. Of the individuals included in the analysis, 70% had a current stroke and 30% had a TIA.
- The odds ratio for reaching the primary endpoint for stroke/TIA was 0.93 (95% CI, 0.70 - 1.23), p = 0.61, for fatal stroke 1.62 (95% CI, 0.67 - 3.93), p = 0.29.
- A significant increase in the incidence of bleeding during triple therapy was noteworthy. The odds ratio for common bleeding was 2.49 (95% CI, 2.00 - 3.10), p < 0.001; for fatal bleeding 2.32 (95% CI, 0.64 - 8.36), p=0.2; for serious bleeding 2.04 (95% CI, 1.16 - 3.60), p = 0.013. At the same time, very few episodes of fatal bleeding were diagnosed - 7 in the intensive therapy group and 4 in the control group.
- A joint assessment of efficacy and safety showed that the overall effect of the treatment was neutral. During triple therapy, the incidence of stroke/major bleeding was 5.9%, in the control group - 4.9% (odds ratio, 1.21 (0.88 - 1.67); p = 0.23). The incidence of death, stroke, myocardial infarction, and major bleeding was 7.1% and 6.8%, respectively (odds ratio, 1.04 (0.79 - 1.38); p = 0.77).
It is interesting to note that the POINT trial is currently being conducted in the United States, which compares the effectiveness and safety of the combination of aspirin and clopidogrel vs. Aspirin monotherapy started within 12 hours of a minor stroke or TIA.
Source:
International Stroke Conference 2021. Abstract LB4.
Anticoagulants (drugs that disrupt secondary hemostasis)
Warfarin
In addition to antiplatelet agents, the process of thrombus formation can be influenced by anticoagulants - drugs that prevent the activation of plasma coagulation factors. Studies performed before the widespread introduction of anticoagulant therapy demonstrated a high incidence of thrombus formation (up to 50%) in the left ventricular cavity in the post-infarction period [5]. The cause of blood clots is not the activation of platelets, but a local violation of myocardial contractility (violation of the rheological properties of blood), which in turn activates plasma coagulation factors. In simple words, platelets have nothing to do with it, and antiplatelet agents will not help. Indications for anticoagulant therapy: prevention of arterial and venous embolism of cardiogenic origin in patients with atrial fibrillation, after heart valve replacement, after extensive transmural MI in patients with parietal thrombosis of the left ventricle, etc.
Vitamin K plays an important role in the production of many plasma coagulation factors. Warfarin blocks the synthesis of vitamin K-dependent blood coagulation factors in the liver, namely factors II, VII, IX and X. The concentration of these components in the blood decreases and the clotting process slows down.
Warfarin has a narrow therapeutic window and requires individual dose selection. It is quite difficult to maintain optimal levels of the drug in the blood during long-term outpatient treatment. It interacts with many medications. A large group of pharmacological drugs can enhance or weaken the effect of Warfarin. The interaction of Warfarin with green vegetables containing large amounts of vitamin K reduces its effectiveness. All this dictates the need for regular monitoring of anticoagulants, which makes their use extremely difficult in areas where such monitoring is not organized or is of poor quality.
To monitor the effect of indirect anticoagulants, INR (International Normalized Ratio) or international normalized ratio (MHO) is usually used. The recommended INR interval for patients with CVD varies depending on the disease, but all heart diseases are in the range of 2-3.5. INR (INR) beyond the recommended level sharply increases the risk of hemorrhagic complications.
Protocol for prescribing antithrombotic therapy in patients with atrial fibrillation and flutter
(intended for therapists, cardiologists, neurologists)
1. Atrial fibrillation (AF) and atrial flutter (AF) are diagnosed using a 12-lead ECG or Holter ECG monitoring.
2. Electrocardiographic criteria for AF:
- completely irregular RR intervals;
- absence of R waves.
Electrocardiographic criteria for TP:
- absence of P waves;
- flutter waves F.
3. If these rhythm disturbances are detected, the attending physician prescribes a consultation with a cardiologist to determine indications for antithrombotic therapy. When examining patients with AF/AFL, the consultant physician should assess:
3.1. Risk of developing systemic thromboembolism according to the CHA2DS2VASc scale
| Risk factor | Points |
| Chronic heart failure or left ventricular dysfunction | 1 |
| Arterial hypertension | 1 |
| Age ≥ 75 years | 2 |
| Diabetes | 1 |
| History of stroke or transient ischemic attack or thromboembolism | 2 |
| Vascular diseases, i.e. peripheral arterial diseases, myocardial infarction, aortic atherosclerosis | 1 |
| Age 65-74 years | 1 |
| Female | 1 |
Total__________ points;
3.2. Risk of bleeding according to the HAS-BLED scale
| Clinical characteristics | Points |
| Arterial hypertension | 1 |
| Impaired liver or kidney function - 1 point each | 1 or 2 |
| History of stroke | 1 |
| History of bleeding or tendency to it | 1 |
| Labile INR | 1 |
| Age >65 years | 1 |
| Taking some medications/alcohol – 1 point each) | 1 or 2 |
Total _________ points:
3.3. Creatinine clearance (CC)_______ml/min (according to the Cockcroft-Gault formula);
3.4. History of gastrointestinal tract diseases.
4. For all types of AF (newly diagnosed, paroxysmal, persistent, long-term persistent, permanent) and AFL, the approaches to antithrombotic therapy are the same.
5. Choice of antithrombotic therapy.
5.1 Patients under 65 years of age and isolated AF/AFL (including women without other risk factors) with 0 points on the CHA2DS2VASc scale should not be prescribed antithrombotic therapy.
5.2 Patients with AF/AFL with 1 or more points on the CHA2DS2VASc- scale are advised to prescribe oral anticoagulants:
5.2. 1. Patients with a low risk of bleeding (HAS-BLED scale ≤ 2); with no history of gastrointestinal pathology; under 75 years of age - prescribe Dabigatran (Pradaxa) at a dose of 150 mg 2 times a day.
5.2. 2. HAS - BLED ≥ 3); with a history of gastrointestinal pathology; over 75 years old - prescribe Apixaban (Eliquis) at a dose of 5 mg 2 times a day.
5.2. 3. The dose of Apixaban (Eliquis) should be reduced to 2.5 mg twice daily in patients having two or more of the following characteristics:
— age over 80 years; — body weight less than 60 kg; — plasma creatinine concentration ≥133 µmol/l.
5.2. 4. Patients taking Warfarin for a long time and demonstrating stable INR values (INR in the therapeutic range at least 70% of the time), with a low risk of intracranial hemorrhage and those who cannot afford oral anticoagulants - continue taking Warfarin under the control of INR (target value from 2 to 3).
5.2. 5. Drug interactions of vitamin K-independent oral anticoagulants (NOACs) are presented in Appendix 1.
6. Special clinical cases:
6.1 Patients with valvular AF (i.e. those with moderate or severe mitral stenosis of rheumatic etiology, mechanical prosthetic heart valves or biological prostheses in the first 6 months after installation) are indicated for Warfarin (target INR values of 2-3 for unoperated valvular disease and artificial valve in the aortic position; 2.5–3.5 – for an artificial valve in the mitral position). The use of NOACs is contraindicated.
6.2. For patients with a history of systemic embolism outside the central nervous system, Rivaroxaban (Xarelto) 20 mg once daily is recommended.
For patients with impaired renal function (creatinine clearance 49–15 ml/min), the dose of Rivaroxaban should be reduced to 15 mg once daily.
6.3 Patients with diabetes mellitus can be prescribed any oral anticoagulant.
6.4. For patients scheduled for catheter ablation , Warfarin should be prescribed for preoperative preparation and the INR should be maintained at target values of 2–3 for at least 3 weeks before surgery and at least 4 weeks after it. Continuous Warfarin therapy during the procedure should be preferred, maintaining the INR at 2 and avoiding heparin bridging. If the risk of ischemic stroke is high, oral anticoagulant therapy after catheter ablation should be continued for life.
As an alternative to Warfarin, Rivaroxaban (Xarelto) 20 mg once daily can be used in patients with nonvalvular AF undergoing catheter ablation for at least 3 weeks before surgery and for at least 4 weeks after surgery. Continuous Rivaroxaban therapy during the procedure should be preferred, without resorting to heparin bridging. If the risk of ischemic stroke is high, oral anticoagulant therapy after catheter ablation should be continued for life.
6.5. In patients with AF who are planning to have a pacemaker (pacemaker) installed , in order to reduce the risk of thromboembolic complications, it is recommended to take oral anticoagulants for at least 1 month before surgery.
6.5. 1.Warfarin
- If the risk of developing hemorrhagic complications is low (0–2 points on the HAS-BLED scale), discontinuation of Warfarin is not required before inserting a pacemaker. The intervention is performed at a therapeutic level of anticoagulation (INR 2–3)
- If there is a high risk of developing hemorrhagic complications (≥3 points on the HAS-BLED scale), warfarin must be discontinued 3 days before surgery before installing a pacemaker.
6.5. 2. PLA
Before pacemaker insertion, NOACs must be discontinued 24–48 hours before surgery (timing of withdrawal varies depending on the drug and renal function - see section 7.2 Interventions Associated with a Moderate Risk of Bleeding for more information).
6.6. For patients with AF undergoing electrical cardioversion , the following anticoagulation regimens are indicated:
6.6. 1. Before performing elective cardioversion, it is recommended to take Warfarin or NOAC for at least 3 weeks before cardioversion and at least 4 weeks after cardioversion. In patients at high risk of developing ischemic stroke, lifelong use of warfarin or NOACs is recommended.
6.6. 2. Before performing emergency cardioversion when AF paroxysm lasts less than 48 hours in patients who have not previously taken oral anticoagulants, anticoagulant therapy with parenteral anticoagulants (low molecular weight or unfractionated heparins) is recommended.
6.6. 3. Before performing emergency cardioversion when AF paroxysm lasts more than 48 hours or is of unknown duration in patients who have not previously taken oral anticoagulants, anticoagulant therapy with parenteral anticoagulants (low molecular weight or unfractionated heparins) or Rivaroxaban (at a dose of 20 mg at least 4 hours before cardioversion) under the mandatory control of transesophageal echocardiography (TE-ECHO).
6.7. In patients with severe renal impairment (creatinine clearance less than 30 ml/min for Dabigatran , less than 15 ml/min for Apixaban and Rivaroxaban ) and/or those on hemodialysis, NOACs are contraindicated . If there are indications for chronic anticoagulant therapy in patients with severe renal dysfunction, Warfarin should be considered (target INR 2-3).
7. Perioperative management of patients with atrial fibrillation and flutter. If a patient is scheduled to undergo surgery, the timing of discontinuation of anticoagulant therapy depends on the type of surgery, the anticoagulant being taken, and renal function.
7.1 Interventions associated with a low risk of bleeding
7.2 Interventions associated with a moderate risk of bleeding:
7.3 Interventions associated with a high risk of bleeding:
8. Resumption of anticoagulant therapy after surgery
- The prescription of anticoagulants after non-cardiac surgery should be adapted to the type of operation and take into account the risk of thromboembolic complications in accordance with the CHA2DS2VASc scale for a particular patient.
- For interventions with a low risk of bleeding, there is no need to interrupt anticoagulant therapy, since the intervention is performed at the end of the drug's action.
- For interventions with a moderate risk of bleeding, anticoagulant therapy should be resumed after 6–8 hours when stable hemostasis is achieved.
- For procedures with a high risk of bleeding, anticoagulant therapy should be resumed after 48–72 hours when stable hemostasis is achieved. Anticoagulant therapy to prevent phlebothrombosis in immobilized patients can be started 6–8 hours after surgery.
- The choice of perioperative anticoagulant therapy strategy should be individualized and always requires collegial discussion.
- Early postoperative period after radiofrequency catheter ablation of the left atrium - it is recommended to take warfarin or NOAC for three months after the procedure (at least 8 weeks). Further decisions on anticoagulant therapy should be made taking into account the risk of thromboembolic complications in accordance with the CHA2DS2VASc scale.
9. Timing of anticoagulant therapy after a transient ischemic attack or ischemic stroke:
Table 1. Timing of anticoagulant therapy after transient ischemic attack or ischemic stroke
Factors contributing to early initiation of OAC use:
- low NIHSS (<8): - small size / no changes in neuroimaging - high risk of relapse, for example, thrombus in the heart cavity (according to ECHO-CG) - no need for percutaneous endoscopic gastrostomy - no need for surgery on the carotid arteries - no hemorrhagic transformation - clinically stable patient - young patient - controlled arterial hypertension
Factors contributing to late initiation of OAC use:
— high NIHSS (≥8)
— large/medium-sized cerebral infarction on neuroimaging — the need to install a gastrostomy tube or perform a “major” surgical intervention — the need for surgery on the carotid arteries — hemorrhagic transformation — unstable neurological status — elderly patient — uncontrolled arterial hypertension
10. Timing of anticoagulant therapy after intracranial hematoma:
Table 2. Timing of anticoagulant therapy after intracranial hematoma
Prescription or resumption of NOACs is recommended for patients with AF 4–8 weeks after intracranial hematoma, taking into account the identification and treatment of the cause of bleeding and correction of risk factors for hemorrhagic complications.
Factors “against” starting OAC: - Bleeding occurred on an adequate dose of NOAC / without taking it / on a low dose - Older age - Uncontrolled arterial hypertension - Lateral hematoma - Severe intracranial bleeding - Multiple microbleeds (for example, > 10 according to MRI in T2* ) - Causes of bleeding cannot be eliminated - Alcohol abuse
— The need for dual antiplatelet therapy after PCI (percutaneous coronary intervention)
Factors in favor of starting OAC: - Bleeding occurred while taking VKAs or an overdose of NOAC was established - Trauma or a removable cause of bleeding - Young age - Well-controlled hypertension
— Medial hematoma — No evidence of leukoaraiosis in the white matter of the cerebral hemispheres — Surgically removed subdural hematoma — SAH: clipped or embolized aneurysm — High risk of ischemic stroke
Changes in serum concentrations of NOACs due to pharmacokinetic interactions:
* Additional risk factors for hemorrhagic complications:
- Age ≥ 75 years
- Weight ≤ 60 kg
- Impaired renal function (GFR according to Cockcroft-Gault 30–49 ml/min/1.73 m2)
- Taking other medications that increase the risk of bleeding (ASA drugs, NSAIDs, glucocorticoids)
- Previous gastrointestinal bleeding
- Recent (<4 weeks) surgery
- Thrombocytopenia
- HAS-BLED score ≥ 3
New generation anticoagulants
Rivaroxaban (Xarelto), Apixaban (Eliquis), and Dabigatran (Pradaxa)
Despite the fact that Warfarin has been a popular oral anticoagulant for more than 50 years, it has many limitations, such as high individual sensitivity and unpredictability of the anticoagulant effect, drug interactions, a narrow therapeutic range, the need for dose selection and constant monitoring of the INR. All this sharply reduces adherence to treatment and increases the risk of severe hemorrhagic complications.
Therefore, in recent years, the FDA has approved new anticoagulants that are safer and have a wider therapeutic range than their predecessors.
Today, a new generation of anticoagulants, which successfully replace Warfarin in many situations (cardiology, prevention and treatment of deep vein thrombosis of the extremities, in the treatment and prevention of strokes), allows us to get away from the problem of INR control. We are talking about three main drugs: Rivaroxaban (Xarelto), Apixaban (Eliquis) and Dabigatran (Pradaxa).
Factor Xa inhibitors (Eliquis and Xarelto) bind directly to the active site of factor Xa, which leads to blocking of the general coagulation pathway. This results in decreased thrombin formation, which in turn inhibits clot formation and platelet activation.
Direct thrombin inhibitors (Pradaxa) bind specifically and reversibly to the active site of thrombin. Due to thrombin inhibition, fibrin clot formation and platelet aggregation are reduced.
The standard test for monitoring patients on warfarin is the prothrombin time or INR, while the standard test for monitoring patients on heparin is the activated partial thromboplastin time or aPTT. Unfortunately, to date there are no laboratory tests to monitor patients taking NOACs. Currently, routine laboratory monitoring in patients taking new oral anticoagulants (NOACs) is not accepted due to the proven effectiveness of fixed doses. Therefore, the real degree of hypocoagulation in such patients is almost impossible to determine, which extremely complicates management tactics during surgical manipulations in the oral cavity. NOACs were developed as a physician- and patient-friendly alternative to vitamin K antagonists (VKAs) that did not require regular laboratory visits.
The number of patients with coronary heart disease (CHD) receiving acetylsalicylic acid (ASA) and/or thienopyridines to prevent thromboischemic complications or for previously implanted coronary stents is steadily increasing. This determines the significance of the imbalance of perioperative hemorrhagic risk and the likelihood of developing vascular thrombosis and thromboembolism when basic therapy is canceled before the upcoming cardiac surgery [7, 15, 23, 25]. From 3.8 to 8.6% of patients after emergency or elective percutaneous coronary intervention and more than half of patients awaiting elective coronary artery bypass grafting (CABG) receive dual antiplatelet therapy in the perioperative period [12]. Discontinuation of such therapy is associated with an increased risk of stent thrombosis, acute myocardial infarction (AMI), and death [18, 20]. Discontinuation of antiplatelet therapy one week before surgery is a common and serious problem for clinicians. 2-2.5% of patients develop recurrent vascular complications during the “waiting period” [10, 21], and the use of perioperative “bridge” therapy regimens in this category of patients remains a topic of debate [16, 26].
In this regard, three preoperative strategies for discontinuing antiplatelet agents are discussed: 1) the use of heparins; 2) use of IIb/IIIa-glycoprotein platelet receptor blockers; 3) prescription of cangrelor [6, 9]. In most clinics in the Russian Federation, before CABG surgery, a strategy of heparin therapy is used with simultaneous withdrawal of oral antiplatelet agents. It should be noted that the introduction of unfractionated and low molecular weight heparins did not demonstrate their effectiveness in this case [13], including due to the possible development of “rebound” hypercoagulation after sudden withdrawal, and due to the proaggregant potential of heparins. Heparin resistance to both unfractionated and low molecular weight heparin was noted in 22% of patients operated on using artificial circulation [1, 19, 22]. The development of heparin-induced thrombocytopenia remains a problem [24]. When studying the need for blood transfusions in four groups of patients (receiving clopidogrel and aspirin, monotherapy with these drugs and not receiving antiplatelet agents), the likelihood of blood transfusions was highest in the clopidogrel and aspirin group [8].
The purpose of the study was to analyze the safety (from the perspective of postoperative hemorrhagic complications) and the effectiveness of maintaining basic antiplatelet therapy before CABG in patients at high risk of developing acute coronary complications.
Material and methods
The study was approved by the local ethics committee of the Federal State Budgetary Institution Research Institute of the Communist Party of the Soviet Union, Siberian Branch of the Russian Academy of Medical Sciences, all patients signed informed consent. A prospective study was conducted, which included 321 patients with coronary artery disease who were consecutively admitted to the clinic during 2013 for direct myocardial revascularization. Initially, all study participants received ASA as part of basic therapy before the main hospitalization.
Depending on the severity of coronary lesions, functional class of angina and preoperative therapy tactics, all patients were divided into two groups:
— ASA group (main) — 103 patients with high risk (coronary artery disease, angina pectoris class III-IV; stenosis of the left coronary artery trunk ≥50% in the proximal/middle segments or three-vessel coronary artery disease with stenoses ≥70%; acute coronary syndrome/ acute ischemic cerebrovascular accident; stenting or angioplasty of coronary or non-cardiac arteries within the previous 12 months), who continued to receive ASA at a dose of 125 mg/day before surgery;
— Heparin group (control) — 218 patients who were discontinued ASA before surgery and were prescribed unfractionated heparin 20,000 units/day.
Clinical characteristics of the groups are presented in Table. 1
.
Patients who underwent emergency interventions were excluded from the study; simultaneous interventions on other arterial basins or valve apparatus; repeated operations; patients with diagnosed coagulopathies.
In all cases, CABG was performed under conditions of artificial circulation with standardized anesthesia and perfusion support: cold blood cardioplegia or Custodiol solution; primary fill volume 1200 ml (modified gelatin, mannitol, sodium bicarbonate, polyion balanced solution) without the use of fresh frozen plasma; induction of anesthesia with propofol (2 mg/kg body weight); maintenance of anesthesia - propofol infusion under the control of BIS monitoring + fentanyl infusion; the beginning of artificial circulation at a level of activated blood clotting time >400 s; heparin binding with protamine sulfate (1 mg per 1 mg); target hemoglobin level 90 g/l with venous blood saturation ≥65%; normothermia during the entire operation.
In the perioperative period (initial, 24 and 48 hours after surgery), general clinical laboratory parameters, platelet count, activated partial thromboplastin time (aPTT), concentration of fibrinogen, soluble fibrin-monomer complexes (SFMC) were assessed using standard laboratory techniques. Clinical indicators were taken into account: the rate of drainage postoperative blood loss (6, 12 and 24 hours after surgery), quantitative and qualitative characteristics of transfusion therapy, the need for repeated sternotomies associated with bleeding, the frequency of perioperative AMI, rhythm disturbances, bleeding from the gastrointestinal tract, the need in renal replacement therapy, pulmonary complications, deaths.
Statistical processing was carried out using the Statistica 6.0 program, nonparametric tests were used (Mann-Whitney, Wilcoxon, Bonferroni correction). Differences were considered statistically significant at p
<0,05.
Results and discussion
The risk of thrombosis increases due to the stress hormonal response and the release of cytokines that develop in response to traumatic operations, which increase the shear stress of atherosclerotic plaques, increase vascular reactivity, increasing the tendency to vasospasm, increase platelet activation and lead to the formation of a hypercoagulable prothrombotic and proinflammatory state that promotes increasing the risk of developing arterial thrombosis [8]. In the preoperative period, not a single case of AMI was registered in the group with preserved disaggregant therapy, while in the group with heparin there were 6 (2.8%) patients, which required a change in tactics: percutaneous coronary intervention for ACS with stenting of the symptom-dependent artery with subsequent complete revascularization in a delayed manner - in 4 and emergency CABG - in 2. These patients were excluded from the study. When surgery cannot be delayed due to a life-threatening condition, it is necessary to minimize the risk of ischemic and hemorrhagic complications by carefully monitoring the patient's condition, ensuring the availability of endovascular intervention and pharmacological prevention of thrombus formation [5].
Both study groups were comparable in terms of the main parameters of the surgical period (Table 2)
.
The average amount of intraoperative blood loss was comparable. The hemoglobin level after surgery also did not differ, although in the main group the frequency of red blood cell transfusions was statistically significantly higher. The latter fact was due to a higher rate of drainage losses in the main group (Table 3)
, but the maximum value of these losses still did not exceed acceptable limits.
According to other authors [4], due to hemorrhagic complications, about 50-60% of cardiac surgical patients receive allogeneic blood components in the perioperative period, and up to 20% of all reserves of preserved blood products are used to treat these patients.
Transfusion of blood components has been an important component of CABG since its inception; previously, almost all patients received blood transfusions. Currently, the need for transfusion therapy is associated with an increase in the number of patients with an unfavorable combination of risk factors - severe somatic pathology and, therefore, a higher risk of developing surgical complications after interventions and the use of antiplatelet and anticoagulant therapy in the preoperative period [11]. According to the protocol adopted in the clinic, in the ASA group, 93 (90.3%) patients underwent transfusion of platelet concentrate in the post-perfusion period; in the Heparin group it was used only in 2 (0.9%) cases. However, it should be noted that with comparable platelet counts in the groups after 48 hours, patients receiving antiplatelet therapy showed statistically significant thrombocytopenia (median platelet count 162·1012) in comparison with the Heparin group (median 252·1012). The existing risk of bleeding associated with the use of antiplatelet agents largely depends on the degree of inhibition of platelet function and the ability of “new” platelets to restore aggregation activity after discontinuation of the drug, which leads to individual variability in the activity of platelet ADP receptors [17].
When analyzing the basic parameters of hemostasis, the initial values of APTT and RFMC did not differ significantly (Table 4)
, and the level of fibrinogen was higher in the Heparin group (
p
= 0.029).
One day after surgery, the content of fibrinogen and APTT in the groups was comparable, and the level of RFMC was statistically insignificantly higher in the Heparin group ( p
= 0.045), generally remaining above normal values in both groups. Moreover, transfusion of fresh frozen plasma was more often used in the ASA group than in the Heparin group - in 21 (20.4%) and 30 (13.8%) cases, respectively.
In 2 (1.9%) patients in the ASA group, AMI developed, the causes of which were not related to the state of hemostasis (coronary bypassography revealed surgical defects in the formation of anastomoses with the coronary arteries, which required percutaneous coronary intervention). When analyzing other complications (Table 5)
It should be noted that some of them (rhythm disturbances, gastrointestinal pathology) in the main group were primarily associated with a more severe initial comorbid background.
The higher incidence of acute lung injury in the same group is explained by more aggressive transfusion tactics, which is consistent with the opinion of some authors [14].
According to the literature [3], repeated operations for bleeding are required in 2-9% of cases after CABG. In the present study, the need for repeated sternotomies due to insufficient surgical hemostasis, as well as mortality rates in the groups, were comparable to each other and to the data of foreign authors. Postoperative bleeding and reoperations to stop it were associated primarily with prolongation of stay in the intensive care unit, an increase in the number of blood transfusions and the development of respiratory complications, which increases the cost of treatment, increases the incidence of postoperative complications (including the risk of AMI, acute renal failure, stroke, wound infections, immunodeficiency) and mortality. [14]. However, there is relative agreement in existing recommendations for the perioperative use of ASA: ASA should be lifelong and not interrupted before surgery if ASA is taken for the purpose of secondary prevention after acute cerebrovascular accident, acute coronary syndrome, AMI or coronary revascularization, regardless of the time that has passed since the drug was prescribed, with the exception of cases associated with a high risk of hemorrhagic complications [2]. Thus, our study shows that continued use of antiplatelet agents before CABG surgery in patients with a high risk of cardiovascular complications, although it required changes in transfusion therapy, was not accompanied by an increase in the volume of blood loss and the frequency of perioperative complications, and also provided prevention of AMI during the period "waiting for surgery." The results obtained make it possible to expand the indications for continuing antiplatelet therapy immediately until the stage of coronary bypass surgery.
Dental appointment
Factors influencing decision making:
- the scale of the planned operation;
- degree of hypocoagulation (monotherapy, dual antiplatelet therapy, Heparin, Warfarin, NOAC, etc.);
- topographic anatomy of the operated area.
Oral surgeries with the highest risk of possible bleeding include:
- Sinus lift.
- Invasive procedures in the floor of the mouth.
- Removal of impacted and dystopic wisdom teeth.
Assessing the risk of thromboembolism (depends on the disease and requires consultation with your doctor).
Medical Internet conferences
Currently, the use of dual antiplatelet therapy with clopidogrel and acetylsalicylic acid (ASA), which affect various mechanisms of platelet aggregation, is relevant in the treatment of patients with myocardial infarction. The advantage of such therapy is a reduction in the relative risk of cardiovascular complications and mortality.
Recently, issues of clinical and biochemical resistance to antiplatelet drugs have been widely discussed. According to literature data, the absence of an antiplatelet effect is detected in 5-40% of patients with long-term use of ASA and in 8-30% when taking clopidogrel (Puchinyan N.F., 2010, Sulimov V.A., Moroz E.V., 2012, Sibbing D., 2009).
One of the reasons (by no means the only one) for such resistance is seen in the genetic characteristics of the body, in the so-called polymorphism of the gene responsible for the synthesis of the enzyme that converts clopidogrel.
In such cases, clopidogrel should either be prescribed in increased doses (both loading and single doses), or other antiplatelet agents (ticagrelor, prasugrel) should be prescribed instead of clopidogrel.
One of the ways to increase the effectiveness of antiplatelet therapy is to carry out genotyping in all patients who are prescribed clopidogrel, in order to identify individuals with so-called genetic polymorphism.
One of the latest achievements of Russian medicine in the management of patients with coronary artery disease is the development of an innovative method of thrombodynamics: a highly sensitive test for diagnosing hemostasis and assessing the risks of thrombosis and hemorrhagic complications, allowing to identify a tendency to hypercoagulable states at an early stage, when other methods are not yet sensitive enough.
Clinical observation can serve as a demonstration of the management tactics of a patient with myocardial infarction receiving antiplatelet and anticoagulant therapy.
Patient Z., 65 years old, in March 2015 suffered an anterior widespread Q-myocardial infarction, complicated by heart failure. Blood test: TC -3.62 mmol/l, HDL cholesterol - 0.9 mmol/l, LDL cholesterol - 2.45 mmol/l, TG - 0.6 mmol/l. KA – 3. Coagulogram: fibrinogen – 3.1 g/l, APTT – 23.1″, TT 17.8″, INR – 1.6, PTT – 15.7″.
Ultrasound of the heart. Local contractility of the left ventricle is characterized by: akinesia of all apical segments, hypokinesia of the middle - anterior, anteroseptal segments of the LV. Global contractility of the left ventricle is reduced - EF: 49%. Moderate nonspecific degenerative changes in the walls of the aorta, aortic valve and fibrous structures of the heart. Slight expansion of the left atrium (DAC - 41mm). Minor aortic regurgitation grade 1. Minor mitral regurgitation grade 1.
As part of standard drug therapy, the patient was prescribed dual antiplatelet therapy with cardiomagnyl and clopidogrel (75 mg/s). During treatment, thrombodynamics studies were carried out. 04/16/2015 Hypercoagulation. An increase in the clot growth rate (31.1 µm/min) is recorded, which also leads to an increase in the final clot size by 30 minutes. (1223µm). An increase in the density of the fibrin clot is recorded (33.405 conventional units). The formation of spontaneous clots is not recorded.
In this regard, a blood test was carried out to determine genetic polymorphisms (resistance to clopidogrel). Result: the AA genotype was identified (“slow” CYP2C19 metabolizer). A different clopidogrel dosage regimen was selected (loading dose per day 600 mg/s, on subsequent days - maintenance dose 150 mg/s). A repeated study of thrombodynamics did not reveal any positive changes; signs of hypercoagulation remained.
2 months after MI, cardiac ultrasound showed signs of dyskinesia and decreased global contractility of the left ventricle—EF 47%. It was found that the perimeter of the LV apex was almost completely lined by a mural thrombus, up to 27 mm high.
The patient underwent coronary angiography. The type of blood circulation is right-sided. The LCA barrel has not been changed. OS - stenosis in the middle segment up to 70%, stenosis in the proximal segment of VTK1 up to 80%. LAD – calcification, diffuse atheromatosis, stenosis in the middle segment up to 80%, in the distal segment – up to 70%. RCA - calcification, diffuse atheromatosis, stenosis in the proximal segment of 70%, a series of stenoses at the border of the proximal and middle segments up to 70%.
The patient was recommended surgical treatment: coronary artery bypass grafting, which he refused.
Subsequently, the patient was prescribed warfarin at a dose of 7.5 mg/s with further dose adjustment, and continued taking cardiomagnyl. INR control indicated achievement of the target level (2.4 - 3.6 - 1.9 - 2.8 - 2.6).
A control ultrasound examination of the heart after 3 months of warfarin treatment revealed a flat mural thrombus lining the apical region.
Warfarin treatment was continued. Repeated cardiac ultrasound 3 months later revealed a small planar aneurysm of the apical localization and increased trabecularity of the apical region. Global LV contractility remained reduced – EF: 49%. No reliable evidence for the presence of a blood clot was identified.
While warfarin therapy is ongoing, the LV cavity must be monitored to confirm the disappearance of the thrombus - a fact that is not easy to explain.
This observation suggests that dose adjustment of clopidogrel did not lead to an improvement in thrombodynamics and, perhaps, this contributed to thrombus formation in the left ventricular cavity, and the presence of an intracardiac thrombus is a direct indication for anticoagulant therapy.
Patient taking antiplatelet drugs
Monotherapy
Recent studies have shown that basic surgical skills and local bleeding control measures are sufficient to control bleeding. Antiplatelet monotherapy does not pose a significant risk during or after surgery.
Recommendations [6]: it is strictly not recommended to interrupt the intake of oral antiplatelet agents during outpatient dental procedures, including surgical procedures.
Dual antiplatelet therapy after stent implantation in patients with stable coronary artery disease or acute coronary syndrome (ACS)
Cardiologists recommend dual antiplatelet therapy for at least six weeks after bare metal (inactive) stent implantation, and for 12 months after an episode of ACS or drug-eluting stent (active stent) implantation.
Recommendations [7]: Low-risk dentoalveolar surgery does not require any changes in the treatment plan.
Dentoalveolar surgery with an average risk of bleeding requires consultation with a cardiologist about temporarily stopping one of the drugs before the surgical procedure (monotherapy does not pose a serious risk). If it is not possible to cancel one of the drugs, you can perform the surgical procedure in stages: divide the surgical procedure with an average risk of bleeding into several smaller interventions.
Dentoalveolar surgical procedures with a high risk of bleeding require consultation with a cardiologist regarding temporary discontinuation of one of the medications before the surgical procedure. If it is not possible to discontinue one of the drugs, major surgical operations should be delayed for 1 year.
Dual antiplatelet therapy: areas of clinical application
Platelets, atherothrombosis and antiplatelet drugs Platelets play an important role both in the development and progression of atherosclerosis and in the pathogenesis of ACS. Coronary intervention, especially associated with stent implantation, is also accompanied by significant changes in the functional state of platelets. Activation of platelets and their subsequent aggregation occur under the influence of various mediators, the most important of which are thromboxane A2 and ADP. Antiplatelet or antiplatelet drugs are used to suppress platelet activation and prevent aggregation [5–9]. Acetylsalicylic acid (ASA) blocks platelet activation by inhibiting cyclooxygenase and thromboxane A2 formation. In most patients with stable coronary artery disease, it is preferable to prescribe ASA in low doses due to the favorable benefit-risk ratio, as well as the low cost of treatment. ASA remains the basis of drug prevention of arterial thrombosis [8, 9]. The mechanism of action of ASA is the irreversible inhibition of platelet cyclooxygenase-1 (COX-1) and disruption of thromboxane synthesis. Complete suppression of thromboxane production is achieved with constant long-term administration of ASA in doses ≥ 75 mg/day. The damaging effect of ASA on the gastrointestinal tract increases as the dose increases. The drug is recommended for all patients with an established diagnosis of coronary artery disease without any restrictions on the duration of use. The optimal balance of benefit and risk is achieved when using ASA in a dose range from 75 to 150 mg/day, and when used as part of DAT, the dose is usually 75–100 mg. The effects of ADP occur through P2Y12 receptors, whose antagonists are currently the second most frequently used group of antiplatelet drugs. Irreversible inhibitors of P2Y12 receptors include thienopyridines (ticlopidine, clopidogrel and prasugrel), and reversible inhibitors include ticagrelor and cangrelor. Clopidogrel is the most famous representative of the thienopyridine group today [5]. The evidence base for the use of this drug in patients with stable coronary artery disease was the CAPRIE study [10]. In this study, which included high-risk patients (recent MI, stroke, and intermittent claudication), clopidogrel 75 mg was more effective and had a better safety profile than ASA 325 mg in preventing vascular complications [10]. Subgroup analysis showed the benefits of clopidogrel only in patients with atherosclerotic lesions of peripheral arteries. Therefore, clopidogrel should be considered a second-line drug prescribed for intolerance to ASA [8, 9] or as an alternative to ASA in patients with advanced atherosclerotic lesions. Clopidogrel, like ticlopidine and prasugrel, is a prodrug. The drug has a complex metabolism. The absorption of clopidogrel in the intestine is controlled by a special protein (P-glycoprotein), encoded by the ABCB1 gene. Only about 15% of absorbed clopidogrel is converted into the active metabolite in the liver. The process is two-step (oxidation and hydrolysis), depending on several isoenzymes of the cytochrome 450 system, the most important of which are CYP2C19 and CYP3A4. It is assumed that the variability of the antithrombotic effect of clopidogrel in different patients may be due to a number of pharmacokinetic factors, including. insufficient shock and maintenance dose of the drug, impaired absorption, formation of an active metabolite from the prodrug (due to polymorphism of genes encoding isoenzymes of the cytochrome 450 system), drug interactions. During treatment with antiplatelet drugs, primarily clopidogrel, there is variability in parameters characterizing residual platelet reactivity. In this regard, the possibility of adjusting antiplatelet therapy based on the results of platelet function studies and the pharmacokinetics of clopidogrel is of interest. It has been established that the preservation of platelet reactivity is determined by factors such as gender, age, the presence of ACS, diabetes mellitus, as well as increased platelet consumption, concomitant use of other medications and low patient adherence to treatment. Specific to clopidogrel is the carriage of single nucleotide polymorphisms associated with decreased absorption of the drug in the intestine (ABC1 C3435T gene) or its activation in the liver (CYP2C19*2 gene). The effect of carriage of these genetic variants on the outcomes of treatment with clopidogrel has been proven for patients with ACS undergoing invasive treatment; There are no similar data for patients with stable coronary artery disease. Therefore, a routine study of the pharmacokinetics of clopidogrel and assessment of ORT in patients with stable coronary artery disease, incl. undergoing elective PCI are not recommended [6–9]. Prasugrel is characterized by a faster onset of therapeutic action compared to clopidogrel [5]. The formation of an active metabolite in the liver also occurs under the influence of isoenzymes of the cytochrome 450 system in several stages. Individual variability in the effect of the drug cannot be excluded. In the TRITON–TIMI 38 study in patients with ACS with and without ST-segment elevation on the ECG and planned PCI, treatment with prasugrel compared with clopidogrel was associated with a significantly lower number of cardiovascular events, but with a greater risk of major bleeding, including fatal [11 ]. Currently, the use of prasugrel for ACS is limited to cases of planned PCI in individuals who do not have a high risk of developing hemorrhagic complications. The use of prasugrel is contraindicated in patients with a history of stroke/transient ischemic attack, and is not recommended in the age group over 75 years (except for those with diabetes mellitus and recurrent myocardial infarction), as well as in those weighing less than 60 kg [6]. Prasugrel should not be prescribed in cases of suspected emergency coronary artery bypass grafting (CABG). The drug has not been studied in ACS patients receiving thrombolytic therapy. The loading dose of prasugrel is 60 mg, the maintenance dose is 10 mg, which can be prescribed in combination with ASA for a period of at least 12 months. in patients who have undergone ACS [6, 7]. Ticagrelor is a pyrimidine derivative (triazolpyrimidine), a direct selective reversible inhibitor of the platelet P2Y12 receptor, does not require preliminary metabolism and cofactors [5]. The drug is an active substance that is metabolized through the CYP3A4 isoenzyme to form an active metabolite. The active metabolite is formed in the liver with the participation of cytochrome P450 3A4. The degree of inhibition of P2Y12 receptors is determined primarily by the plasma levels of ticagrelor and, to a lesser extent, its active metabolite. The half-life is about 12 hours, and therefore the drug is prescribed 2 times a day. Like prasugrel, ticagrelor has a faster onset of therapeutic action and provides more pronounced and persistent inhibition of platelet activation compared with clopidogrel. At the same time, restoration of platelet function after discontinuation of ticagrelor occurs faster compared to clopidogrel. Along with the effect on platelet function, ticagrelor has other effects that are associated with its ability to stimulate adenosine receptors (shortness of breath, pauses in ventricular contractions, increased levels of creatinine and uric acid in the blood, and possibly others). The presence of more attractive pharmacological properties, as well as the problems associated with taking clopidogrel, were studied in the large-scale PLATO (Platelet inhibition and patient outcomes) study, which compared the effectiveness and safety of ticagrelor with those of clopidogrel in patients taking ACS [12] (see. Further). DAPT PCI in patients with stable angina pectoris Optimal drug therapy for stable coronary artery disease, including ASA, statins, angiotensin-converting enzyme (ACE) inhibitors, β-blockers and other antianginal drugs, improves the prognosis and allows long-term effective control of angina attacks [3, 8, 9]. However, if angina attacks persist or there are episodes of silent myocardial ischemia, angiographic examination and invasive treatment are indicated. If PCI is performed to restore coronary blood flow, antiplatelet therapy before and after the intervention has a number of features. Before implantation of coronary stents, especially drug-eluting stents (DES), patients should be counseled about the need for long-term DAPT, the associated bleeding risks, and the use of alternative treatment options if patients are unwilling or unable to adhere to the recommended duration of DAPT [ 6]. If the patient has had an angiographic study and it is decided to perform a planned PCI, then he continues to receive ASA at a dose of 75–150 mg/day, and 5–7 days before the intervention, clopidogrel 75 mg 1 time/day is added to the treatment. According to the updated recommendations of the American College of Cardiology/American Heart Association/Interventional Cardiology Specialists (ACC/AHA/SCAI), the dose and duration of ASA administration depend on both the type of stent and the risk of bleeding in a given patient [6]. In the early period after stenting – after implantation of a bare metal stent (BMS) – 1 month, DES – 3–6 months. (depending on the drug) patients should take ASA at an increased dose of 162–325 mg/day. Subsequently, its use should be continued on an ongoing basis at a dose of 75–162 mg/day. regardless of the type of stent. In case of an increased risk of bleeding, it is allowed to use ASA at a dose of 75–162 mg/day. and immediately after stenting. The recommendations of the European Society of Cardiology (ESC) for the use of DAPT in patients with stable coronary artery disease during invasive treatment are presented in Table 1. If a decision is made to perform PCI immediately after angiographic treatment, and the patient was only on ASA therapy, it is given directly in the cath lab a loading dose of clopidogrel 300 mg, after which endovascular intervention is performed. In both cases, after invasive treatment, the patient receives DAPT ASA and clopidogrel 75 mg/day. The third generation thienopyridine - prasugrel, as well as a drug with a reversible mechanism of P2Y12 receptor blockade - ticagrelor, as noted earlier, cause a stronger inhibition of platelet aggregation compared to clopidogrel, however, there have been no clinical studies studying prasugrel and ticagrelor in patients with stable ischemic heart disease. . It should be noted that the duration of taking thienopyridines is the most important preventive measure for the development of stent thrombosis, incl. and later. According to current recommendations, after DES implantation, in the absence of a high risk of bleeding, patients should take clopidogrel at a dose of 75 mg/day. for at least 12 months. Given the importance of DAPT in the prevention of late DES thrombosis, it is recommended to postpone elective operations (for example, elective cholecystectomy) until the end of the course of clopidogrel. After BMS implantation, patients should take clopidogrel at a dose of 75 mg/day. for at least 1 month, ideally up to 12 months, and in case of a high risk of bleeding, clopidogrel should be taken for at least 2 weeks. DAPT for stable CAD without PCI The use of DAPT for stable CAD could provide more effective prevention of coronary thrombosis. In the CHARISMA study, which included stable patients with atherosclerotic lesions of various vascular territories or multiple cardiovascular risk factors, the addition of clopidogrel to ASA did not bring additional benefit [13]. Subgroup analysis of this study revealed a positive effect of the combination of ASA and clopidogrel only in patients with coronary artery disease who had suffered a myocardial infarction. The 2012 new American guidelines for the treatment of stable coronary artery disease in the section “Antiplatelet therapy” note that ASA 75–162 mg and clopidogrel 75 mg can be prescribed in certain patients with a high risk of complications - class IIb recommendations, level of evidence B [8 ]. The 2013 European Society of Cardiology guidelines indicate that DAPT is only beneficial in certain categories of patients at high risk of ischemic events. Routine administration of this therapy to patients with stable coronary artery disease is not recommended [9]. Platelets and the development of acute coronary thrombosis As already noted, the administration of DAPT, which includes ASA and a P2Y12 receptor inhibitor (clopidogrel), is recognized as mandatory in patients with ACS, as well as in persons who have undergone PCI. However, in some patients, despite regularly taking a combination of these antiplatelet drugs, there is a lack of the expected effect in preventing the risk of thrombotic events, on the one hand, and observed bleeding, on the other. The presence of more attractive pharmacological properties, as well as existing problems associated with taking clopidogrel, were the main reasons for organizing the large-scale PLATO (Platelet inhibition and patient outcomes) study, which compared the effectiveness and safety of ticagrelor with those of clopidogrel among patients with ACS [12]. The study included 18,624 patients with ACS with and without ST segment elevation on ECG [12]. The study was a double-blind, randomized, prospective study with a follow-up period of 6–12 months. The main exclusion criteria included: contraindications to the use of clopidogrel, thrombolytic therapy within 24 hours before randomization, the need to take indirect anticoagulants, the index event (acute complication of PCI) or PCI performed before taking the first dose of the study drug, increased risk of bradycardia, concomitant prescription of strong inducers or inhibitors of the CYP3A4 isoenzyme. Patients were randomized to receive ticagrelor at a loading dose of 180 mg, followed by a maintenance dose of 90 mg 2 times a day. or clopidogrel 300–600 mg – loading dose and 75 mg/day. - supportive. All patients received ASA at a dose of 75–100 mg, with the exception of those with known intolerance to the drug. For patients who had not previously received ASA, a loading dose of 325 mg was recommended (doses from 160 to 500 mg were allowed). A dose of 325 mg of ASA was also approved for daily use for 6 months. after stent implantation. In cases where CABG was necessary, the study drug was discontinued: clopidogrel – 5 days, and ticagrelor – 24–72 hours before surgery. During therapy with ticagrelor, compared with clopidogrel, there was a significant reduction in the total number of events of the primary end point (cardiovascular death, MI or stroke): 9.8 versus 11.7%, a risk reduction of 16%, p < 0.001 (Fig. . 1). In those receiving ticagrelor, compared with those treated with clopidogrel, there was a significant decrease in the incidence of MI from 6.9 to 5.8%, and cardiovascular death from 5.1 to 4%. At the same time, the total number of strokes was the same in both subgroups: 1.5 and 1.3%. Hemorrhagic stroke was slightly more common when taking ticagrelor than clopidogrel - 23 (0.2%) and 13 (0.1%). However, their total number was insignificant and the difference was unreliable. The incidence of a combined secondary endpoint (death from vascular causes, myocardial infarction, stroke, recurrent myocardial ischemia, transient ischemic attack or other types of arterial thrombosis), as well as death from all causes, was also significantly lower in the ticagrelor group compared with clopidogrel: 14. 6 versus 16.7% and 4.5 versus 5.9%, respectively. The beneficial effect of ticagrelor was greater in patients with a positive initial troponin level compared with troponin-negative patients, and in those with a final diagnosis of myocardial infarction compared with those with a discharge diagnosis of unstable angina. Greater efficacy of ticagrelor, compared with clopidogrel, was observed in various subgroups of patients, but was lower in patients with low body weight, in those not taking lipid-lowering therapy, and in residents of North America. There were no significant differences between the groups in the incidence of major bleeding or fatal or life-threatening bleeding. The risk of major bleeding, including fatal intracranial bleeding not related to the CABG procedure, was slightly higher in the ticagrelor group compared with clopidogrel (4.5 vs. 3.8%, p=0.03). However, the incidence of CABG-related bleeding was lower among those receiving ticagrelor (7.4 vs. 7.9%, p-nd). The results of 13,408 (72%) patients with an invasive treatment strategy planned at the randomization stage were analyzed separately [14]. 49.1% of patients were diagnosed with ACS with ST segment elevation on the ECG and in 50.9% - ACS without ST segment elevation on the ECG. During the first hospitalization, PCI was performed in 10,298 (72%) patients and CABG in 782 (5.8%). The average time to PCI was 2.4 hours (0.8–20.1) after randomization in patients with ACS without ST segment elevation on the ECG and 0.5 hours (0.2–1) in patients with ACS with ST segment elevation on ECG. The average time to CABG was 6 days [3–10]. The total number of MIs, strokes and cardiovascular deaths during therapy with ticagrelor decreased to 9%, compared with clopidogrel - 10.7%, i.e. the risk reduction was 16% (p<0.0025). It is important to emphasize that the benefit of ticagrelor on the primary endpoint was observed in different subgroups and was independent of the loading dose of clopidogrel. Large bleeding was equally often found both in those who took Tikagrelor and in the treated clopidogrels (11.6 versus 11.5%, pp). The number of cases of stent thrombosis was significantly lower in the Tikagrelor group, and, both when using SLP and without it. The incidence of certain stent thrombosis in patients receiving ticagrelor was significantly lower at both 30 days and 360 days of follow-up compared with those treated with clopidogrel, including those patients taking a loading dose of 600 mg or more. When analyzing a fragment of a study, which included 1261 patients subjected to AKSh, within 7 days from the last administration of the drug under study, a reliable difference in reducing the number of primary end points was not revealed (10.6% in the group of ticagrelor and 13.1% - clopidogrel). At the same time, among those who took Tikagrelor, a reliable decrease in total mortality was observed by 51%, and the cardiovascular-by 48%, both in the early and later stages of operation. In 5 216 (28%) out of 18,624 patients entering the hospital with OKS, non -invasive tactics of treatment were chosen. 2 183 (41.9%) patients conducted coronary angiography during their hospitalization in the clinic, 1065 (20.4%) - ChKV and 208 (4%) patients - AKSh. Ultimately, 3,143 (60.3%) patients were carried out conservative therapy until the study was completed. The frequency of reaching the primary final point against the background of Tikagrelor was taking was lower compared to clopidogrel (12% (n = 295) V 14.3% (346); relative risk 0.85, 95% CI 0.73–1.00; p =0.04). The total mortality rate was also lower (6.1% (147) compared with 8.2% (195); 0.75, 0.61–0.93; P = 0.01). The total frequency of large bleeding (11.9% (272) compared with 10.3% (238); 1.17, 0.98–1.39; P = 0.08) and the frequency of large bleeding not associated with AKSh (4% (90) compared with 3.1% (71); 1.30, 0.95–1.77; p = 0.10), was numerically higher for the background of the intake of ticagrelor, compared with the clopidogrel. Thus, in patients with ACS with an initially planned non-invasive strategy, the advantage of ticagrelor therapy, compared with clopidogrel, is consistent with the data obtained in the Plato study in general, which indicates significant advantages of the inhibition of P2Y12-receptors with ticagrelor, regardless of the planned treatment strategy. RLATO was the first large -scale study, in which the clinical efficiency of thicrelor was demonstrated in relation to a decrease in the frequency of development of the main vascular events in patients with ACS, without a significant increase in the risk of bleeding. The advantages of Tikagrelor over Clopidogrel were observed regarding the risk of developing him, general and cardiovascular mortality, but not with respect to a stroke. The high efficiency of Tikagrelor was noted in comparison with Clopidogrel both in the early (in the first 30 days) and in a later date (from the 31st to the 31st of the 360th day) of treatment. The positive effect of ACS patients was obvious regardless of the planned treatment strategy (invasive or conservative), as well as from taking higher load doses of clopidogrel. A more significant reduction in the risk of developing thrombotic episodes on therapy with ticagrelor, apparently, is due to faster and intense inhibiting P2Y12-receptors of platelets. When prescribing a loading dose of a clopidogrel of 600 mg, 2-4 hours are required to achieve 50% inhibiting platelet aggregation, and the same effect is achieved after 30 minutes. When taking 180 mg of ticagrelor or 60 mg prasugrel. In addition, there is a fairly large group of patients with the presence of defective options for alleles of the cytochrome system 450, which is associated with a slowdown in the formation of an active metabolite of clopidogrel, insufficient suppression of platelet function when taking it, as well as with a higher risk of cardiovascular complications after ACS and CCV. The advantages of ticagrelor also include the reversible nature of inhibition of platelet P2Y12 receptors, which means a more rapid cessation of the antiplatelet effect after discontinuation of the drug. This circumstance seems important during invasive interventions, as well as before the upcoming CABG procedure. Although the incidence of major bleeding was no lower with ticagrelor than with clopidogrel, it should be noted that the greater inhibition of platelet function was not associated with an increase in the incidence of major bleeding. This distinguishes ticagrelor from prasugrel, whose more pronounced antiplatelet effect is accompanied by an increased risk of major bleeding. Tikagrelor was more more often than a clopidogrel, was canceled in connection with the development of undesirable phenomena: in 7.4 and 6% of patients, respectively (p <0.001). Shortness of breath was more often recorded in the Tikagrelor group than clopidogrel - 13.8 VS 7.8%, p <0.001. The appearance of shortness of breath, as a rule, was of a transient character, was not associated with a deterioration in the function of the cardiovascular system and lungs. Shortness of breath caused the abolition of the drug only in 0.9% of patients taking ticagrelor. During the monitoring of the ECG, the Tikagrelor in the first week was reliably more likely to have pauses than those treated with clopidogrel. Pauses, as a rule, were observed in people who have already had violations of the conducting system of the heart. By the 30th day of treatment, the number of pauses has significantly decreased in both groups, and the difference was leveled. With a possible cause of shortness of breath and pauses, a violation of the reverse capture of adenosine by red blood cells under the influence of thicrelor is associated. As already noted, in 1814 patients from the USA and Canada, the positive effect of Tikagrelor was not confirmed. The number of primary endpoints for those taking ticagrelor in comparison with Clopidogrel was 11.9% VS 9.6%, p-n. The difference in the results obtained from patients in North America and other regions is possibly explained by the features of the population and unequal approaches in clinical practice, in particular, taking higher doses of ASK. Experts of the European Cardiological Society recommended taking a ticagrelor (in a load dose - 180 mg and 90 mg 2 times/day - in supporting) all patients with ACS, regardless of the planned treatment strategy (invasive or conservative). If patients received clopidogrel at the very beginning of the disease, it should be replaced with ticagrelor. The intake of clopidogrel in patients with ORS with invasive or conservative strategies is possible in cases of lack of ticagrelor or prasugrel. The duration of therapy with P2Y12 receptors inhibitors in patients who have undergone ACS is 12 months. In patients on therapy, the P2Y12 receptors inhibitors, in cases of planned surgery (including AKSh), ticagrelor and clopidogrel are canceled in 5 days, and prasogrel for 7 days. This does not apply to people with a high risk of ischemic vascular events. In some cases, in the therapy, genotyping and studying the function of platelets can be carried out in clopidogreel [7]. In the recommendations of the American College of Cardiologists/American Association of Heart/Interventional Cardiology (ACC/AHA/SCAI), it is noted that patients with OKS underlying Tikaggrelor are equally permissible for the use of thicrelor, prasugrel and clopidogrel - first in the loading and then in the supporting dose. The use of ticagrelor was not studied in persons who received thrombolytic therapy. In patients without ACS subjected to the ChKV procedure, it is recommended that the appointment of clopidogrel is recommended. Routine genotyping and studying the function of platelets in all patients receiving clopidogrel is not recommended, although in some cases in high -risk patients and with the alleged inadequate anti -aggregate effect of clopidogrel, such a campaign is possible. In this situation, a ticagrelor and prasogrel can be prescribed as an alternative to clopidogrel. The dose of ASK, with its combination with ticagrelor, should not exceed 75–100 mg/day. The duration of the therapy by the P2Y12 blocker after the stent implantation should be as follows: in patients who were implanted, a stent (HMS or SLP) was implanted during the ChKV for the OCS, the P2Y12 blocker should continue at least 12 months: Options include clopidogrel in a dosage of 75 mg/day . and ticagrelor in a dosage 90 mg 2 times/day. Features of antitromobocyte therapy, according to the recommendations of specialists of the European Cardiological Society for the treatment of patients with ACS without lifting the ST segment, are presented in table 2 [7]. The conclusion of the dates, including ASK in combination with tyenopyridin pester (clopidogrel, prasogrel) or thikagrelor, is an effective method of treating patients with both ACS and stable coronary heart disease subjected to scheduled ChKV. The choice of the second anti -cargant is determined by the form of IBS, the treatment method and the degree of provenness in a specific clinical situation, which is reflected in modern clinical recommendations.
Literature 1. Guide to atherosclerosis and coronary heart disease / Ed. E.I. Chazova, V.V. Kukharchuk, S.A. Boytsova. M.: Media-Medika, 2007. 736 p. 2. Rational pharmacotherapy of cardiovascular diseases / Ed. E.I. Chazova, Yu.A. Karpova. M.: Litterra, 2014. pp. 28–36. 3. Karpov Yu.A., Sorokin E.V. Stable coronary artery disease: strategy and tactics of treatment. 3rd ed. M.: MIA, 2012. 270 p. 4. Karpov Yu.A., Samko A.N., Buza V.V. Coronary angioplasty and stenting. M.: MIA, 2010. 312 p. 5. Kei A.A., Florentin M. et. al. Antiplatelet Drugs: What comes next? // Clin. Applied Thrombosis/Hemostasis. 2011. Vol. 17(1). P. 9–26. 6. Levine GN et al. 2011 ACCF/AHA/SCAI Guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions // J. Am. Coll. Cardiol. 2011. Vol. 58. P. e44–122. 7. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST segment elevation. The Task Force for the management of acute coronary syndromes in patients presenting without persistent ST segment elevation of the European Society of Cardiology // Eur. Heart J. 2011. Vol. 32. P. 2999–3054. 8. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines // Circulation. 2012. Vol. 126. P. e354–e471. 9. 2013 ESC guidelines on the management of stable coronary artery disease. The Task Force on the management of stable coronary artery disease of the European Society of Cardiology.https://eurheartj.oxfordjournals.org/content/early/2013/08/28/eurheartj.eht 10. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischemic events (CAPRIE). CAPRIE Steering Committee // Lancet. 1996. Vol. 348. P. 1329–1339. 11. Wiviotti SD Braunwald E. et al. for the TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes // N. Engl. J. Med. 2007. Vol. 357. P. 2001–2015. 12. Wallentin L, Becker RC, Budaj A et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes // N. Engl. J. Med. 2009. Vol. 361. P. 1045–1057. 13. Bhatt DL, Flather MD, Hacke W. et al. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial // J. Am. Coll. Cardiol. 2007. Vol. 49. P. 1982–1988. 14. Cannon CP, Harrington RA, James S. et al. Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomized double-blind study // Lancet. 2010. Vol. 375. P. 283–293. 15. James SK, Roe MT, Cannon CP at al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes intended for non-invasive management: substudy from prospective randomized PLATelet inhibition and patient Outcomes (PLATO) trial // BMJ. 2011. Vol. 342. P. d3527.