There are contraindications. Specialist consultation is required.
Forms of the disease Causes of development Symptoms Who is at risk? Classification What will happen if left untreated? Diagnostics Treatment methods and techniques Prevention
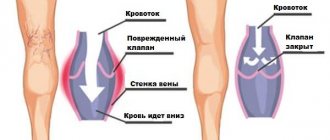
Chronic venous insufficiency, as well as its acute form, is associated with impaired blood flow. Thanks to the valve system, as well as the muscles of the body, blood from the lower extremities flows to the heart, overcoming the force of gravity. Therefore, moderate physical activity, gymnastics during sedentary work, and regular walks are recommended for each person. If, due to an incorrect lifestyle or poor heredity, varicose veins appear or the lumen of the vessel is clogged with a blood clot, venous insufficiency of the legs begins to develop. First, the affected vein expands, then the function of the valves is disrupted (they do not close completely), and the result is reflux, that is, reverse flow of blood - it flows not only to the heart, but also back to the legs. Due to the accumulation of blood, a person feels heaviness in the legs and swelling appears. These symptoms should not be attributed to fatigue; you need to urgently contact a phlebologist, otherwise chronic venous insufficiency will develop.
Important! Varicose veins and venous insufficiency are not the same thing. Varicose veins are only one sign of insufficiency, although the most common.
Forms of venous insufficiency
The course and treatment of venous insufficiency depend on what form of the disease is present in the patient. The SM-Clinic employs some of the best phlebologists in St. Petersburg and has advanced diagnostic equipment. Thanks to this, we provide qualified assistance even at the first symptoms.
Acute venous insufficiency (AVI)
Appears due to a sharp disruption of blood flow in deep-lying vessels. For example, due to blockage of a vein by a blood clot. Also, its causes are the peculiarities of blood clotting, various diseases, and intoxication when taking certain medications.
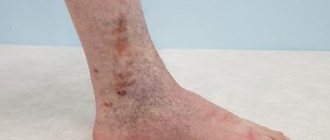
The disease develops rapidly, its signs are immediately visible: bluish skin, rapid and severe swelling of the legs, and a visually easily noticeable venous pattern. In addition, the person feels pain in the legs. OVN has no stages.
Chronic venous insufficiency (CVI)
It develops slowly, at first it does not even give symptoms, but gradually leads to serious problems. May be a consequence of varicose veins left without treatment. Leads to trophic ulcers and various complications, including infection of the limb.
Has several stages:
- At zero there are no unpleasant symptoms, only spider veins are visible. Unfortunately, people at this stage rarely go to the doctor.
- In the first stage, in the evening a person feels heaviness in his legs and notices swelling. This is caused by small lesions in the superficial veins.
- On the second, pigment spots appear, swelling does not go away after a night's rest, and eczema (its parts) occurs.
- At the third stage, trophic ulcers appear at the site of eczema - weeping, non-healing wounds.
CVI at a high degree of development is accompanied by bleeding, thrombophlebitis, and blockage of deep veins by blood clots.
Risk factors for CVI
A number of factors significantly increase the risk of developing chronic venous insufficiency, these include:
- Genetic predisposition, including pathologies of connective tissue and weakness of the vascular wall.
- Taking hormone-containing drugs, including hormonal contraceptives.
- Low physical activity, sedentary lifestyle, constant heavy lifting, excess weight.
- Chronic constipation.
CVI is more often diagnosed in women, since the formation of venous insufficiency is influenced by a high concentration of estrogens (female steroid sex hormones). The period of pregnancy and childbirth and the use of hormonal contraceptives have a negative impact. With age, the likelihood of developing the disease increases in both sexes due to prolonged exposure to adverse factors.
What are the causes of venous insufficiency?
All causes of the disease can be divided into groups:
- Negative heredity. These include structural features of blood vessels, insufficiency of the vascular wall and valve apparatus.
- Blood flow disorders: varicose veins, thrombosis, consequences of injuries, postthrombophlebitis syndrome, constantly high pressure in the lower extremities, phlebitis, pregnancy.
- Lifestyle: obesity, lack of physical activity, excessive strength loads (both uncontrolled lifting of weights in the gym and, for example, working as a loader), sedentary lifestyle or standing work.
- Hormonal status: women suffer from venous insufficiency more often than men, this also includes taking OCs (oral contraceptives) and hormonal therapy.
- Age factor: the older the patient, the higher the likelihood of the disease.
The mechanism of the onset and development of the disease
Under the influence of gravity, the blood in the vessels descends to the lower extremities, the body has to make efforts to lift it. Venous valves prevent blood from flowing downwards, which are actively helped in this by physical activity, muscle contraction and bending of the knees. The combination of these factors ensures normal blood flow.
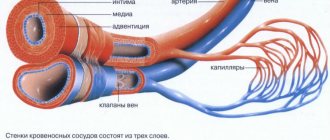
Maintaining a constant resistance to gravity is possible due to physiological changes in the lumen of blood vessels when changing body position, the operation of the valve apparatus and the tone (elasticity) of the venous wall. If one of these component mechanisms is disrupted, pathological processes begin to affect the entire system as a whole. Loss of elasticity of the section of the vein below the valve, and its expansion leads to valvular incompetence, the inability to maintain blood flow for subsequent rise. Stagnation of fluid leads to increased pressure to move blood upward. But, over time, increased pressure increases the volume of the part of the vein that has lost its elasticity.
Venous reflux (reverse flow of blood from top to bottom) can join the pathological process. The liquid begins to stagnate and put pressure on the walls of the vessel. As a result, blood plasma leaks into the surrounding tissue, causing swelling. The situation develops similarly with initial valvular insufficiency.
Simultaneously with circulatory failure, the lymphatic system is also overloaded. Trophic disorders contribute to the formation of trophic ulcers. Trophic ulcers are long-term non-healing wounds (6 months or more) affecting the skin and tissues. They form on the lower leg, are surrounded by an area of inflammation, and have a high risk of infection.
Symptoms of the disease
Let's look at the general symptoms first. It should be remembered that in the initial stages of the disease they appear one at a time and are often “blurred”. The more the disease progresses, the more pronounced the symptoms, and they most often appear in groups. So:
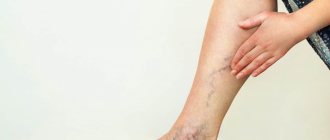
- pain in the legs, feeling of heaviness, fullness;
- spider veins;
- swelling - first passing after rest, then permanent;
- night cramps in the legs;
- dryness, unhealthy shine of the skin, age spots or areas of discoloration;
- trophic ulcers at an advanced stage.
Consultation with a surgeon: when to contact, how is the appointment?
Symptoms of CVI by stage
| Stage | Description |
| 0 | The person is able to work, there are no symptoms, they do not appear after sports and other activities. |
| 1 | Slight pain, heaviness in the legs and swelling at the end of the day, which disappears in the morning, mild cramps. A person is not limited in physical activity and can work at the same pace. |
| 2 | Manifestations of venous insufficiency become pronounced. These are skin pigmentation, dermatological diseases, severe swelling, skin necrosis in some areas. It becomes difficult for a person to work physically or play sports. |
| 3 | Trophic ulcers appear and tissue metabolism is completely disrupted. A person loses his ability to work. |
Symptoms of AHF
Signs of the disease are more pronounced and appear much faster compared to CVI:
- Pain in the legs that increases with movement;
- just below the site of vein blockage there is large swelling;
- difficulties in any physical activity;
- pallor or bluishness of the skin;
- at the site of pathology, skin temperature decreases by 2-3℃;
- body temperature can rise to 40℃;
- increasing pain to unbearable;
- “referring” pain to the groin and pelvis area.
Important! To reduce the likelihood of a blood clot breaking off with subsequent thromboembolism, the patient needs to limit movement. Bed rest is recommended for up to 10 days; the affected leg should be higher than the body.
Types of vein reflux
Depending on how the blood is distributed, there are 2 types of reflux:
- Vertical.
It can develop in deep and superficial vessels. With this type of reflux, the blood moves in a downward direction. In deep veins it occurs during postthrombophlebitic syndrome and provokes severe venous insufficiency.
It is very difficult to identify this disease in the early stages. Even complex therapy does not guarantee complete recovery, and therefore patients with this disease need to be regularly checked by a doctor.
- Horizontal.
In this case, blood flows from deep veins to superficial ones. Horizontal reflux is considered the main cause of varicose veins. Diagnosed in the initial stages of development.
Classification of the disease
Acute venous insufficiency has no classification by stages. Let's consider the degrees and stages of the chronic form of the disease. To fully describe the patient’s diagnosis, doctors use the CEAP classification, that is, assigning a kind of “code”, for example: C4a, S, Eс, Ad, Pr, 8, 10, 11, 17. It is deciphered based on the following classification characteristics.
C – pathology class:
- C0 – no visible symptoms;
- C1 – spider veins;
- C2 – dilated veins from 3 mm;
- C3 – swelling in the areas of the legs and ankles;
- C4a – dermatitis, pigmentation and other skin lesions;
- C4b – increased pigmentation, thickening of the skin;
- C5 – self-healing skin wounds;
- C6 – non-healing trophic ulcers.
The following index indicates whether the patient has complaints: A – without symptoms or complaints, S – there are complaints.
E – cause of disease:
- EC – congenital;
- Er – etiology unknown;
- Es – the reason is known.
A – localization:
- As – saphenous veins;
- Ar - vessels that connect the deep veins and subcutaneous veins;
- Ad – deep veins;
- Аn – there are no pathologies of the venous system.
P – type of pathology:
- Po – cessation of blood movement through the veins;
- Pr – valve insufficiency;
- Pr,o – both;
- Рn – no disturbances in the movement of blood through the veins were detected.
The number is the area of the venous system where the pathology is detected: from 1 to 18.
Causes of CVI
Most often, venous insufficiency occurs with varicose veins, as well as against the background of such diseases and pathological conditions as:
- congenital pathology of the venous system;
- congenital aplasia and hypoaplasia of the deep veins;
- congenital arteriovenous fistulas;
- congenital osteohypertrophic nevus with varicose veins;
- suffered acute thrombosis of the main veins;
- Klippel-Trenaunay syndrome;
- previous phlebothrombosis.
In recent years, a new cause for the development of chronic venous insufficiency has emerged, which is increasingly being identified - phlebopathy. This name refers to the state of venous stagnation in the absence of clinical signs of pathology. In rare cases, CVI begins to develop after a trauma (bruise, rupture, deep burns or hypothermia).
Treatment of CVI
To treat pathology, a conservative method is used, if possible, and an operative one.
Conservative technique
Such therapy is possible when there are no irreversible changes in the veins. Treatment primarily involves wearing compression garments (knitwear), which creates a frame for the affected veins and prevents them from overstretching. It also increases blood flow through the veins, preventing the formation of blood clots.
Compression knitwear is knee socks, leg warmers, stockings, tights. It varies in compression strength. Selecting the optimal option individually is the task of a phlebologist.
Patients are also advised to lose weight if they are overweight and especially obese. Losing body weight means unloading the veins. When prescribing a diet, attention is also paid to the prevention of constipation in order to prevent constantly increased venous pressure.
The patient must be prescribed therapeutic exercises or exercise therapy. Swimming is especially beneficial.
In addition, drug therapy is carried out with the following types of drugs:
- angioprotectors;
- bioflavonoids;
- anticoagulants;
- local anesthetics;
- glucocorticosteroids.
An additional measure is external agents, that is, heparin-based gels and ointments.
Surgery
Radical method of treating chronic venous insufficiency. Indications for surgery are severe symptoms, pathological reflux, progression of trophic ulcers, low effectiveness of conservative treatment, pronounced progression of CVI.
Basic techniques:
- laser;
- surgical;
- ablative;
- bypass;
- phlebectomy, endoscopic phlebectomy.
Preparing for surgery
The patient undergoes a duplex scanning of the veins, as well as a standard examination, which includes:
- ECG;
- fluorography;
- coagulogram;
- general and biochemical blood tests;
- general urine analysis;
- blood tests for infections, group and Rh factor.
The test results are provided to the therapist. You also need to consult with an anesthesiologist.
Before the procedure, the patient should remove hair from the intended intervention area. The last meal is 4-6 hours before.
Progress of the operation
- Laser technique . Allows you to eliminate varicose veins and significantly enlarged veins. The affected vessel is irradiated from the inside with a laser at high temperature. As a result, its walls “stick together”, and gradually it completely disappears. No incisions are needed - just punctures. The procedure is carried out under ultrasound control.
- Surgery . To access the affected veins, several small incisions are made in the skin and soft tissue. The surgeon either completely removes the damaged vessel or ties the veins that connect the deep and superficial (in the area of the legs). Surgery is performed when the veins are significantly dilated.
- Phlebectomy . Removal of small veins. Several small incisions are made on the skin under local anesthesia, through which the doctor gains access to the affected vessel and removes it.
- Endoscopic phlebectomy . A microcamera is inserted into the vessel, allowing the doctor to remove the affected vein under visual control. The method is applicable when ulcers are present.
- Ablation . A flexible catheter is inserted into the vein. The heating electrodes, which are located at its end, thermally act on the venous wall, destroying it.
- Bypass surgery . An artificial vessel is used that connects the veins so as to allow blood flow to bypass the damaged area. Usually performed with the development of CVI and severe venous damage.
Rehabilitation
In the first days, swelling, hematomas and compactions in the affected area are possible. If stitches were applied, they are removed after 7-10 days.
From 1 to 2 months you need to constantly wear compression garments, and then - for the period recommended by your doctor - only during the day. Also, depending on the type and scale of the intervention, the patient is prescribed medications (primarily painkillers), physiotherapy, and exercise therapy. Walking at a calm pace is recommended.
Overheating of the intervention area and heavy physical activity are completely eliminated for about 2 months. During sleep, your legs should be placed on a slight elevation - about 15 cm.
Classification of CVI
- 1st degree:
is expressed in a feeling of heaviness, aching pain, passing, minor swelling and night cramps.
- 2nd degree:
persistent swelling, hyperpigmentation (darkening of the skin of the affected limb), lipodermatosclerosis (dystrophic changes in fatty tissue), dry and weeping eczema (serous inflammation of the dermis).
- 3rd degree:
To the above signs of stage 2, trophic ulcers, current or already healed, are added.
- There is also 0 degree
when the patient does not observe changes, there are no complaints, however, the pathological processes have already started. The treatment method for patients with zero degree CVI differs from the treatment of patients at other stages.
In international practice, chronic venous insufficiency is divided into 6 degrees according to clinical manifestations, including zero (CEAP system). The disease also has an etiological classification based on its causes:
- EC is a congenital pathology.
- ES is an acquired form as a result of thrombosis, varicose veins, or trauma.
- EP is an unclear cause of venous insufficiency.
There is also an anatomical classification based on location, taking into account pathophysiology, disability scale and a number of other factors.
Prevention of venous insufficiency
Preventive measures:
- refusal of high-heeled shoes;
- wearing comfortable clothes that do not constrict the body;
- moderate tan;
- extremely rare visits to the sauna, bathhouse;
- maintaining normal body weight;
- proper nutrition;
- drink plenty of fluids (1.5-2 liters of water, unsweetened tea per day);
- giving up alcohol and smoking;
- active lifestyle;
- regular gymnastics during sedentary or standing work;
- when working sedentarily, place your feet on a not very high stand.
Make an appointment with a phlebologist at SM-Clinic on the website or by phone. Prices for services are indicated on the website.
Diagnostic methods in the department of vascular surgery
Several research options are used for diagnosis:
· Doppler ultrasound: assessment of blood flow in the veins without assessment of the structure of the veins.
· Doppler ultrasound angioscanning (color mapping of blood flow) is the best diagnostic method for a comprehensive assessment of the condition of the venous system.
· Phleboscintigraphy: a study by injecting into a vein a drug that is labeled with a radioactive isotope that has a short half-life.
· Emission CT, performed to evaluate venous pathology.
· Phlebography: X-ray examination using a contrast agent.
Prevention
It is necessary to fight excess weight, eat right, avoid prolonged static loads, move a lot, wear shoes with low heels, play sports (swimming, cycling, skiing).
If it is impossible to remove the provoking factors (heredity or work characterized by prolonged static loads), doctors recommend resorting to preventive compression hosiery, and for pregnant women - therapeutic ones.
Preventive measures help eliminate risk factors for CVI.
Varicose veins of the lower extremities (LVL) is the most common disease of the venous system. The frequency of detection of this pathology is 10-20% in men and 25-33% in women [1, 2]. In Russia, according to some data, its frequency reaches 60% [3, 4].
The leading link in the development of the disease is the formation of pathological veno-venous reflux, leading to varicose vein transformation, venous stagnation and tissue damage. The main importance is given to the discharge of blood through the trunks of the main (great and small) saphenous veins and perforating veins (PV) [5-12]. In this case, reflux through the perforators is given extreme importance - the discharge of blood through them is believed to lead to an overload of the associated tributaries of the main saphenous veins, their varicose transformation and, accordingly, the manifestation of the disease (“ascending form of varicose veins”).
This view of the pathogenesis of VVVV led to the formation of a unified surgical concept recognized by the majority of surgeons in the world: surgery for varicose veins should be of a pathogenetic nature, i.e. first of all, eliminate its cause—trunk and perforator reflux [11, 13-16]. According to this concept, phlebosurgery is currently developing: new methods of trunk phlebectomy are proposed, alternative methods of thermal obliteration of trunks are studied, the technique of interventions on perforators is honed, and the possibilities of their sclerosis or thermal obliteration are explored.
At the same time, a very significant drawback can be found in the general pathogenesis scheme described above. A careful, unbiased look at the role of perforant reflux in pathogenesis shows that there is practically no indisputable evidence of the significance of PV failure. We wish to present and analyze both well-known and relatively recently published data regarding this issue.
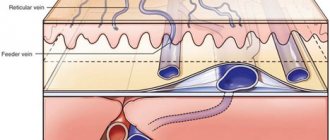
History of the study, anatomical and functional features of perforating veins
The first scientist to demonstrate the presence of PV on the lower extremities was Kh.I. Loder, Russian anatomist [17]. Following him, for a century and a half, dozens of researchers studied the anatomy of the PV, describing in detail their morphological characteristics [18-24]. The PVs connect the superficial and deep veins of the lower extremities and normally direct blood flow from the former to the latter. They are called perforators because they perforate the muscle fascia. PVs are often duplicated. A small artery runs parallel to the PV [25]. Perforators have valves (from 1 to 4), which are located mainly in the subfascial segment of the vessel [22]. The number of perforators on each lower limb is very large. One of the first and most complete works on this problem is a study conducted by Russian surgeon I.A. Kostromov [26]. He detected up to 112 perforators on one lower limb. These data were confirmed by other studies [27].
The localization of PVs is quite constant, which is reflected in the long-existing names of the most significant of them by the names of the authors who described their location in detail. At the same time, modern conciliation documents do not recommend the use of nominal names for these vessels in practice [28]. The current classification of PV is based on the territorial-anatomical principle. According to it, PVs of the lower extremities can be divided into six groups: PVs of the foot, ankle joint, lower leg, knee joint, thigh and perigluteal area [29].
From a clinical point of view, perforators located on the lower leg, in the popliteal fossa and on the thigh are of greatest importance [30]. The most famous for clinicians are the PVs of the medial tibia, which for many years were called Cockett’s perforators. These include four veins that have a very constant localization and connect the tributaries of the great saphenous vein (GSV) with the posterior tibial veins. It is reflux, according to PV data, that is traditionally recognized as one of the main causes of varicose veins and subsequent trophic disorders, and therefore most surgical techniques are designed to eliminate these vessels [20].
In the upper third of the leg, along the medial surface, there are paratibial veins, also called the veins of Sherman and Boyd. They connect the GSV with the posterior tibial veins. The appearance of varicose veins in this area is also often associated with perforating reflux [30].
In the hip, the most clinically interesting sites are the femoral canal perforators (also called Dodd's or Hunter's PVs), which connect the GSV to the superficial femoral vein. In some patients, these PVs have no connection with the GSV [31].
Functionally, the PVs play an important role in directing blood from the superficial venous network to the deep veins using their valves [32]. During muscle contraction (muscle systole), when there is a rapid significant increase in pressure, the valves in the PV close, which prevents blood from flowing from the deep to the superficial system. During muscle relaxation (diastole), the pressure decreases and the valves in the PV open, allowing blood to drain from the superficial to the deep system. The flow of blood in the intact PV in the direction from the superficial to the deep veins has long been considered an axiom. I.A. Kostromov in 1951 reported that from 3 to 10% of the intact PVs he studied on the thigh and leg were valveless, which means blood flow through them could be carried out in both directions. However, the author [26] also noted that if there were valves in the PV, they were always oriented in such a way that the blood flow occurred in the direction of the deep veins. Studies by R. Bjordal [33, 34] also demonstrated the presence of bidirectional blood flow in the PV, but in patients with primary varicose veins. The advent of ultrasound imaging in clinical practice has made it possible to observe bidirectional blood flow along the PV in real time both in patients with venous pathology [35, 36] and in healthy individuals [36, 37]. Nevertheless, there are researchers who do not consider it possible for such blood flow through perforators to exist normally [35].
Insufficiency of perforating veins
The first scientist to express an opinion on the pathogenetic significance of insufficiency of perforating veins, apparently, was R. Linton [23]. He examined patients with trophic disorders and concluded that horizontal venous reflux plays an important role in their development. It should be said that R. Linton's research was devoted to postthrombotic disease. Nevertheless, the idea was picked up by specialists from many countries around the world and, as a result, perforating reflux was recognized as the cause of not only trophic disorders in postthrombotic disease, but also primary varicose veins [12, 26, 38, 39]. Dozens of papers devoted to perforator reflux have been published. The main problems of interest to specialists were methods for identifying and criteria for the failure of PV, as well as the search for effective ways to eliminate perforating discharge.
Clinical diagnosis of PV failure.
No pathognomonic clinical signs have been found to establish the fact of reflux by the PV and accurately localize it. Focus on the location of conglomerates of dilated veins and data from functional tests (three-strand Sheinis, Thalmann, Pratt-2) [40] have long been the main diagnostic criteria, but, from the standpoint of modern requirements for scientific data, these methods do not have an evidence base.
Another method for identifying perforator reflux was palpation of defects in the fascia of the leg, accompanied by painful sensations for the patient in the presence of insufficient PV [14, 26, 41]. At the same time, there is also no evidence of the accuracy of this method [42, 43], in particular, no comparative studies have been conducted with the most accurate method of localizing PV, which is ultrasound scanning.
X-ray contrast venography in the diagnosis of PV failure.
Ascending venography and varicography were the first instrumental methods for detecting perforator reflux, which is manifested by the penetration of contrast from the deep system into the superficial one. According to a number of authors [44-47], with the help of X-ray contrast studies it is possible to identify from 65 to 90% of existing incompetent PVs.
Ultrasound angioscanning in the diagnosis of PV failure.
In recent years, this method has become the leading one in the diagnosis of perforator reflux. It can be used to visualize PVs, measure the diameter of veins, and describe the direction of blood flow [36]. The only difficulties arise in visualizing and determining blood flow in PVs smaller than 1 mm in diameter, although it is not entirely clear whether PVs of this size are clinically or hemodynamically significant. The accuracy of diagnosing perforant reflux based on the most hemodynamically significant PVs of the medial group is comparable with venography [48]. E. Pierik et al. [49] examined 42 patients with venous ulcers and reported 100% specificity and 79% sensitivity for duplex testing.
Radioisotope phleboscintigraphy in the diagnosis of PV failure.
In the arsenal of modern phlebology there is another minimally invasive, safe and highly informative method for detecting venous circulation disorders - radionuclide phleboscintigraphy. The method was developed in the work of the clinic V.S. Savelyev [50], who showed the enormous importance of the technique in identifying insolvent PVs. The advantage of radiophlebography is the ability to record the movement of the isotope with natural blood flow at the moment the patient imitates walking. This makes it possible to identify and localize perforating discharge.
Criteria for the insolvency of PV.
This question is in many ways key, since it has a direct impact on both the formation of views on the development of varicose veins and on surgical tactics. In the pre-ultrasound era, when the leading diagnostic method was x-ray venography, the criterion was considered to be the visualization of the PV and the contrasting of the saphenous veins associated with the movement of blood through them. A similar criterion is used when performing radioisotope venography [51]. Significant hopes were placed on ultrasound angioscanning, which appeared in the arsenal of phlebologists in the late 80s of the last century, which makes it possible to visualize the PV and determine the direction of blood movement along it. However, despite the long history of studying the anatomy and function of the PV, scientists have not been able to come to a common opinion on the basis of what signs can be considered a failed PV [36, 37, 52-54].
The most commonly used three criteria are the presence of reflux, its duration and the diameter of the vessel. Since ultrasound angioscanning occupies a dominant position among diagnostic methods in phlebology today, the assessment of the mentioned signs occurs at the time of this study.
The presence of reflux, at first glance, is the most reliable criterion for PV insufficiency. It has been shown that the number of PV with reflux increases with the progression of chronic venous disease [35, 37, 55].
The duration of reflux as a criterion for failure varies from 0.3 s [56] to 1 s [57], however, most authors [55, 58-61] consider reflux of more than 0.5 s to be a sign of failure of PV.
As for the diameter, some authors [23, 36, 37, 53, 59] consider a certain diameter to be a sign of PV failure. In addition, a number of studies [35, 55, 59] have shown that incompetent PVs have a larger diameter compared to intact vessels. At the same time, many experts [57, 62] reasonably believe that diameter cannot serve as a criterion for the failure of the PV, pointing to both a certain subjectivity in assessing the diameter of the vein and the fact that the PV is large in diameter (0.6-0.7 cm ) are often competent, and veins of small diameter (0.2-0.3 cm) may be insufficient.
Other criteria characterizing PV failure have also been proposed: in a prospective study of 265 PVs on 90 limbs using duplex ultrasound scanning, K. Delis [55] concluded that the criteria for determining perforator failure are not only the diameter and the presence of reflux, but also the maximum and average velocity, volume of flow, time to peak velocity, and venous volume moved into the superficial system. Such criteria have not found supporters due to the cumbersome and noticeable subjectivity of such an assessment.
Frequency of detection of PV failure.
Data on the frequency of PV failure in patients with VLNK vary markedly across historical periods. In an era when X-ray phlebography was recognized as the “gold” diagnostic standard, insufficient perforators were identified in the vast majority (80-90% or more) of patients [63]. With the introduction of ultrasound angioscanning into practice, the situation changed; PV failure began to be recorded less frequently - from 3.3% with clinical class C1 to 30% with class C2 [7, 8, 62].
Studies [35, 37, 55] in recent years have demonstrated that as the severity of the disease increases, the frequency of detection of insufficient PT also increases. According to Yu.A. Belkova et al. [64], the frequency of hemodynamically significant reflux in the PV in various clinical classes of chronic venous diseases ranges from 33 to 85%. A study conducted by the staff of the V.S. Savelyev [62], showed that in patients with trophic disorders (classes C4-C5) with VBNK, the frequency of detection of incompetent PVs reaches 100%, but, on the other hand, with an uncomplicated course of the disease (class C2), perforator reflux is recorded only in 28% cases.
Disadvantages and limitations of instrumental methods for diagnosing PV failure.
It is impossible not to mention the fundamental disadvantages of ultrasound angioscanning, which are of significant importance in assessing the state of PV. The study evaluates blood flow only in a small segment of the venous bed and over a short period of time [62]. Thus, when obtaining sufficiently detailed anatomical information about the PV, an integrative assessment of its function is practically impossible. The proposed technique of scanning the PV in a vertical position of the patient during contraction and relaxation of the calf muscles (imitation of walking) to some extent eliminates this disadvantage of ultrasound scanning [62, 65], and yet only the fact of bidirectional blood flow along the PV is more accurately recorded. The hemodynamic significance of perforator reflux cannot be established using ultrasound angioscanning.
This drawback is equally inherent in radiopaque venography - it records the failure of the PV only in a separate period of time. In addition, this study [14] is traumatic, accompanied by a significant number of complications and is currently practically excluded from the diagnostic arsenal for chronic venous diseases.
In contrast to these two methods, radiophlebography makes it possible to record precisely the function of the PV, since during the study the movement of the isotope with the bloodstream is recorded over a fairly long period of time in the process of almost accurate imitation of walking by the patient [50]. At the same time, the conditions in which the research is carried out cannot be called fully physiological - a tourniquet is applied over the patient’s ankle, thereby changing the hemodynamic conditions in the segment being studied (lower leg). In addition, using radiophlebography it is difficult to accurately localize incompetent PVs, which makes it difficult to use the method for mapping perforators in surgical practice.
Methods for correcting insufficiency of perforating veins
The development of methods for surgical correction of PV incompetence is associated with the name of R. Linton [23], who proposed dissecting them through a long longitudinal incision along the medial surface of the leg. This intervention received his name [14, 66-68]. D. Felder et al. [69] in 1955 modified the intervention by proposing to perform ligation of the PV from an approach along the posterior surface of the leg. This option, by removing the incision from trophically altered tissues, made it possible to reduce the frequency of purulent-necrotic complications [66]. The Linton operation, as modified by Felder, was widely used in many phlebological and surgical centers around the world and became one of the most famous in phlebosurgery [14, 68]. At the same time, the high frequency of severe purulent-necrotic complications led to a gradual abandonment of its use [70].
F. Cockett [20] proposed to perform not sub-but suprafascial ligation of the PV in the lower and middle third of the leg along the inner surface. However, the search for PV in the subcutaneous tissue with pronounced trophic changes is traumatic, causes significant technical difficulties, and therefore is often accompanied by postoperative necrosis of the wound edges and its suppuration [9, 66], and therefore the use of this intervention also had to be abandoned. However, having undergone a certain transformation, it remained in surgical practice in the form of suprafascial ligation of perforators from separate small incisions and is still used by many doctors. It is this option that is known to domestic surgeons as the Cockett operation, although this is not an entirely accurate name.
In 1985, G. Hauer [71] first published work on endoscopic dissection of the PV
. He used a mediastinoscope or rigid proctoscope to insert instruments under the fascia propria of the leg. Later, the technique was improved, and special endoscopes and instruments were developed for isolating and cutting the PV [72, 73]. The advantages of the method are a significantly lower number of complications, small scars and a shorter period of patient stay in the hospital [56, 58, 74, 75]. The incidence of wound complications is 4-26% [76, 77]. Postoperative deep vein thrombosis is rare [74]. Pain (1.5%) and paresthesia (6%) may also develop after surgery, especially when electrocoagulation is used in the subfascial space [78]. Isolated cases of iatrogenic ulcers [79] and arteriovenous fistulas [80] have been reported.
Endoscopic PV dissection is considered effective in patients with venous trophic ulcers (from 84 to 100% of ulcer healing and 0-22% of relapses within 2 years) [56, 81-84].
Methods of endovasal laser and radiofrequency obliteration
appeared in clinical practice in the late 90s of the last century. They are minimally invasive outpatient procedures requiring only local anesthesia. Their initial area of application was the elimination of reflux through the great or small saphenous veins [85-89].
In recent years, reports have appeared about the possibility of using laser and radiofrequency techniques for obliteration of the PV. For this, manufacturers have developed additional accessories that allow puncture and obliteration of vessels of small caliber and length, i.e. PV [90]. The advantage of this approach is that there is no need to make any incision at all (only percutaneous puncture is required), which reduces trauma to a minimum. Nevertheless, the intervention has not yet become widespread, perhaps due to its technical complexity (it is not always possible to quickly, easily and, most importantly, accurately puncture such a small vessel as the PV) and the need to use expensive equipment and consumables. In addition, there is an even less traumatic and much more economical alternative method of PV obliteration - sclerotherapy.
Phlebosclerosis
It has long been used by many doctors to eliminate failed PV [31, 91-93], but to date there is no common point of view on the effectiveness and role of this technique [93-95].
One of the pioneers of sclerosis of perforators was W. Fegan [96], who developed the “empty” vein technique, which also involved obliteration of incompetent perforators, the localization of which was determined exclusively visually and by palpation. The author’s experience was enormous, and his authority was indisputable, however, it should be recognized that from a scientific point of view, his data are not sufficiently substantiated - it is impossible to judge the accuracy of injections into the PV without ultrasound control, as well as to evaluate long-term results.
When assessing the effectiveness of PV sclerotherapy, it must be recognized that there are very few high-quality studies [28, 61, 97] devoted to this problem. Two prospective studies of ultrasound-guided sclerotherapy for failed PVs have been conducted. P. Thibault and W. Lewis [98] sclerosed the PV in 36 patients with 0.5-1.0 ml of 3% sodium tetradecyl sulfate. In 15% of cases, there was a need for repeated injections. Compression was applied for 4 weeks. After 6 months, from 73 to 100% of perforators were obliterated. The best results were achieved when injecting into insufficient PV of the leg. The authors concluded that further refinement of the technically complex procedure is required and the development of a needle guide device that allows accurate localization of the needle tip at a depth of 0.5 to 3 cm is required. No complications (deep vein thrombosis, pulmonary embolism, or intra-arterial insertion) were noted.
In another open-label, prospective study by M. Schadeck [99], 3% sodium tetradecyl sulfate was used in 51 patients. First of all, the saphenous veins were sclerosed. Observation from 1 to 7 months showed 92% of favorable outcomes with one session. No compression was applied. In 40% of cases, complications such as redness, pain or thickening were observed. No intra-arterial injections, venous thrombosis, or nerve injuries were noted.
J.-J. Guex [100] reported achieving PV occlusion in 90% of cases with three or fewer treatment sessions. Veins with a diameter of more than 8 mm, in his opinion, are resistant to sclerosing effects. According to J.-J. Guex, further studies should include measurements of the diameter and duration of reflux before and after the procedure, and plethysmographic evaluation of therapy.
Complications of sclerotherapy for PV are identical to those for obliteration of the saphenous veins: hyperpigmentation, phlebitis, necrosis, allergic reactions, intraarterial injections [101]. Contrary to the danger mentioned by many authors [102] of getting into the artery accompanying the perforator, such complications during sclerosis of the PV are less common.
It is obvious that the possibilities of sclerotherapy of the PV require careful further study. The long-term results of these manipulations are unknown [100], since most researchers [103] report only short follow-up periods.
Regarding long-term results
surgical elimination of insufficient PV, then if we leave aside sclerotherapy, which fundamentally cannot provide lasting long-term results, and modern methods of thermoobliteration, which are rarely used for this purpose and not so long ago, then we can consider the effectiveness of only traditional or endoscopic interventions. However, when analyzing the literature data, attention is drawn to their extreme paucity. As a rule, the result of treatment as a whole is assessed, or, if we are talking about relapses of the disease, the authors report that a certain percentage of cases were associated with perforating reflux. This does not allow any valid conclusions to be drawn because the baseline incidence of PT failure is usually not reported. Only in studies devoted to endoscopic subfascial dissection of the PV can one find information suitable for analysis - this method is used only when perforator reflux is undoubtedly present. J. Sybrandy et al. [56] found a large number of newly formed PVs 4 years after endoscopic dissection. This may seem quite unusual, since a more logical explanation would be the errors of the operation - the veins were not found and were not ligated. Nevertheless, this point of view is confirmed by A.G. Khitaryan et al., who discovered PV neoplasm after classical Linton-Felder interventions [104]. A number of authors [105] explain the persistence and detection of PV in the long-term period after endoscopic intervention, in addition to possible technical errors, also by the expansion of veins that were healthy at the time of surgery, and therefore not ligated. A.I. Kiriyenko et al. noted an interesting fact - despite the formally not entirely satisfactory results (incompetent PVs were found in 50% of the operated limbs), the clinical results of treatment were good in most cases. A.V. reports something similar. Andriyashkin [106], who identified incompetent PVs in more than 90% of patients with recurrent VLNK before surgery, and 1 year after surgery, with obvious successful results, PVs were detected during control ultrasound examination in 38% of cases.
Such observations make us think, firstly, about the possibilities of surgical elimination of PV, and, secondly, about the pathogenetic validity and expediency of these interventions.
Contradictions in the theory of the pathogenetic significance of perforating discharge in varicose veins
Already in the late 80s - early 90s of the last century, many scientists began to wonder how the opinion about the pathogenetic significance of the failure of PV corresponded to the actual state of affairs. At the same time, it is not the very fact of the presence of a perforator that is called into question - the failure of the PV in patients with varicose veins undoubtedly develops. The question is whether perforating reflux can be the cause of varicose veins or is it only its consequence, which means whether surgical interventions on the PV are necessary [53, 107].
Proponents of the latter version, who believe that the expansion of the PV is a compensatory mechanism [108], ensuring the return of blood volumes that increase during varicose transformation to the deep venous system, have several very serious arguments.
First, there is no evidence that PV ligation improves surgical outcomes. Since surgery for varicose veins includes, in addition to ligation of the varicose veins, the elimination of incompetent trunks and their tributaries, it is impossible to say that the elimination of perforating discharge led to more favorable treatment results [41, 70, 109-111].
A solution could be to conduct an isolated dissection of the PV in patients with VLNK with subsequent evaluation of the effect, but, obviously, such an experiment is unlikely to be decided by any of the clinicians who value their reputation - it is absolutely clear that it is impossible to perform the intervention, leaving the patient with with VBNK there is an insolvent trunk and tributaries. However, a number of studies have obtained data indicating that selective ligation of PV in the absence of deep venous reflux does not improve venous hemodynamics [12, 113], which to some extent casts doubt on the pathogenetic significance of PV. Some studies [87, 114-116] have shown the absence of a statistically significant improvement in venous function after ligation of perforators in patients with post-thrombotic disease, although this can also only serve as indirect confirmation of the first argument.
Since the above-mentioned research design is fundamentally impossible, specialists who sought to determine the true role of PV incompetence went “from the opposite direction”, resulting in the second important argument in their hands - isolated removal of pathologically altered saphenous veins leads to the disappearance of reflux in those that were incompetent at the time of the operation, but remaining unligated perforators [109, 117-120]. Elimination of pathological venous capacity reduces the hemodynamic load on the PVs, which as a result return to their normal state.
Proponents and opponents of the theory of “guilt” of perforators, if viewed from the standpoint of modern evidence-based medicine, can be judged by randomized studies in which some patients will be ligated with PV, while others will not. This work was carried out by R. Fitridge et al. [112]. They examined 38 limbs of VVNK patients with insufficiency of the GSV and calf perforators, but without signs of deep vein insufficiency. The saphenofemoral anastomosis was ligated, the GSV was extirpated, and all visible varicose veins were removed. Patients were randomly assigned to have incompetent perforators ligated or left intact. Hemodynamic function was assessed using air plethysmography before and 2 months after surgery. No significant hemodynamic differences were shown between the two groups. The investigators concluded that there was no additional hemodynamic benefit of perforator ligation in patients with uncomplicated variceal veins over a 3-month follow-up period. It should be noted that the number of patients included in the study was small and the follow-up period was short.
And finally, the third argument is the fact that the frequency of detection of failed PV increases with increasing clinical class of the disease [62, 64]. According to the researchers who obtained such data, this indicates the secondary nature of the changes in the perforators - the more pronounced the varicose transformation of the saphenous veins, the greater the hemodynamic load falls on the PVs, which as a result expand and naturally become insolvent.
Of course, all the above arguments can only serve as indirect evidence that the failure of PV in varicose veins does not have the importance that is usually attached to it. However, these data cannot be ignored.
Concluding the review of literature data on PV insufficiency, it must be said that in this seemingly fully studied area of phlebology, more questions remain than answers, and the problem seems very far from being resolved. First of all, there is no strong evidence of the pathogenetic significance of PV failure in varicose veins, and emerging indirect evidence suggests rather the opposite - the absence of such significance. The effectiveness of interventions aimed at eliminating the insolvency of PV is low, and the feasibility of their implementation is increasingly being questioned. There is an urgent need for a targeted study of this problem from the standpoint of our current knowledge about PVs and their physiology and taking into account the capabilities of modern instrumental diagnostics.