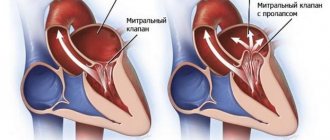
Hypoxemic crisis (dyspnea-cyanotic attack)
A shortness of breath-cyanotic attack is an attack of hypoxia (paroxysmal shortness of breath with severe cyanosis) in a child with blue-type congenital heart disease, most often with tetralogy of Fallot. It occurs as a result of a sudden decrease in pulmonary blood flow associated with spasm of the outflow tract of the right ventricle of the heart, an increase in blood discharge from right to left and hypoxemia in the systemic circulation.
Attacks of hypoxia develop mainly in young children - from 4–6 months to 3 years. Provoking factors for a dyspnea-cyanotic attack can be psycho-emotional stress, increased physical activity, intercurrent diseases accompanied by dehydration (fever, diarrhea), iron deficiency anemia, neuro-reflex excitability syndrome with perinatal damage to the central nervous system, etc.
A dyspnea-cyanotic attack is characterized by a sudden onset. The child becomes restless, moans, cries, and cyanosis and shortness of breath increase. Takes a forced position - lies on its side with its legs brought to the stomach or squats. On auscultation of the heart there is tachycardia, a decrease in intensity or disappearance of systolic murmur over the pulmonary artery. The duration of a hypoxic attack ranges from several minutes to several hours. In severe cases, hypoxemia, acidosis, convulsions, loss of consciousness, even coma, and death are possible.
Heart failure with restored ejection fraction: diagnostic criteria and treatment strategies
Current recommendations for the diagnosis and treatment of heart failure (HF) distinguish three variants depending on the ejection fraction: HF with reduced ejection fraction (<40%), HF with intermediate ejection fraction (40-49%) and HF with preserved ejection fraction ( ≥50%). However, it is clear that in a significant proportion of patients, the ejection fraction may change as the disease progresses. Moreover, this change often occurs not in the direction of decreasing it, but, on the contrary, in the direction of increasing it. Thus, according to observational studies, from 10 to 40% of patients move from the category of HF with low ejection fraction to the category of HF with intermediate/preserved ejection fraction. Various guidelines provide little discussion of the management of these patients, and the JACC Advisory Panel has prepared a document addressing the issue of HF with reduced ejection fraction.
It is noted that the reason for issuing this document was the almost complete lack of information discussed above about the management of such patients in standard recommendations for HF, as well as an attempt to draw attention to the problem of HF with restored ejection fraction.
Speaking about the diagnosis of heart failure with reduced ejection fraction, experts noted that the working definition should be the presence of three criteria:
1. Information about a decrease in ejection fraction <40% in the anamnesis;
2. Absolute improvement in ejection fraction ≥10%;
3. Re-measurement ejection fraction >40%.
These are the criteria used in the vast majority of studies of heart failure with reduced ejection fraction.
Given the lack of information on the effects of long-term treatment withdrawal in these patients, as well as data from the TRED-HF study, which demonstrated worsening of HF even after recovery of ejection fraction when treatment was discontinued in patients with dilated cardiomyopathy, it is emphasized that all patients with HF and restored ejection fraction should continue taking the therapy indicated in the recommendations for patients with heart failure and low ejection fraction.
Regarding the management of such patients, as the authors note, it should be remembered that despite the restored ejection fraction, such patients have an increased risk of developing cardiovascular complications. In addition, it is possible that the ejection fraction will decrease again. In this connection, it is recommended to perform echocardiography every 6 months, including assessment of left ventricular deformation, every 6-12 months - electrocardiography (ECG), as well as a study of the concentration of brain natriuretic peptides. If there is reason to believe that there is an increased risk of developing cardiac arrhythmias (for example, with transthyretin amyloidosis), Holter ECG monitoring is recommended every 1-2 years. It has been discussed that after a year of clinically stable HF with restored ejection fraction, MRI may be considered if it was not performed during HF with low ejection fraction.
Source:
Wilcox JE, et al. J Am Coll Cardiol. 2021 Aug 11;76(6):719-734.
Chronic heart failure: shifting focus to the initial stages of the disease
Chronic heart failure (CHF) is one of the most severe and prognostically unfavorable complications of cardiovascular diseases [1–4]. Today, the prevalence of functional class III–IV CHF in the European part of Russia is 2.3%, and FC I–II CHF reaches 9.4%, which significantly exceeds similar foreign indicators [5]. The number of patients with left ventricular (LV) dysfunction in the country as a whole is approaching, according to some estimates, 12% (16 million people) [6]. From 55 to 295 billion rubles per year are spent on the treatment of CHF in Russia, and the cost of hospitalization for exacerbations of CHF reaches 184.7 billion rubles [7].
CHF is a progressive syndrome, and patients with asymptomatic CHF within 1–5 years can become the most severely ill patients who are difficult to treat. Therefore, early diagnosis of CHF and left ventricular (LV) dysfunction, and therefore early initiation of treatment for such patients, is the key to success in preventing mortality from heart failure. Unfortunately, in Russia, CHF is extremely rarely diagnosed at the initial stage, which indicates the absence of clear criteria for diagnosing CHF in the earliest period of its development [8].
The need to optimize the management of patients with CHF on an outpatient basis, the complexity of this work and the true state of affairs largely became obvious after the completion of the EPOCHA-O-CHF study [5]. This study was based on an analysis of visits from 4586 patients with symptoms of CHF to hospitals and clinics. The study was conducted in 22 regions of the Russian Federation for 3 months. About 2/3 (63%) of all patients with symptoms of CHF sought help in a hospital and only 1/3 (37%) - in a clinic. This can be explained by the fact that patients with CHF seek help only when decompensation becomes clinically significant and requires hospitalization and inpatient treatment. Another reason is the underestimation of the manifestations of the initial stages of CHF, especially in patients with arterial hypertension (AH) and coronary heart disease (CHD). The results of the EPOCHA study clearly demonstrate that in our country the main efforts are aimed at inpatient treatment of decompensated CHF, and not at its early diagnosis and prevention of progression in an outpatient setting. This is precisely what explains the sad fact that Russia has the worst rates in Europe of re-hospitalization of patients with CHF (31% within a month after discharge) and the length of a hospital day for the treatment of decompensation - 27 days. For comparison, similar figures in Europe are 16% and 10–12 bed days, respectively [8].
Another important point was the discovery of the fact that deterioration of systolic function was no longer a mandatory criterion for CHF. Moreover, low contractility in outpatients with CHF is rather an exception to the rule: LV ejection fraction (EF) less than 40% is detected in only 8.4% of patients. The most common finding is normal or almost normal EF in the range of 40–60% (in 52.4% of patients). And finally, 38.8% of outpatients with CHF have a hyperkinetic type of blood circulation with LVEF > 60%, which is associated with the presence of hypertension, LV enlargement (mainly due to myocardial hypertrophy), and normal cavities.
It is not surprising that in 2005, the ACC (American College of Cardiology) and the AHA (American Heart Association) proposed to classify CHF not only by exercise tolerance, but also by the degree of evolution of organ changes, as if combining the internationally accepted NYHA classification with the long-used one in our country using the Obraztsov–Strazhesko–Vasilenko classification (table).
The problem of heart failure in patients with preserved systolic function has recently received much attention. According to the Rochester Epidemiological Study, more than 43% of patients with CHF have an LVEF >50% [9]. A similar picture was observed in the Framingham study: 51% of patients with CHF had an LVEF of more than 50% [10]. Heart failure in patients with preserved systolic function is more common in older people. In this regard, according to experts, the projected number of such patients in developed countries will increase due to an increase in the proportion of elderly patients in the overall structure of CHF. Data from the EPOCHA-O-CHF study show that the situation expected in the future for Europe and America has already arrived in Russia: the proportion of patients with CHF with preserved LVEF (systolic function > 40%) exceeded 80% for outpatients [11].
For a long time, there was no clear concept for the diagnosis and treatment of patients with CHF with preserved systolic function, but with impaired diastolic function. Back in the middle of the century, in the experimental works of E. Sonnenblick, E. Braunwald, F. Z. Meerson, the postulate of the unity of systolic and diastolic disorders underlying the development of heart failure was substantiated. By the beginning of the 80s, a lot of clinical evidence had accumulated, boiling down to the fact that poor contractility and low LVEF do not always clearly determine the severity of decompensation, exercise tolerance, and even the prognosis of patients with CHF.
What are the main difficulties associated with resolving the issue of diastolic CHF today? Firstly, the “Achilles heel” of diagnosis continues to be the lack of an accurate and safe method for assessing diastolic cardiac function. Another problem is the lack of developed approaches to the treatment of diastolic CHF: despite a wide range of drugs that are potentially effective for the treatment of such patients, none of them can be considered ideal. Finally, the last and probably the most important problem is the lack of attention of researchers and doctors to this issue. Simple logic dictates that, based on the prevalence of the phenomenon, at least 1/3 of all large multicenter studies assessing the survival of patients with heart failure should be devoted to patients with diastolic CHF. In fact, such studies are very few (PEP-CHF, CHARM) [29].
According to the recommendations for the diagnosis of CHF with normal LVEF, proposed by the Association of Heart Failure and Echocardiography of the European Society of Cardiology in 2007, diastolic heart failure is also classified as heart failure with normal LVEF.
Normal or moderately reduced LVEF implies both LVEF > 50% and LV end-diastolic volume < 97 ml/m2. For diagnostic confirmation of LV diastolic dysfunction, both invasive (LV end-diastolic pressure > 16 mm Hg or pulmonary capillary wedge pressure > 12 mm Hg) and non-invasive methods can be used: tissue Doppler sonography (E/E` > 15). If the E/E` indicator is > 8 but < 15, then additional non-invasive studies are required to confirm LV diastolic dysfunction. These include determination of transmitral or pulmonary venous flow, LV myocardial mass index or left atrial mass index by echocardiography, atrial fibrillation by ECG, or plasma levels of brain natriuretic peptide [30].
In accordance with the modern model of the pathogenesis of CHF, this condition is considered, first of all, as a pathology of neurohumoral mechanisms of blood circulation regulation, one of which is an increase in the activity of the sympathoadrenal system (SAS) [12]. The initial activation of the SAS is compensatory in nature, but is subsequently characterized by a whole complex of maladaptive adverse consequences [13]. In the appearance and progression of symptoms of CHF, an important place is played by the activation of the sympathetic nervous system, which, along with an increase in the activity of the renin-angiotensin-aldosterone system, leads to the retention of sodium and water ions, to vasoconstriction and a decrease in the contractile function of the LV of the heart [17].
In this regard, to study the role of dysfunctions of the autonomic nervous system involved in regulatory mechanisms, the assessment of heart rate variability (HRV) seems to be a promising direction [14, 18]. In recent years, the method of studying HRV has begun to be used to assess the sympathetic and parasympathetic components of the regulation of cardiac activity in patients with CHF [15]. Thus, in the UK-HEART study it was shown that the standard deviation index (SDNN) is an independent predictor of overall mortality and the most significant predictor of mortality from progression of CHF [14, 16].
In patients with CHF with preserved LVEF, dyspnea is often the earliest sign due to pulmonary congestion, while skeletal muscle fatigue is characteristic of CHF with reduced LVEF due to decreased cardiac output, impaired vasodilation capacity, and decreased skeletal muscle perfusion. Dyspnea is especially difficult to interpret in the elderly and in obese patients, and these patients represent a large percentage of patients with CHF who have preserved LVEF.
Objective confirmation of a decrease in exercise tolerance can be provided by the use of a stress test in such patients - spiroergometry - with determination of maximum oxygen consumption (VO2max) (reduced VO2max < 25 ml/kg/min; low VO2max < 14 ml/kg/min) and a test with 6- minute walk (distance <300 m has an unfavorable prognosis) [30].
The functional classification of CHF (NYHA), based on a subjective assessment of symptoms by the patient and the doctor, allows only an approximate assessment of physical performance (PF), and objective and widely used indicators of the pumping function of the heart at rest, in particular, LVEF, correlate with it very weakly . The most accurate and reproducible quantitative parameter is exercise oxygen consumption, directly measured by gas analysis.
The maximum individual FR is characterized by maximum oxygen consumption (VO2max) - the highest value of oxygen consumption, which cannot be exceeded with a further increase. In patients with CHF, although it is theoretically possible to achieve it, in practice it is extremely rare, since shortness of breath or weakness stops them much earlier than this level. You can focus on peak oxygen consumption (VO2), but it should be taken into account that the duration and power of the load depend on the motivation of the patient and the doctor. The patient’s effort is considered sufficient and the test is informative if the anaerobic threshold (AT) is reached, usually 60–70% of VO2max. The anaerobic threshold (AT) is the level of O2 consumption above which energy production is supplemented by anaerobic mechanisms. With spiroergometry, it is determined at the moment when the rate of CO2 release begins to exceed the rate of O2 consumption. In stable patients with CHF, peak VO2 and AP are highly reproducible indicators.
Features of the hemodynamic effects of drugs (for example, β-blockers) can lead to differences in the assessment of their effect on RF based on the results of maximal and submaximal tests, therefore, comparison of oxygen consumption and exercise performed is of particular importance. It should be noted that multicenter studies (SOLVD, V-HeFT) did not reveal a clear connection between the effectiveness of drugs according to the results of FN tests and their effect on survival or LV contractility parameters [27]. Carrying out stress tests in patients with CHF is justified not to clarify the diagnosis, but to assess the patient’s functional status and the effectiveness of treatment, as well as to determine the degree of risk. However, a normal exercise test result in a patient not receiving specific treatment may make the diagnosis of CHF unlikely [28].
A number of studies have examined HRV and oxygen supply to the load in patients with CHF. P. Ponikovski et al. examined 102 patients with CHF (mean age 58 years, NYHA I-IV, LVEF 26%, maximum oxygen consumption (VO2max) 16.9 ml/kg/min). Within one year, 19% of the patients included in the study died. The main predictors of mortality were: NYHA functional class (p = 0.003), VO2max (p = 0.01), LVEF (p = 0.02), ventricular arrhythmias (p = 0.05), as well as such parameters of temporary and spectral analysis of HRV as SDNN (p = 0.004), SDANN (p = 0.003) and LF (p = 0.003). The study authors found that the one-year survival rate of patients with SDNN less than 100 ms was lower compared to those with SDNN greater than 100 ms (78 and 95%, respectively, p = 0.008). The combination of SDNN less than 100 ms and VO2max less than 14 ml/min/kg made it possible to identify 18 patients with the highest risk of death. The authors conclude that reduced HRV is an independent prognostic factor for the risk of mortality and complications in patients with CHF [19].
The study of the prognostic significance of HRV in comparison with LVEF and VO2max during a cardiopulmonary training test was devoted to the work of C. Kruger et al. The study included 222 patients with sinus rhythm (mean age - 54 ± 1 year, LVEF less than 40%), of which 151 were with dilated and 71 with ischemic cardiomyopathy. Over 15 ± 1 month, 17% of patients died and 20% were hospitalized due to progression of CHF. In these patients, the SDNN value was significantly lower than in patients without complications (118 ± 6 and 142 ± 5 ms, respectively). In addition, they significantly differed in LVEF (18 ± 1 and 23 ± 1%) and VO2max (12.8 ± 0.5 and 15.6 ± 0.5 ml/min/kg), respectively. Univariate analysis showed that each of these parameters is independent of the other two and significantly predictively significant for both groups. According to multivariate analysis, SDNN had greater predictive value than LVEF and VO2max. The authors believe that measuring HRV improves risk stratification in patients with CHF [20].
HRV analysis is an accessible and highly informative method for determining the state of the autonomic nervous system in patients with CHF. Along with determining parameters such as maximum VO2 and LVEF, HRV research allows us to better characterize the severity of CHF and predict the survival of this category of patients. In patients with the initial stages of CHF, as a rule, normal values of HRV indicators are detected with signs of autonomic imbalance and a predominance of the sympathetic nervous system - an increased ratio of the power of low- and high-frequency oscillations (LF/HF). As the disease progresses, both temporal and spectral indicators of heart rate variability decrease [22].
The most interesting is the correction of increased SAS activity with the help of highly selective b-blockers, which is accompanied by an improvement in both the clinical condition of patients with CHF and their prognosis.
Thus, in the work of Yu. N. Belenkov and V. Yu. Mareev, a significant increase in SDNN was noted in patients with FC II–III CHF who took carvedilol for 6 months. An increase in SDNN by 40% of the initial level indicates a positive effect of the drug on overall HRV [23].
In a study by EC Keeley et al. involved patients with post-infarction cardiosclerosis who took metoprolol for a year, against the background of which an increase in the activity of the parasympathetic nervous system was noted [24].
The SADKO-CHF study included 63 patients with CHF (class II–III) with EF <40%, randomized into groups that differed in the combination of bisoprolol, quinapril and valsartan, and bisoprolol was present in all study groups. The study revealed that the combination of drugs bisoprolol + quinapril has the effect of improving HRV parameters and sympathoadrenal activity [25].
I. V. Nesterova et al. examined 38 men (mean age 61 ± 2 years) who had suffered an MI, with class II–III CHF (NYHA) and EF < 45%. Patients were randomized into 2 groups, receiving in Group I, in addition to standard therapy, metoprolol tartrate at an average daily dose of 54.4 mg, and in Group II, nebivolol 2.3 mg. The results of the study showed that therapy with metoprolol tartrate and nebivolol leads to a decrease in the FC of CHF and normalization of the ratio of HRV indicators [26]. The studies involved patients with reduced ejection fraction, which does not answer the question related to the effect of β-blockers on the course of CHF with preserved systolic function. Potentially, β-blockers can improve the course of CHF with preserved systolic function through several mechanisms: slowing the heart rate (HR) and, as a result, improving LV diastolic filling, reducing LV hypertrophy and inhibiting renin release. However, on the other hand, activation of beta-adrenergic receptors is compensatory in nature, helping to reduce diastolic dysfunction, therefore the effectiveness of long-term use of beta-blockers in patients with EF above 45% requires further study.
Treatment of patients with CHF in the initial stages (stages A and B according to the ACC/AHA classification, 2005; functional class I according to NYHA and a risk group for the development of heart failure) with b-blockers requires further research, and it is likely that patients can receive a number of advantages, including in relation to reducing the risk of mortality and major cardiovascular complications, due to the normalization of HRV indicators. Thus, further study of heart rate variability and oxygen supply to the load in patients with the initial stages of CHF and the effect of b-blockers on these indicators is necessary to clarify adequate therapy for this strategically important group of patients.
For questions regarding literature, please contact the editor.
D. A. Napalkov , Candidate of Medical Sciences N. M. Seidova V. A. Sulimov , Professor, Doctor of Medical Sciences MMA named after. I. M. Sechenova , Moscow