Hepatitis C is a disease caused by an RNA virus. The virus can be transmitted through blood, through unsterile medical instruments, during organ and tissue transplantation, through sexual contact, from mother to child during pregnancy and childbirth. It multiplies in liver cells and causes the development of acute or chronic hepatitis. The hepatitis C virus has several genotypes; due to frequent mutations, it is resistant to human immune defense mechanisms.
Viral hepatitis C is most often asymptomatic. Only in 15% of cases of acute disease are nausea, signs of intoxication, lack of appetite observed, and jaundice is rare. A minority of patients experience hepatitis C as an acute disease and fully recover; the majority of those infected develop chronic hepatitis C. Long-term chronic disease can lead to cirrhosis or liver cancer.
At the start of the disease, antibodies to the components of the virus begin to form, first immunoglobulins M, later immunoglobulins G. Specific antibodies to the hepatitis C virus can be detected in the blood 3–8 weeks after the virus enters the body, but in some cases they may be absent for several months. In this case, infection can be confirmed by testing the blood for hepatitis C virus RNA. After infection in recovered patients, specific antibodies persist for many years with a gradually decreasing concentration and can be detected in low quantities throughout life. Antibodies to hepatitis C do not protect against re-infection when exposed to the virus again.
Those at risk for hepatitis C include doctors and nurses, people who use medical and cosmetic services, drug addicts, patients who have undergone blood transfusions or organ transplants, and children born to infected mothers who are carriers of the hepatitis C virus.
An analysis for total antibodies to the hepatitis C virus (HCV) allows you to identify specific immunoglobulins, the presence of which indicates a possible infection or a previous disease. The test is a screening test; it is mandatory to take it during hospitalization, before planned operations and during pregnancy.
Antibodies to hepatitis C virus (total)
Antibodies to the hepatitis C virus are normally absent in the serum. Total antibodies to the hepatitis C virus are antibodies of the IgM and IgG classes directed to the complex of structural and non-structural proteins of the hepatitis C virus. This study is a screening test to identify patients with VSH.
Total antibodies to the hepatitis C virus can be detected in the first 2 weeks of the disease, and their presence indicates possible infection with the virus or a previous infection. It is impossible to obtain an unambiguous answer based on the results of this test, since the test determines total IgM and IgG antibodies. If this is an early period of acute viral hepatitis C, then this is indicated by IgM antibodies, and if this is a period of convalescence or a condition after suffering from HCV, then this is indicated by IgG antibodies.
IgG antibodies to HCV can persist in the blood of convalescents for 8-10 years with a gradual decrease in their concentration. Late detection of antibodies is possible a year or more after infection. In chronic hepatitis C, total antibodies are constantly determined. Therefore, to clarify the timing of infection, it is necessary to separately determine IgM antibodies to HCV.
Evaluation of the study results
The result of the study is expressed qualitatively - positive or negative. A negative test result indicates the absence of total antibodies (JgM and JgG) to HCV in the blood serum. A positive result - the detection of total antibodies (JgM and JgG) to HCV indicates the initial stage of acute viral hepatitis C, the acute period of infection, the early stage of convalescence, previous viral hepatitis C or chronic viral hepatitis C.
However, the detection of total antibodies to HCV is not sufficient to make a diagnosis of HCV and requires confirmation to exclude a false-positive test result. Therefore, when a positive result of a screening test for total antibodies to HCV is obtained, a confirmatory test is performed in the laboratory. The final result of determining total antibodies to HCV is issued together with the result of the confirmatory test.
Anti-HCV, antibodies, ELISA
Anti-HCV are specific immunoglobulins of the IgM and IgG classes to the proteins of the hepatitis C virus, indicating possible infection or a previous infection.
Synonyms Russian
Total antibodies to hepatitis C virus, antiHCV.
English synonyms
Antibodies to Hepatitis C Virus, IgM, IgG; HCVAb, Total.
Research method
Chemiluminescent immunoassay.
What biomaterial can be used for research?
Venous blood.
How to properly prepare for research?
Do not smoke for 30 minutes before donating blood.
General information about the study
Hepatitis C virus (HCV) is an RNA virus from the Flaviviridae family that infects liver cells and causes hepatitis. It is able to multiply in blood cells (neutrophils, monocytes and macrophages, B-lymphocytes) and is associated with the development of cryoglobulinemia, Sjogren's disease and B-cell lymphoproliferative diseases. Among all the causative agents of viral hepatitis, HCV has the greatest number of variations, and due to its high mutational activity, it is able to evade the protective mechanisms of the human immune system. There are 6 genotypes and many subtypes of the virus, which have different implications for the prognosis of the disease and the effectiveness of antiviral therapy.
The main route of transmission of infection is through blood (during transfusion of blood and plasma elements, transplantation of donor organs, through non-sterile syringes, needles, instruments for tattooing, piercing). Transmission of the virus through sexual contact and from mother to child during childbirth is possible, but this occurs less frequently.
Acute viral hepatitis is usually asymptomatic and remains undetected in most cases. In only 15% of those infected, the disease is acute, with nausea, body aches, lack of appetite and weight loss, and is rarely accompanied by jaundice. 60-85% of those infected develop a chronic infection, which is 15 times higher than the frequency of chronicity with hepatitis B. Chronic viral hepatitis C is characterized by “undulation” with an increase in liver enzymes and mild symptoms. In 20-30% of patients, the disease leads to cirrhosis of the liver, increasing the risk of developing liver failure and hepatocellular carcinoma.
Specific immunoglobulins are produced for the core (nucleocapsid protein core), shell (nucleoproteins E1-E2) and fragments of the virus genome (NS non-structural proteins). In most patients with HCV, the first antibodies appear 1-3 months after infection, but sometimes they can be absent from the blood for more than a year. In 5% of cases, antibodies to the virus are never detected. In this case, HCV will be indicated by the detection of total antibodies to virus antigens.
During the acute period of the disease, antibodies of the IgM and IgG classes are formed to the nucleocapsid protein core. During the latent course of the infection and during its reactivation, IgG antibodies to the non-structural proteins NS and the nucleocapsid core protein are present in the blood.
After an infection, specific immunoglobulins circulate in the blood for 8-10 years with a gradual decrease in concentration or remain for life in very low titers. They do not protect against viral infection and do not reduce the risk of re-infection and development of the disease.
What is the research used for?
- For the diagnosis of viral hepatitis C.
- For differential diagnosis of hepatitis.
- To identify previously transmitted viral hepatitis C.
When is the study scheduled?
- For symptoms of viral hepatitis and increased levels of liver transaminases.
- If you know about hepatitis of unspecified etiology.
- When examining people at risk for infection with viral hepatitis C.
- During screening examinations.
What do the results mean?
Reference values
Result: negative.
S/CO ratio (signal/cutoff): 0 - 1.
Reasons for the positive result:
- acute or chronic viral hepatitis C;
- previously suffered viral hepatitis C.
Reasons for negative results:
- absence of hepatitis C virus in the body;
- early period after infection;
- absence of antibodies in viral hepatitis C (seronegative variant, about 5% of cases).
What can influence the result?
- If the material is taken and stored incorrectly, an unreliable result may be obtained.
- Rheumatoid factor in the blood contributes to a false positive result.
Important Notes
- If the result is positive, to confirm the diagnosis of hepatitis C virus, a test is performed to determine the structural and non-structural proteins of the virus (NS, Core).
- If there are existing risk factors for infection and suspicion of viral hepatitis C, it is recommended to determine the RNA of the virus in the blood by PCR, even in the absence of specific antibodies.
Also recommended
- Anti-HCV, antibodies
- Antibodies to structural and non-structural proteins of the hepatitis C virus
- HCV, RNA [real-time PCR]
- HCV, RNA quantitative [real-time PCR]
- HCV, genotyping, RNA [real-time PCR]
- anti-HAV, IgM
- HBsAg
- anti-HBc, IgM
- Gamma-glutamyl transpeptidase (gamma-GT)
- Alanine aminotransferase (ALT)
- Aspartate aminotransferase (AST)
- Total alkaline phosphatase
- Serum albumin
- Total bilirubin
- Total cholesterol
- Thrombin time
- Fibrinogen
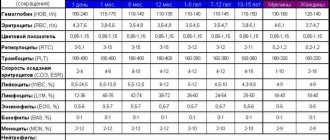
- Complete blood count (without leukocyte formula and ESR)
- Leukocyte formula
- Erythrocyte sedimentation rate (ESR)
Who orders the study?
Infectious disease specialist, hepatologist, gastroenterologist, therapist.
Literature
- Vozianova Zh. I. Infectious and parasitic diseases: In 3 volumes - K.: Health, 2000. - T.1.: 600-690.
- Kishkun A. A. Immunological and serological studies in clinical practice. – M.: MIA LLC, 2006. – 471-476 p.
- Harrison's Principles of Internal Medicine. 16th ed. NY: McGraw-Hill; 2005: 1822-1855.
- Lerat H, Rumin S, Habersetzer F, and others. In vivo tropism of hepatitis C virus genomic sequences in hematopoietic cells: influence of viral load, viral genotype, and cell phenotype. Blood. 1998 May 15;91(10):3841-9.PMID:9573022.
- Revie D, Salahuddin SZ. Human cell types important for hepatitis C virus replication in vivo and in vitro: old assertions and current evidence. Virol J 2011 Jul 11;8:346. doi:10.1186/1743-422X-8-346. PMID:21745397.
Antibodies to hepatitis C virus JgM
Antibodies to the hepatitis C virus JgM are normally absent in the serum. The presence of JgM class antibodies to HCV in the patient’s blood makes it possible to verify active infection. Antibodies of the JgM class can be detected not only in acute HCV, but also in chronic hepatitis C.
Antibodies of the JgM class to HCV appear in the patient’s blood 2 weeks after the development of the clinical picture of acute viral hepatitis C or exacerbation of chronic hepatitis and usually disappear after 4-6 months. A decrease in their level may indicate the effectiveness of drug therapy.
Complexes with this research
Preparation for partner childbirth for a man Tests necessary to accompany a woman in labor 3,680 ₽ Composition
Preparation for IVF for a man Examination to prepare a man for the IVF procedure 6,060 ₽ Composition
Women's check-up No. 1 38 studies for annual preventive examination RUB 19,290 Composition
IN OTHER COMPLEXES
- Pregnancy planning. Diagnosis of infections RUB 8,620
- Men's check-up No. 1 RUB 18,570
- Male infertility. Extended examination RUB 29,030
- Joining IVF RUB 23,020
- Hospital complex 2,100 ₽
Detection of hepatitis C virus by PCR (qualitative)
The hepatitis C virus is normally absent in the blood. Unlike serological methods for diagnosing HCV, where antibodies to HCV are detected, PCR allows you to directly detect the presence of HCV RNA in the blood, both qualitatively and quantitatively. The detectable fragment in both is a conserved region of the hepatitis C genome.
The detection of only antibodies to HCV confirms only the fact of infection of the patient, but does not allow one to judge the activity of the infectious process (virus replication) or the prognosis of the disease. In addition, antibodies to the HS virus are found both in the blood of patients with acute and chronic hepatitis, and in those patients who were sick and recovered, and often antibodies in the blood appear only several months after the onset of the clinical picture of the disease, which makes diagnosis difficult. Detection of the virus in the blood using PCR is a more informative diagnostic method.
Qualitative detection of HCV by PCR in the blood indicates viremia, allows us to judge the reproduction of the virus in the body and is one of the criteria for the effectiveness of antiviral therapy.
The analytical sensitivity of the PCR method is at least 50-100 viral particles in 5 μl of DNA sample isolation; specificity is 98%. Detection of hepatitis C virus RNA using PCR in the early stages of the development of a viral infection (possibly as early as 1-2 weeks after infection) against the background of the complete absence of any serological markers can serve as the earliest evidence of infection.
However, isolated detection of hepatitis C virus RNA in the absence of any other serological markers cannot completely exclude a false-positive PCR result. In such cases, a comprehensive evaluation of clinical, biochemical and morphological studies and repeated repeated confirmation of the presence of infection by PCR is required.
According to WHO recommendations, to confirm the diagnosis of viral hepatitis C, triple detection of hepatitis C virus RNA in the patient’s blood is necessary.
- Detection of hepatitis C virus RNA by PCR is used for the following purposes:
- resolution of questionable results of serological studies;
- differentiation of hepatitis C from other forms of hepatitis;
- identification of the acute stage of the disease in comparison with previous infection or exposure; determining the stage of infection of newborns from mothers seropositive for the hepatitis C virus;
- monitoring the effectiveness of antiviral treatment.
What is hepatitis C?
The hepatitis C virus was discovered relatively recently. If left untreated, the disease can progress to liver cirrhosis or cancer. Until a certain time, it was widely believed that this was a death sentence. This is all due to the low medical literacy of the population, as well as the fact that for a very long time medications for this disease were very expensive and inaccessible to the average person. However, now the cost of treatment is also quite high. However, it is possible to recover now.
The incubation period is up to 60 days. The main difficulty in diagnosing hepatitis C at the first stage is that symptoms may be mild or completely absent. In addition, symptoms may masquerade as other diseases. In general, the disease in its early stages can manifest itself as follows:
- Increased body temperature.
- Aches in the joints.
- Heaviness in the stomach area.
- Painful sensations in the right hypochondrium.
- Fast fatiguability.
- Digestive disorders.
- Stool or urine that is too light.
- General weakness.
The peculiarity of the disease is that all these signs appear separately and from time to time and pass relatively quickly. Therefore, detection in the early stages is very difficult. Therefore, it is so important to determine the presence of antibodies to hepatitis in time.
Detection of hepatitis C virus by PCR (quantitative)
A quantitative method for determining the content of hepatitis C virus RNA in the blood provides important information about the intensity of the disease, the effectiveness of treatment, and the development of resistance to antiviral drugs. The analytical sensitivity of the method is from 5.102 copies/ml of viral particles in blood serum, specificity is 98%.
The level of viremia is assessed as follows: when the HCV RNA content is from 10^2 to 10^4 copies/ml – low; from 10^5 to 10^7 copies/ml – medium and above 10^8 copies/ml – high.
Quantitative determination of HCV RNA in blood serum by PCR is important for predicting the effectiveness of interferon-alpha treatment. It has been shown that individuals with a low level of viremia have the most favorable prognosis of the disease and the greatest likelihood of a positive response to antiviral therapy. With effective treatment, the level of viremia decreases.
Other types of diagnostics for hepatitis C
The hepatitis C virus primarily affects liver cells, so when it is detected, a full examination of this organ is necessary.
- Virological diagnostics are supplemented by clinical and biochemical indicators (ALT, AST, total and direct bilirubin, GGTP, alkaline phosphatase and others - the list is compiled by the attending physician). This set of tests is taken regularly: before starting, once a month during and for a year after treatment. This is necessary to monitor the condition of the liver and its response to therapy.
- Instrumental diagnostics , primarily involving ultrasound of the abdominal organs with liver elastography. In conclusion, the ultrasound diagnostic doctor describes the shape, size, position, structure of the liver and gallbladder, and using elastography, the stage of liver fibrosis/cirrhosis.
- Additionally, the doctor may prescribe special tests FibroMax or FibroActiTest. This is a comprehensive blood test, also necessary for diagnosing fibrosis, liver steatosis and the activity of destructive processes.
Regardless of whether your symptoms bother you, hepatitis C can be treated . Without treatment, over time the disease can lead to cirrhosis, liver cancer and even death. Severe cirrhosis may require a liver transplant.
At the EXPERT clinic you will receive a full and only necessary examination, detailed consultation with a doctor and assistance at all stages of hepatitis C treatment.
How is blood taken for hepatitis C testing?
The initial blood test for hepatitis C is performed in the morning on an empty stomach.
The procedure is quick and painless in a resting state, “sitting” or “lying down.”
For this type of diagnosis, venous blood is used, which is taken using a sterile syringe from the ulnar vein.
Further actions will be determined by the attending physician based on the results of the analysis.