Pneumonia is an acute infectious disease of the lower respiratory tract. Pneumonia or pneumonia is a common pathology, the development of which is caused by the activity of microorganisms.
Atypical pneumonia is a form of pneumonia in which the pathological process develops under the influence of atypical pathogens. In this case, the patient exhibits signs that are uncharacteristic of this disease. Atypical pneumonia is caused by mycoplasma, legionella or chlamydia. An inflammatory process in the lungs, if untreated or poorly treated, can cause serious harm to health.
You can undergo examination and receive treatment for pneumonia at the Yusupov Hospital. The clinic’s doctors follow European recommendations for the treatment of this disease and prescribe modern drugs registered in the Russian Federation to patients. To improve the patient’s condition and his recovery, rehabilitation doctors are developing a set of unique measures.
The Yusupov Hospital employs candidates and doctors of medical sciences with knowledge and experience in the treatment of atypical pneumonia. They take an individual approach to the selection of drugs for combination therapy. Patients with severe atypical pneumonia are hospitalized around the clock in the intensive care unit. Resuscitation doctors monitor their condition using modern cardiac monitors. Oxygen is supplied centrally to each room. Artificial ventilation is performed using expert-class devices.
Features of the disease
Atypical pneumonia has an acute onset of symptoms. This type of pneumonia is being actively studied by pulmonologists, since this term appeared in medicine relatively recently - in the early 30s of the 20th century. Atypical pneumonia is common among young people, and the pathological process can also develop in children.
One of the tasks that modern pulmonologists solve is the selection of effective drugs that suppress the activity of viruses and bacteria. When prescribing therapeutic measures, pulmonologists use clinical recommendations for the treatment of atypical pneumonia.
The range of treatment measures prescribed to patients at the Yusupov Hospital depends on many factors, one of which is the staged nature of the process. It has been revealed that the development of atypical pneumonia occurs in several stages:
- The incubation period lasts from the moment the pathogen enters the body and the first signs of the disease appear. The average duration of this period is 8 days;
- the period of precursors lasts up to 3 days. The patient develops signs of respiratory diseases that are not specific to pneumonia;
- the period of the height of the disease is characterized by the appearance of an inflammatory process in the lungs;
- The period of convalescence is characterized by a decrease in the inflammatory process and normalization of the patient’s condition.
Treatment of atypical pneumonia in adults at the Yusupov multidisciplinary hospital is carried out in comfortable conditions. Patients who are indicated for inpatient treatment are accommodated in spacious rooms with the necessary hygiene supplies. The Yusupov Hospital is located in a place with developed infrastructure, so clients can arrive by public transport. For the convenience of patients arriving by car, a parking area is provided.
Make an appointment
Atypical forms of lichen planus: clinical observation
Lichen planus (LP) (lichen ruber planus) is a chronic disease of the skin and mucous membranes, which is characterized by monomorphic rashes in the form of papules, accompanied by itching of varying intensity.
The disease was first described by the Austrian dermatologist F. Gebra in 1860 as itchy dark red nodules and therefore called “leichen ruber” (ancient Greek leichen - crawl, lick and lat. ruber - red). The English dermatologist E. Wilson in 1869 for the first time, using the example of 50 patients, gave a more complete clinical description of the disease: flat polygonal papules with a purple tint, an umbilical depression in the center and a characteristic waxy sheen - and proposed the term “leichen planus” (from the Latin planus - flat). The first report on LP in the domestic literature was made by V. M. Bekhterev and A. G. Polotebnov in 1881. Despite the fact that dermatosis was described more than 150 years ago, its clinical recognition presents certain difficulties in some cases. Diagnostic errors are primarily due to the huge variety of clinical manifestations and often the atypical course of dermatosis.
Etiology and pathogenesis
In modern literature, various theories of the development of LP can be traced, such as viral, neurogenic, hereditary, intoxication and immunoallergic. Currently, LP is considered to be a multifactorial disease.
There is no generally accepted classification of LP. The common typical (classical) form of LP is observed in 76% of patients [1], and a large number of atypical forms have been described.
By type of eruptive elements: atrophic, hypertrophic (verrucous, warty), follicular, pigmented, pemphigoid (bullous), erosive-ulcerative, hyperkeratotic.
According to the relative arrangement of the eruptive elements: ring-shaped, moniliform (necklace-like, coral-shaped), linear, zosteriform.
According to the localization of the rashes: damage to visible mucous membranes, hair follicles, nail plates, palms and soles.
Separate forms of LP of the mucous membrane are distinguished (typical, exudative-hyperemic, erosive-ulcerative, hypertrophic, bullous). In rare cases, one patient may experience combinations of several clinical forms [2].
Among the atypical forms, one of the rarest is the pemphigoid (bullous) form. In 1881, Baker-London first described a type of lichen planus that occurs with the formation of blisters. Kaposi in 1891 gave such cases the name pemphigoid red lichen. The literature describes manifestations of bullous LP in cancer patients (combination with thymoma, malignant tumors in the retroperitoneal space), and therefore some authors included LP in the paraneoplastic syndrome [3, 4].
The hypertrophic form occurs in 15% of patients with LP. This form is characterized by papules and plaques localized on the anterior surface of the legs, with a warty surface of a pink-red color, covered with a small number of scales [5]. The lesions are round or oval in shape, with uneven edges and clear boundaries. Subjectively, patients are bothered by painful itching. The elements are resistant to therapy and exist for a long time. This form is extremely rarely disseminated, spreading to the skin of the trunk and limbs [6].
Clinical case
We present a clinical observation of a patient with long-term hypertrophic lichen planus, in the process of evolution the addition of a bullous form. The patient was born in 1937 and has been ill since 1967, when isolated rashes first appeared on the skin of the legs, accompanied by minor itching. I did not go to a dermatologist and did not undergo treatment. In 2001, she noted the spread of rashes and increasing itching, and contacted the Surgut Clinical Dermatovenerologic Dispensary (SKVD), where she was diagnosed with lichen planus, hypertrophic form. Repeatedly (1–2 times a year) she received outpatient treatment in the form of antihistamines and topical glucocorticosteroids, with a slight positive effect in the form of reduced itching and blanching of the rashes. Repeatedly (1–2 times a year) she received inpatient treatment at the SKKVD: antimalarials, antihistamines, topical glucocorticosteroids, physiotherapy, and was discharged with slight improvement. I did not notice complete regression of the rash. Exacerbation regardless of season. The last exacerbation was from January 2015. She did not treat herself. Over the past week, I have noticed the appearance of blisters on the skin of my legs. I contacted a dermatologist at SKVD. She was hospitalized in the inpatient department due to the prevalence of the skin process.
Life history: native of the Omsk region, in the North since 1965. Opisthorchiasis - in 1966, 1980. sanitized Chronic diseases at the moment: seen by a general practitioner at the place of residence with a diagnosis: “Arterial hypertension of the 2nd degree, stage 3, risk 4. CHF 2. Deforming osteoarthritis of both knee joints.” Takes constantly: lisinopril 10 mg, 1 tablet 2 times a day, metoprolol tablets 50 mg/day, hydroxychloroquine tablets 25 mg in the morning, acetylsalicylic acid + magnesium hydroxide tablets 75 mg/day. Operations: thyroidectomy in 1981, denies any previous injuries. Allergy history is not burdened.
Objectively: the general condition is of moderate severity, due to concomitant therapeutic pathology. Position: active - passive. The patient has a hypersthenic build, high nutritional status, height 172 cm, weight: 140 kg. BMI 47.3. Movement in the knee joints is limited. Swelling in the lower legs. Local status: the pathological skin process is widespread, localized on the skin of the anterior surface of the abdominal wall and lower extremities. On the skin of the abdomen in the navel area there are lenticular and numular hypertrophied papules, dark pink in color, the surface of the papules is uneven and lumpy. There is a Wickham grid on the surface (Fig. 1). On the skin of the lower extremities, mainly on the skin of the legs, there are multiple hypertrophied papules, pink-bluish in color with a lilac tint, with an umbilical depression in the center, prone to merging into plaques up to 10.0 cm with pronounced infiltration at the base. The surface of the plaques is uneven, lumpy, dotted with warty protrusions with many depressions (Fig. 2). On the surface of the plaques there is erosion, oval in shape, up to 7.0 cm in diameter, with serous discharge, multiple serous crusts, single blisters, up to 1.5 cm in diameter, with a dense covering with transparent contents (Fig. 3). Peripheral lymph nodes are not enlarged. Dermographism is mixed. There are no other pathological rashes on the skin or visible mucous membranes.
During the examination: general blood test, general urinalysis, biochemical blood test - without pathology. Examination for hepatitis HBsAg - not detected. HIV ELISA is negative.
Consultation with a therapist. DS: IHD. Atherosclerotic cardiosclerosis. Angina pectoris FC II. Atrial fibrillation. Tachysystolic form. Hypertension stage 3, risk 4, degree 2. CHF III A-B. FC IV. Decompensation of heart failure. Obesity III degree (BMI 47). Deforming osteoarthritis of the knee joints, NFS I. Therapy was adjusted for concomitant diseases.
Prescribed treatment: intravenous drip solution of meglumine sodium succinate 1.5%, 400 ml No. 5 every other day; clemastine solution 0.1% - 2.0 ml IM 2 times a day No. 10, hydroxychloroquine tablets 200 mg 2 times a day orally for 5 days, then a break of 2 days - 2 courses (10 days), lisinopril 20 mg 1 tablet - 2 times a day, metoprolol 50 mg, 1 tablet 1 time a day, hydrochlorothiazide 25 mg, 1 tablet 1 time a day, acetylsalicylic acid + magnesium hydroxide 75 mg, 1 tablet 1 time a day. Externally: open the blisters, treat, spray betamethasone on the skin of the legs 2 times a day - 2 days, betamethasone ointment 2 times a day on the skin of the legs, abdomen.
During treatment: subjective reduction of itching. The rashes on the skin of the abdomen and legs became slightly pale, erosions became epithelialized, and new blisters did not appear.
Due to the developed decompensation of concomitant therapeutic pathology, it was decided to urgently transfer the patient to the therapeutic department of the budgetary institution of the Khanty-Mansi Autonomous Okrug-Yugra “Surgut District Clinical Hospital”. Recommendations were given: diet (limit the intake of fried, smoked, salty foods, alcohol), clinical observation by a dermatologist, systematic courses of specific therapy, avoid skin trauma, limit physical and psycho-emotional stress, limit contact with household chemicals, consultations with specialists: infectious disease specialist, gastroenterologist , oncologist; correction of treatment of concomitant pathology, rehabilitation of foci of chronic infection.
Conclusion
The presented clinical case of widespread LP with a combination of atypical forms of the disease (hypertrophic and bullous) is notable for the long duration of the disease (about 48 years), torpid course (the rash did not completely regress), and the insufficient effect of the therapy used. Thus, the case we described of a combination of atypical forms of LP can be considered as a rare clinical variant of the course of dermatosis.
Literature
- Molochkov V. A., Sukhova T. E., Molochkova V. E. Clinical features of lichen planus // Clinical dermatology and venereology. 2013; 4: 34–42.
- Mikheev G.N., Krasnoselskikh T.V., Yastrebov V.V., Grigoryan A.E. A rare form of lesions of the palms and soles with lichen planus // Bulletin of Dermatology and Venereology. 2014; 6: 136–143.
- Slesarenko N. A., Utz S. R., Artemina E. M., Shtoda Yu. M., Karpova E. N. Comorbidity in lichen planus // Clinical dermatology and venereology. 2014; 5:4–10.
- Molochkova Yu. V., Selezneva E. V., Prokofiev A. A. Pemphigoid lichen planus // Russian Journal of Skin and Venereal Diseases, 2013; 3:19–22.
- Gadzhimuradov M.N., Gunasheva A.A. Atypical forms of lichen planus: clinical manifestations, differential diagnosis, treatment // Clinical dermatology and venereology, 2009. pp. 85–90.
- Abdrakhmanov R.M., Khismatulina I.M., Sadykova F.G. Clinical case of warty form of lichen planus // Medline.ru. Dermatology. 2010; (11): 37–43.
E. A. Vasilyeva* E. N. Efanova*, 1, Candidate of Medical Sciences Yu. E. Rusak*, Doctor of Medical Sciences, Professor I. V. Ulitina**
* BU VO KHMAO-Yugra Surgut State University, Surgut ** BU KHMAO-Yugra Surgut KVD, Surgut
1 Contact information
Key words: lichen planus, atypical form, itching, hypertrophic form, papule, vesicle, malignancy.
Keywords: lichen ruber planus, atypical form, itch, hypertrophic form, papule, bubble, malignization.
Reasons for development
The development of the inflammatory process in the lungs is associated with the vital activity of pathogenic microorganisms with different characteristics. This causes a variety of forms of atypical pneumonia. Atypical pneumonia is most often detected in children and young people. The causes of atypical pneumonia are the following pathogens:
- coronavirus;
- mycoplasma;
- chlamydia;
- legionella;
- Coxiella;
- influenza viruses A, B;
- respiratory syncytial virus;
- hantavirus.
In the early stages, diagnosis of the disease is difficult. Therefore, pulmonologists recommend that if signs of an inflammatory process in the respiratory tract appear, you should go to the Yusupov Hospital for an examination under the guidance of candidates and professors of medical sciences.
The main difficulty in the treatment of atypical pneumonia is mutations of pathogens, as a result of which their resistance to drugs increases. When treating patients at the Yusupov Hospital, drugs are used whose effectiveness has been confirmed by many studies. At the same time, patients are prescribed only those medications that are certified in the Russian Federation.
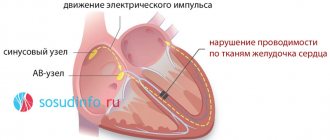
Causes of myocardial infarction
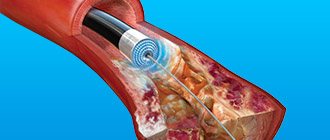
The main reason for the disruption of oxygen delivery to the heart muscle is the partial or complete obstruction of the “transport routes” - the blood vessels supplying the heart. Obstruction may be caused by:
- blockage of the lumen of the vessel (thrombosis, atherosclerosis of the heart arteries, fat embolism);
- vascular spasm.
In turn, obstacles to blood flow often appear in blood vessels due to metabolic disorders (the leading role is played by lipid metabolism, including increased levels of LDL - the so-called “bad cholesterol”), which arise due to:
- low physical activity;
- obesity (and diabetes);
- drinking alcohol and smoking;
- nutrition inadequate for age, constitution, physical activity.
There are situations when the cause of blockage of a vessel by a blood clot or fatty drop is injury or surgery.
Other risk factors for acute myocardial ischemia are:
- acute and chronic infectious diseases (staphylococcal and streptococcal infections), which, among other things, can lead to rheumatic carditis;
- arterial hypertension;
- male gender;
- age, etc.
- a previous heart attack.
Most often, excluding cases of heart attack provoked by surgical intervention, the causes of this disease are complex, and physical and/or mental stress most often plays the role of the “last straw”. And vasospasm is a direct consequence of the stress reaction.
Classification
SARS infection occurs through airborne droplets when pathogenic microorganisms enter the body. Experts distinguish several forms of atypical pneumonia depending on the type of pathogen:
- chlamydial pneumonia. The development of this form in children can occur from the mother, since chlamydia can cause vaginal chlamydia;
- acute respiratory syndrome;
- Mycoplasma pneumonia most often affects children and adolescents;
- Q fever;
- legionella pneumonia.
Atypical forms of lobar pneumonia have pronounced clinical manifestations. Patients experience a sharp rise in body temperature and pain in the chest. Pulmonologists at the Yusupov Hospital regularly improve their skills and study the experience of other specialists in this field, which allows them to improve the quality of therapy and carry out highly accurate diagnostics of those forms of the disease that do not have pronounced symptoms.
Atypical forms of myocardial infarction
Sometimes there is an atypical course of myocardial infarction with localization of pain in atypical places (in the throat, fingers of the left hand, in the area of the left scapula or cervicothoracic spine, in the epigastrium, in the lower jaw) or painless forms, the leading symptoms of which may be cough and severe suffocation, collapse, edema, arrhythmias, dizziness and confusion.
Atypical forms of myocardial infarction are more common in elderly patients with severe signs of cardiosclerosis, circulatory failure, and secondary myocardial infarction.
However, only the most acute period usually proceeds atypically; further development of myocardial infarction becomes typical.
The erased course of myocardial infarction is painless and is accidentally detected on an ECG.
Symptoms of the disease in children
In childhood, the causative agents of pneumonia in most cases are mycoplasma bacteria. This type of disease is characterized by a poorly expressed temperature reaction and rapid deterioration of the condition.
If atypical pneumonia develops in children, the symptoms intensify quickly; it is this sign that should alert parents and become a reason to contact a medical facility. The main symptoms of atypical pneumonia in children:
- apathy and lethargy;
- drowsiness;
- increased sweating;
- decreased appetite;
- dyspnea;
- diarrhea;
- vomit.
A polymorphic skin rash is one of the signs of atypical pneumonia in children; in addition, with the development of the disease, enlargement of the liver and spleen may occur. SARS causes chest pain, so children can lie on the side of the affected lung to reduce pain.
Emphysema is a common complication of atypical pneumonia in newborns. The danger of the pathology at this age is that it is extremely difficult and treatment is complicated by the inability to use potent drugs.
Pulmonologists recommend that parents, if pathological signs appear, contact specialists for advice and diagnostics. Treatment with drugs without a doctor’s prescription can cause various complications, since the disease is most pronounced in childhood.
Signs of the disease in adults
Atypical pneumonia, the symptoms of which in adults may differ from ordinary pneumonia, is characterized by the appearance of pronounced symptoms at its height. Symptoms of atypical pneumonia in adults:
- low-grade fever, with an increase in inflammation, body temperature can reach 41˚C;
- moderate headache;
- slight noise in the ears;
- profuse sweating at night;
- severe weakness;
- pain localized in the chest;
- dry cough;
- sputum discharge.
These signs are common to various forms of atypical pneumonia, but patients may experience other symptoms caused by a specific strain of the microorganism. When examining an x-ray, the focus of inflammation may be dispersed, so a complex of high-precision measures is used to make a diagnosis.
The most severe form of atypical pneumonia occurs in the form of acute respiratory disease syndrome. The symptoms of the disease at the initial stage are similar to those of the flu, but after a few days the patient may experience tachycardia and severe shortness of breath. The course of the disease depends on the body’s immune response; with weakened immunity, the patient’s condition quickly deteriorates. At the Yusupov Hospital, patients in serious condition are provided with emergency care. In case of pronounced symptoms, immediate diagnosis and hospitalization are carried out.
Pulmonologists note cases when atypical pneumonia develops, and the symptoms in adults without fever are similar to the manifestations of other inflammatory diseases of the respiratory system. High-precision diagnostics allows specialists to establish the causes of the pathological process and identify the pathogen.
Mycoplasma atypical pneumonia
The causative agent of the disease is mycoplasma. Atypical pneumonia is diagnosed in children in 21% of cases, in adults – in 25%. Children aged 3 to 14 years are most often affected. The infection is spread by airborne droplets.
The incubation period lasts from 3 to 11 days. This is followed by a short prodromal period, which lasts up to 1 day. The main symptoms of mycoplasma atypical pneumonia are:
- moderate headache;
- general malaise;
- dry, sore or sore throat;
- dry cough;
- low-grade fever.
During the examination, the doctor reveals redness and enlargement of the follicles on the back wall of the pharynx. The cough that appears at the beginning of the disease gradually increases. It becomes paroxysmal, but remains unproductive for 2-3 weeks. When lung tissue is damaged, the patient's condition worsens, body temperature rises, chills and signs of intoxication appear.
A feature of mycoplasma atypical pneumonia is the discrepancy between physical changes and x-ray data and the lack of effect of treatment with penicillin antibiotics or cephalosporins. On days 3-5, during auscultation, weakened breathing and a small amount of moist rales are heard. Mycoplasma atypical pneumonia can be suspected by the presence on radiographs of a low-intensity or moderate-intensity heterogeneous shadow of infiltration, which is visible against the background of a sharply changed pulmonary vascular pattern.
To diagnose mycoplasma atypical pneumonia, doctors at the Yusupov Hospital use the following methods:
- microbiological (culture of sputum and nasopharyngeal swabs on special nutrient media);
- serodiagnosis (complement fixation reaction);
- detection of mycoplasma antigens in human biological environments.
Express diagnosis of atypical pneumonia is carried out using DNA and RNA probes.
For the treatment of mycoplasma atypical pneumonia in adults, antibacterial drugs of the macrolide group and lincosamides are used. Children over 8 years of age and adults are prescribed doxycycline. With the development of broncho-obstructive syndrome, the use of bronchodilators is indicated.
Typical and atypical forms of diabetic polyneuropathies
Diabetic polyneuropathy, one of the common complications of diabetes mellitus, is manifested by damage to various parts of the peripheral nervous system. There are typical and atypical forms of diabetic polyneuropathies, which have different pathogenesis, symptoms, course and prognosis. The article discusses clinical features, modern diagnostic methods and approaches to the treatment of generalized and atypical polyneuropathies.
Differential diagnosis of DPN with atypical symptoms and additional research methods
Diabetes mellitus (DM) is a common disease, the incidence of which is steadily increasing every year. According to the results of a Russian epidemiological study, 6 million people suffer from type 2 diabetes. Every fifth resident of Russia is in a state of prediabetes [1]. The most common chronic complication of diabetes, diabetic polyneuropathy (DPN), is characterized by symptoms and signs of peripheral nerve dysfunction when other causes of polyneuropathy have been excluded. Depending on the severity of symptoms, DPN can significantly reduce quality of life. DPN develops on average five years after the onset of type 1 diabetes and is detected in 20% of cases of newly diagnosed type 2 diabetes. DPN can also occur at the stage of prediabetes. Thus, impaired glucose tolerance was observed in 25–62% of patients with “idiopathic” polyneuropathy [2]. In patients with impaired glucose tolerance, symptoms of DPN were found in 11–25% of cases, and neuropathic pain in 13–26% of cases.
Classification
There are various variants of polyneuropathies with various clinical manifestations, course and pathogenesis. The American Diabetes Association offers the following classification of DPN (2017) [3].
1. Generalized polyneuropathies.
A. Distal symmetrical DPN:
- predominant damage to thin nerve fibers;
- predominant damage to thick nerve fibers;
- damage to both thin and thick nerve fibers (combined damage, mixed polyneuropathy).
B. Autonomic neuropathies:
- cardiac - decreased heart rate variability, resting tachycardia, orthostatic hypotension, sudden death (malignant arrhythmia);
- gastrointestinal – diabetic gastropathy, diabetic enteropathy, decreased colon motility (constipation);
- urogenital – neurogenic bladder, erectile dysfunction, sexual dysfunction in women;
- impaired sweating - distal hypohidrosis (anhidrosis), increased sweating after eating;
- impaired pupillary reactions;
- hypoglycemia unrecognized (by the patient).
2. Mononeuropathies (multiple mononeuropathies): neuropathy of the cranial nerves, nerves of the upper and lower extremities and multiple mononeuropathies.
3. Radiculopathies and radiculoplexopathies: cervical and lumbosacral radiculoplexopathy, thoracic radiculopathy.
In diabetes, non-diabetic polyneuropathies are also common: compression paralysis, chronic inflammatory demyelinating polyradiculoneuropathy, radiculoplexopathy, acute painful neuropathy with damage to thin nerve fibers (during treatment).
Generalized forms
The most common type of DPN (about 75% of all DPN) is distal symmetrical sensorimotor DPN [4]. Depending on the type of nerve fibers affected, clinical symptoms can vary significantly. For example, patients with predominantly damage to thin nerve fibers are mainly concerned about neuropathic pain, manifested by burning, tingling, and shooting pains. The painful form is observed in at least 25% of cases [5]. A neurological examination reveals “positive” sensory symptoms: hyperalgesia, allodynia, hyperpathia. Tendon reflexes may remain intact. As DPN progresses and nerve fiber degeneration occurs, pain symptoms decrease. In DPN with predominant damage to thick nerve fibers, numbness, tingling, sensory ataxia, decreased or absent Achilles reflexes are noted. In some cases, weakness in the foot extensor muscles may be noted, but in general muscle weakness is an uncommon symptom for distal symmetric sensorimotor DPN.
Autonomous DPN occurs in 60% of patients with diabetes duration of more than five years [6]. Damage to autonomic fibers is often combined with damage to thin nerve fibers. With autonomous DPN, any organs and systems can be affected: cardiovascular, gastrointestinal, genitourinary. The most common and serious type of autonomic DPN is cardiac autonomic neuropathy. It is characterized by symptoms such as resting tachycardia, fixed pulse, orthostatic hypotension, silent ischemia and myocardial infarction, arrhythmias, sudden cardiac arrest during surgery, obstructive sleep apnea syndrome. It should be noted that cardiac autonomic neuropathy can be asymptomatic for a long time, but at the same time increases the risk of sudden death, heart attacks and strokes - it can be called the “silent killer” of a patient with diabetes [7]. This is why it is so important to regularly conduct screening tests for the timely diagnosis and treatment of cardiac autonomic neuropathy.
The following symptoms are not typical for generalized DPN:
- severe muscle weakness, multiple mononeuropathies, damage to cranial nerves;
- pronounced asymmetry of sensory and motor disorders;
- acute onset and rapid development of neurological disorders;
- predominance of neurological symptoms in the upper extremities.
If similar symptoms are observed in a patient with diabetes and other causes of peripheral nerve damage have been excluded (table [8], as modified), then an atypical form of DPN should be suspected.
Atypical forms
Mononeuropathies of the upper extremities
The most common among atypical forms of DPN are neuropathies of the nerves of the upper extremities, which are tunnel neuropathy due to compression of the nerves in places typical of compression (neuropathy of the median nerve at the level of the carpal tunnel, neuropathy of the ulnar nerve at the level of the cubital tunnel). In diabetes, as a result of hyperglycemia, axonal transport in the nerve fiber is disrupted and other biochemical changes in the axon occur [9]. Nerves become more vulnerable in anatomically narrow areas, and even a slight external impact (compression, tension) can lead to subclinical or clinical damage.
Mononeuropathies begin gradually, can be asymptomatic and detected only with electroneuromyography (ENMG). Often, tunnel neuropathies of the upper extremities precede the development of generalized DPN and may be the first symptoms of diabetes. Thus, carpal tunnel syndrome is an early complication of diabetes, developing in the first five years. Tunnel neuropathies have also been described in patients with impaired glucose tolerance [10].
The most common tunnel DPN is median nerve neuropathy with compression at the level of the carpal tunnel - carpal tunnel syndrome. The incidence of carpal tunnel syndrome increases with the duration of diabetes: 28% at the onset of diabetes and 63% with a disease duration of 14.5 years [10, 11]. Like the idiopathic form of carpal tunnel syndrome, this pathology most often affects women. Typical clinical symptoms include numbness, tingling and pain in the hand (in the zone of innervation of the median nerve), worsening at night. As it progresses, clumsiness and muscle weakness in the finger flexors occur. To diagnose carpal tunnel syndrome, the Tinel, Phalen, and elevator tests are used. The diagnosis is confirmed by ENMG examination.
Tunnel neuropathy of the ulnar nerve at the level of the cubital canal was diagnosed in 34% of cases in patients with diabetes. If nerve compression is localized in the retrocondylar groove (76%), then demyelination of the nerve is observed, and if in the area of the brachial-ulnar aponeurotic arcade (17%), then axonopathy is observed [9]. Clinically, ulnar nerve neuropathy manifests itself as painful paresthesia in the fourth and fifth fingers and/or weakness of the hypothenar and interosseous muscles. Often, ulnar nerve neuropathy occurs subclinically and is combined with carpal tunnel syndrome.
Mononeuropathies of the cranial nerves
Patients with diabetes often have damage to the oculomotor and facial nerves. Risk factors for the development of cranial neuropathies include the duration of diabetes and the age of the patient. Most often, paresis of the abducens nerve occurs, less often of the oculomotor nerve, and even less often of the trochlear nerve.
The oculomotor nerves are usually affected in elderly people (over 50 years old). The onset of the disease is acute. With damage to the third pair of oculomotor nerves, in contrast to paresis of the VI and IV pairs, pain in the retro-orbital region is often observed, which often precedes paresis of the oculomotor muscles. The prognosis is favorable: oculomotor functions are fully restored within three to six months [8]. A distinctive feature of oculomotor nerve paresis is the preservation of pupillary reactions to light. This is due to the fact that during ischemia, the motor fibers in the central part of the nerve are primarily affected. At the same time, in a third of patients with diabetes, the pupil size changed; in such cases, magnetic resonance angiography is recommended to exclude an aneurysm of the posterior communicating artery.
The prevalence of facial paresis in patients with diabetes varies widely. In general, facial neuropathy and diabetes are considered comorbid conditions, and facial neuropathy is not an indication for testing to exclude diabetes in the absence of other indicators, such as concomitant hypertension (Level of Evidence: C).
Proximal diabetic radiculoplexopathy
Another form of atypical DPN is proximal diabetic amyotrophy (proximal diabetic radiculoplexopathy, Bruns-Garland syndrome). This is an autoimmune disease caused by ischemic damage to nerves and nerve roots due to microvasculitis and subsequent degeneration of nerve fibers. This form most often affects the lumbosacral plexus, but the cervical and brachial plexuses may also be involved. Proximal diabetic amyotrophy is somewhat more common in men with type 2 diabetes.
The onset of the disease is acute or subacute in the form of intense neuropathic pain in the lower back and anterior thigh, intensifying at night. The nature of the pain can be shooting, deep aching, burning, reminiscent of electric current and is often accompanied by allodynia. In most cases, the pain is unilateral and local, but gradually it spreads to the distal parts and the opposite side. On average, the symptoms in most patients switch to the other side within three months and the lesion becomes bilateral, but with preservation of asymmetry. Over time (from several days to several weeks), muscle weakness and atrophy develop in the affected limb. Muscle weakness often reaches severe levels. Almost half of the patients required auxiliary equipment due to severe paresis [8].
Depending on the affected roots, distal muscle weakness may also be observed, which should be distinguished from distal DPN. There is a decrease or loss of knee reflexes. Achilles reflexes may also be reduced due to distal muscle involvement or concomitant DPN. Sensory disturbances are variable and are represented by hypoesthesia on the anterior thigh or in the distal parts of the legs due to concomitant DPN. Characteristic loss of body weight. Autonomic dysfunction often occurs. The development of orthostatic hypotension, sexual dysfunction, gastroparesis, diarrhea, constipation and impaired sweating has been described. Sometimes there is bilateral damage to the roots and plexuses with the development of “diabetic paraplegia.” The level of protein in the cerebrospinal fluid may increase - more than 1 g/l.
Along with painful forms, painless forms of proximal diabetic amyotrophy are also occasionally found. The painless form, especially with bilateral symptoms, may clinically resemble chronic inflammatory demyelinating polyneuropathy (CIDP) in the classical or atypical form. CIDP is the most common (from 4.3 to 30%) non-diabetic polyneuropathy in patients with diabetes [8, 11, 12]. The classic version of CIDP is characterized primarily by proximal symmetric muscle weakness, decreased reflexes and sensory disturbances with a predominance of deep sensory disturbances that progress over more than two months. Atypical variants of CIDP include isolated sensory and motor forms, multifocal sensory-motor form, isolated damage to the plexus or nerves of one limb [13]. The fundamental difference between proximal diabetic amyotrophy and CIDP is a monophasic course with good recovery even without immunosuppressive therapy. With CIDP, a relapsing or progressive course is more often observed; self-healing is uncharacteristic. Differential diagnosis is also based on ENMG examination. Signs of segmental demyelination in the form of a significant decrease in the speed of propagation of excitation, blocks of excitation conduction, or loss of F-waves are not typical for DPN. In both forms of polyneuropathy, the level of protein in the cerebrospinal fluid may increase, but with CIDP the increase is usually significant (1.5–2 g/l and above), and with proximal diabetic amyotrophy it is insignificant (up to 1.2 g/l) [8].
To confirm the diagnosis of proximal diabetic amyotrophy, the following examinations are recommended:
- magnetic resonance imaging of the lumbosacral spine (to exclude a herniated disc or pelvic mass);
- ENMG/electromyography (signs of denervation are detected in the paraspinal muscles, as well as proximal and distal leg muscles, axonal damage to peripheral nerves);
- examination of cerebrospinal fluid (increased protein level more than 1 g/l, absence of cell-protein dissociation);
- nerve biopsy (non-routine examination indicated only in atypical cases).
Thoracic radiculopathy
A rare form of atypical DPN is thoracic radiculopathy, which occurs in middle and old age and more often among men. The disease begins suddenly, with burning pain in the chest or abdominal area, worsening at night. Allodynia is noted in the affected area, accompanied by unpleasant sensations from the touch of clothing. Clinical examination reveals hyperpathy and other symptoms of sensory impairment, as well as weakness of the abdominal muscles and, occasionally, weight loss. Spontaneous recovery is observed within several months. If the condition does not improve during this period, magnetic resonance imaging of the thoracic and lumbar spine should be performed to exclude pathology of the spinal cord or intervertebral discs.
All atypical forms of DPN can be accompanied by generalized forms of DPN (distal symmetric diabetic and/or autonomic polyneuropathy).
Diagnostics
Today, in the instrumental diagnosis of DPN, ENMG is mainly used - an objective, sensitive, reproducible and standardized method. In DPN, it is necessary to determine the status of motor and sensory fibers in the arms and legs (eg, sural, peroneal, and tibial nerves in the legs and median nerve in the arms). With symmetrical DPN, it is enough to conduct an examination on one side.
The earliest ENMG sign of DPN is axonal damage to the sural nerve with a subsequent decrease in the speed of propagation of excitation as the polyneuropathy progresses. Some authors suggest using the speed of excitation propagation as the most sensitive ENMG marker of DPN, especially at the subclinical stage [4]. An ENMG study of tunnel neuropathies reveals signs of local nerve damage in the form of an increase in distal (residual) latency or a local decrease in the speed of propagation of excitation. Ultrasound examination reveals an increase in the cross-sectional area of the nerve both in places typical for compression and in other places without clinical symptoms. The results obtained confirm morphological changes in peripheral nerves in patients with diabetes, which makes them sensitive to compression [11].
However, with an ENMG study, it is possible to evaluate the function of only thick myelinated nerve fibers (A-alpha and A-beta types). In case of DPN with predominant damage to thin nerve fibers, ENMG indicators will be within normal values. In these cases, techniques developed specifically for the diagnosis of small nerve fiber neuropathies should be used: quantitative sensory testing, skin biopsy, confocal microscopy of the cornea.
The quantitative sensory testing method is based on determining the threshold values of heat (C-fiber) and cold (A-gamma fiber) sensitivity, heat and cold pain (C-fiber). This sensitive and non-invasive test provides information about the function of thin and thick nerve fibers. However, at the same time, this method is not standardized, depends on the attention and mood of the patient, his willingness to cooperate, anthropometric data and is characterized by variability in results.
Skin biopsy has become widespread in a number of countries. This method involves examining a 3 mm diameter area of skin on the lateral part of the leg closer to the distal part and assessing the density of intraepidermal nerve fibers. In patients with DPN, there is a significant decrease in the density of intraepidermal nerve fibers, and in the early stages of the painful form of DPN with predominant damage to small fibers, the indicators are lower compared to the painless form, which indicates a greater involvement of thin nerve fibers in the painful form of DPN. Skin biopsy has a level of evidence for diagnosing small nerve fiber neuropathies. However, this is an invasive method, and in Russia it is used only in scientific work.
A promising method for diagnosing small nerve fiber neuropathy appears to be confocal microscopy of the cornea, a fast and non-invasive technique that has a fairly high sensitivity. This technique evaluates the length and density of corneal nerve fibers, which are significantly lower in patients with DPN compared to the healthy population.
Treatment
Treatment of DPN includes glycemic control, active lifestyle, and correction of risk factors. However, according to the study results, optimizing blood glucose control only prevents or slows the progression of type 1 diabetes, but not type 2. In this regard, the prescription of drugs that directly affect the pathogenetic mechanisms of the development and progression of DPN is justified and justified.
Hyperglycemia triggers a cascade of metabolic and vascular disorders leading to DPN. Oxidative stress, increased formation of glycosylation end products, activation of lipid peroxidation and inducible NO synthase lead to excessive formation of free radicals - molecules with increased reactivity. Free radicals disrupt the integrity of cellular structures, primarily the endothelium, causing endoneurial hypoxia and, as a consequence, the development of neuropathy. The activity of the body's antioxidant system (in particular, superoxide dismutase, catalase, glutathione) is reduced in patients with DPN. Exogenous antioxidants are required to overcome oxidative stress. The first place among antioxidants and drugs used for the pathogenetic treatment of DPN is alpha-lipoic (thioctic) acid.
The effectiveness of intravenous alpha-lipoic acid for 15 days in the treatment of DPN has been demonstrated in clinical placebo-controlled studies (ALADIN, ALADIN III, SYDNEY I and NATHAN II), one meta-analysis and has a grade of recommendation of A in European guidelines [5, 14, 15]. When prescribing alpha lipoic acid, improvement was noted in relation to positive symptoms (pain, burning, numbness, paresthesia) according to the Total Symptom Score (TSS), negative symptoms (decreased sensitivity of all modalities, leg reflexes and leg muscle strength) , as well as data from ENMG studies. For oral therapy of DPN, the most optimal dose is considered to be 600 mg/day [14]. One of the representatives of alpha-lipoic acid is the drug Thiogamma (Werwag, Germany). Thiogamma is prescribed at a dose of 600 mg in the form of a ready-made solution of 50 ml for 15 days, followed by 600 mg orally once a day 30-40 minutes before meals for 2-12 months, depending on the initial severity of DPN.
Vitamin B1 (thiamine) is involved in the functioning of neurons, ensuring axonal transport, conducting nerve impulses, and nerve regeneration. In patients with diabetes types 1 and 2, plasma thiamine levels are significantly lower than in healthy individuals. Benfotiamine – The fat-soluble form of thiamine provides a much higher concentration of thiamine in tissues than the water-soluble form. Prescribing benfotiamine to patients with diabetes at a dose of 300 and 600 mg/day for six weeks led to a decrease in the severity of DPN symptoms according to TSS [16]. Benfotiamine contains drugs such as Milgamma compositum (100 mg benfotiamine + 100 mg pyridoxine) and Benfogamma (150 mg benfotiamine) (Werwag, Germany).
Therapy with alpha-lipoic acid and benfotiamine is also of great importance in the treatment of diabetic tunnel neuropathies. The effectiveness of intravenous administration of Thiogamma was demonstrated in the work of I.A. Strokova et al. [17]. In addition to pathogenetic agents, treatment may include therapeutic blockades with anesthetic in places where nerves are compressed. It is important to change motor patterns (decreasing movements in the hand, carrying heavy objects). It is possible to immobilize the limb at the site of nerve compression. If treatment is ineffective and movement disorders progress, surgical intervention is indicated. The outcome of surgery for tunnel neuropathy in patients with diabetes is less successful than in patients without diabetes [8].
Proximal diabetic amyotrophy has a monophasic course, and regression of symptoms can be observed even in the absence of treatment. However, in the group of patients receiving immunomodulatory therapy, the recovery rate was higher and the prognosis was better [8]. Based on the autoimmune mechanism of development of diabetic radiculoplexopathies, the effectiveness of immunomodulatory therapy (glucocorticosteroids, intravenous immunoglobulin G, plasmapheresis) has been established in the treatment of the latter, especially at the onset of the disease and without pronounced axonal degeneration. The safest treatment method is intravenous administration of immunoglobulin G in a total dose of 2 g/kg body weight, but the use of this method is limited by the high cost. A good effect was described when glucocorticosteroids were administered intravenously at a dose of 1000 mg for five days under the control of blood glucose levels [8, 18]. Complex therapy for proximal diabetic amyotrophy should also include alpha-lipoic acid and benfotiamine. There are currently no specific clinical recommendations for the treatment of proximal diabetic amyotrophy.
Treatment of neuropathic pain is recommended to begin if the severity of the pain syndrome is above 4 points on the Visual Analogue Scale. To relieve neuropathic pain, the use of pregabalin, gabapentin, and duloxetine is indicated. The best tolerability profile was observed for gabapentinoids: gabapentin (Gabagamma) and pregabalin - fat-soluble amino acids, chemical structure similar to the endogenous inhibitory transmitter gamma-aminobutyric acid (GABA), a neurotransmitter involved in the transmission and modulation of pain. Our own clinical observations of the effectiveness and safety of gabapentin (Gabagamma) in the treatment of neuropathic pain syndrome in distal sensorimotor polyneuropathy confirm its effectiveness against all types of spontaneous and stimulus-dependent neuropathic pain. The advantage of Gabagamma compared to other gabapentinoids is its flexible dosage range: an intermediate dosage of 400 mg allows you to individually select therapy depending on the clinical characteristics of the patient and his pain syndrome. The initial recommended dose of gabapentin is 300 mg on the first day, 600 mg (in two divided doses) on the second day, and 900 mg (in three divided doses) on the third day. Subsequently, the daily dose is increased by successive additions of 300 mg to an optimal dose of 1800 mg/day. To achieve maximum effect, it is possible to further increase the daily dose to 2400 mg with normal tolerability starting from the 15th day of use and up to 3600 mg starting from the 21st day. However, according to our observations, in large (above average height and/or obese) patients it is better to start taking Gabagamma with 400 mg, adding 400 mg per day accordingly. Thus, in the first week of titration, the dose is increased to 1200 mg/day and insufficient effectiveness of therapy - up to 2400 mg/day in the second and up to 3600 mg/day in the third week of taking Gabagamma. This allows you to quickly achieve the maximum effective daily dose of the drug without losing compliance and without causing significant side effects. Gabapentin (Gabagamma) does not lead to drug dependence and is not included in the list of drugs for medical use that are subject to subject-quantitative recording, so the use of this drug for the treatment of neuropathic pain is currently optimal. Selective serotonin reuptake inhibitors (sertraline, fluoxetine, paroxetine, etc.) are less likely to cause significant side effects, but the analgesic activity of most drugs of this class for chronic pain syndromes does not exceed the effectiveness of placebo [19, 20].
Conclusion
For distal sensory or sensory-motor polyneuropathy, it is necessary to order a glucose tolerance test or determine the level of glycated hemoglobin to identify diabetes or prediabetes as the most common causes of polyneuropathy.
If clinical symptoms are atypical for DPN, measures should be taken to diagnose non-diabetic polyneuropathies (see table).
In the treatment of DPN, especially in patients with type 2 diabetes, the leading place is occupied by pathogenetic therapy: alpha-lipoic acid (Tiogamma) and benfotiamine (Milgamma compositum, Benfogamma).
Chlamydial pneumonia
Atypical pneumonia can develop when chlamydia enters the body. The disease is transmitted by airborne droplets. The infectious agent enters the external environment when talking, coughing, or sneezing. The portal of entry is the upper respiratory tract. Within 48 hours after infection, the villi of the bronchial epithelium are completely immobilized.
Chlamydial atypical pneumonia begins with an increase in body temperature to 38-39°C. Patients complain of joint and muscle pain, headache, and sore throat. From the first days of the disease, a dry cough appears, sometimes with a small amount of mucous sputum. On auscultation, wet and dry rales are heard. The radiograph shows interstitial or small focal infiltration.
Clinical diagnosis of chlamydial atypical pneumonia in children and adults presents significant difficulties, since the symptoms of the disease are similar to signs of damage to the respiratory tract by other microorganisms. The diagnosis can be made using serological research methods. To identify the pathogen, the Yusupov Hospital uses microscopy of smears stained according to Romanovsky-Giemsa, polymerase chain reaction, cultural methods, and enzyme-linked immunosorbent assay based on monoclonal antibodies. Macrolides and fluoroquinolones are used to treat chlamydial pneumonia. Vibramycin is also used.
Legionella pneumonia
Legionella pneumonia is an acute infectious disease caused by microorganisms belonging to the Legionella genus. The entry point for infection is the mucous membrane of the respiratory tract. Pathogens enter the body by inhaling water aerosols (air conditioners, showers, baths, humidifiers, mechanical ventilation systems, ultrasonic water sprays, fountains). No evidence of transmission of infection from person to person has been established.
Pathological changes cover at least one lobe of the lung and occur in the form of confluent pneumonia. The duration of the incubation period is from 2 to 10 days. Symptoms of Legionella SARS are:
- moderate headache;
- malaise;
- temperature rise within 1-2 days to 40°C;
- severe chills.
At the same time, a dry cough appears, and then mucous or mucopurulent sputum begins to separate. Some patients develop shortness of breath, muscle and pleural pain, nausea, vomiting and abdominal pain. During a physical examination, pulmonologists do not detect signs of compaction of the pulmonary parenchyma. Radiographs show a much larger volume of the lesion than is determined during the physical examination. In the early stages of the disease, unilateral infiltrates can be detected. They are rounded shadows that tend to merge, occupying at least one lobe.
Almost 30% of patients experience symptoms of severe hypoxemia and hyperventilation. At the Yusupov Hospital, artificial ventilation of the lungs is carried out using modern stationary and portable devices. Erythromycin is administered intravenously to treat Legionella SARS. In severe cases of the disease, it is combined with rifampicin. Reserve drugs are doxycycline, ciprofloxacin and ciprolet.
Symptoms of SARS
The disease occurs in two stages.
During the first, the following symptoms are observed:
- headache;
- chills;
- strong increase in temperature;
- muscle pain;
- loss of appetite;
- general weakness;
- diarrhea (not in all cases).
During the second stage of atypical pneumonia, the following symptoms are observed:
- dyspnea;
- dry cough;
- respiratory failure, which causes low oxygen levels in the blood.
Who is at risk for SARS?
People who have come into contact with a sick person (for example, medical personnel or relatives).
Those at risk for more severe disease include:
- Patients suffering from diabetes, hepatitis, diseases of the cardiovascular system;
- People over 50 years old.
Severe acute respiratory syndrome
Human coronavirus plays a leading role in the development of severe respiratory syndrome. It causes damage to the upper respiratory tract in adults, and to the bronchi and lungs in children. Patients develop atypical pneumonia.
The incubation period is 3-10 days. The disease begins with the following symptoms:
- unstable increase in body temperature to 38-39°C;
- slight chills;
- ailments;
- sweating;
- muscle pain;
- headache;
- sore throat.
These signs of a viral infection persist for several days or become less pronounced. As the disease progresses, the body temperature rises, weakness and headache increase, and the patient begins to feel short of breath. Breathing becomes difficult and rapid. Patients complain of palpitations and chest tightness.
Acute period
In the acute period of myocardial infarction, pain syndrome usually disappears. The persistence of pain is caused by a pronounced degree of ischemia of the peri-infarction zone or the addition of pericarditis.
As a result of the processes of necrosis, myomalacia and perifocal inflammation, fever develops (from 3-5 to 10 or more days). The duration and height of the temperature rise during fever depend on the area of necrosis. Arterial hypotension and signs of heart failure persist and increase.
Diagnostics
Infection with pneumonia can occur both through airborne droplets and through the use of things with which the patient has been in contact. Caution should be exercised by people who interacted with the patient, since the spread of the disease often occurs in groups. Doctors, when examining a patient and studying clinical signs, may suspect the development of atypical pneumonia, and then prescribe high-precision studies and laboratory tests to make a diagnosis.
Laboratory research methods to detect atypical pneumonia are:
- general blood test with leukocyte formula;
- studying the content of enzymes in the blood: AST and ALT, the concentration of which increases during atypical pneumonia;
- enzyme immunoassay, which determines the presence in the body of antibodies to the causative agent of pneumonia;
- detection of virus RNA in blood and secretions from the respiratory tract.
Percussion in the inferolateral and posterior parts of the chest reveals dullness of the pulmonary sound. During auscultation, against the background of weakened breathing, crepitus and moist fine bubbling rales are heard. Due to the deterioration of blood oxygen saturation, cyanosis (cyanosis) of the nasolabial triangle appears, and deafness of heart sounds is noted.
Changes during X-ray examination in some patients can be detected only at the height of the disease. They are characterized by the presence of infiltrates in the peripheral parts of the pulmonary fields. As the disease progresses, the infiltrates increase in size and become bilateral.
The following changes are noted in peripheral blood:
- leukopenia;
- lymphopenia;
- increase in the relative number of neutrophils.
A biochemical blood test determines an increase in the activity of lactate dehydrogenase, transaminase, and a decrease in the concentration of sodium in the blood plasma. In proportion to the severity of lung damage, blood oxygen saturation decreases.
If atypical pneumonia is suspected, a chest x-ray is indicated. Changes in the lungs, which can be detected by a specialist, appear 10 days after infection. At the Yusupov Hospital, a differential diagnosis of atypical pneumonia is carried out, in which diseases with similar symptoms are excluded.
High-precision diagnostics make it possible to identify the pathogen strain and begin treatment of atypical pneumonia according to an individual plan. Therapy is carried out in a hospital setting, so the patient receives medical care around the clock.
Treatment
Treatment of atypical pneumonia is carried out in two directions. The first is aimed at eliminating symptoms, the second is to combat the causative agent of pneumonia. Antibiotics are prescribed to destroy microorganisms; the course of treatment is 10-14 days. The choice of drug is determined by the type of pathogen. Antibacterial therapy for this disease is also a measure to prevent secondary inflammation. In addition to drug therapy, massages and breathing exercises are prescribed when the condition has stabilized.
To eliminate the manifestations of the disease, mucolytics, anti-inflammatory and antipyretic drugs are prescribed. Connecting the patient to a ventilator is an emergency measure for respiratory failure. With atypical pneumonia, patients should follow a diet and drink sufficient fluids. In addition, patients may be prescribed various vitamins.
In the intensive care unit, for the purpose of detoxification, infusion therapy with crystalloid solutions is carried out, and immunoglobulins are slowly administered intravenously. With the development of respiratory distress syndrome, special modes of artificial ventilation are used, surfactant preparations are administered by intubation or inhalation. With the help of cardiac monitors, the basic parameters of the balance of the internal environment of the body are monitored. To maintain sufficient urine output, optimal infusion therapy is carried out and diuretics are prescribed.
The Yusupov Hospital provides inpatient treatment for atypical pneumonia. Patients are accommodated in comfortable rooms with a modern ventilation system, which is of particular importance for respiratory diseases. In addition, each patient receives the necessary hygiene products.
Make an appointment
Treatment of atypical pneumonia
There are currently no effective methods to cure atypical pneumonia. Specialists use drugs that belong to different groups: glucocorticoids (the so-called adrenal cortex hormones with a pronounced anti-inflammatory effect), broad-spectrum antibiotics, antivirals, etc.
Treatment reduces the severity of symptoms of the disease, prevents secondary infections (for example, the appearance of other infectious agents that affect various organs), and supports those body functions that have been impaired (for example, artificial ventilation of the lungs can be performed).
Scientists continue their work to create a drug that can fight the virus that causes this disease. They are also developing a vaccine to prevent SARS.
Forecast
The prognosis of how atypical pneumonia will develop is determined by the symptoms and type of pathogen. With timely therapy and a strong immune response, the prognosis is favorable. In childhood, pneumonia can be complicated by damage to the alveoli and the development of emphysema. In addition, self-medication may cause the disease to become chronic.
If left untreated, death is possible, but modern therapeutic methods make it possible to provide quality care to patients with various forms and severity of the disease. Doctors at the Yusupov Hospital carry out emergency hospitalization and take measures necessary to improve the patient’s condition.
Patients can go to the Yusupov Hospital if their condition worsens at any time of the day. You can make an appointment with candidates and doctors of science with many years of experience at a convenient time, and find out about the cost and terms of service by calling the Yusupov Hospital.