© Author: A. Olesya Valerievna, candidate of medical sciences, practicing physician, teacher at a medical university, especially for SosudInfo.ru (about the authors)
Hyalinosis is understood as one of the types of protein metabolism disorders, in which structural disorders affect the stroma of organs and the walls of blood vessels. During this dystrophy, dense protein deposits accumulate, which are similar in appearance to hyaline cartilage, which is where its name comes from.
Hyalinosis is characteristic of the connective tissue that makes up the supporting framework of parenchymal organs and vascular walls, therefore it is classified as a type of so-called stromal-vascular dystrophy. The appearance of hyalinosis marks a serious, irreversible stage of morphological changes, one way or another affecting the functioning of organs.
Every minute, billions of biochemical processes take place in our body, aimed at the proper functioning of cells, tissues and organs, and the most important life support mechanism - nutrition - is performed by blood, lymph, intercellular fluid, ensuring the interaction of the structural elements of tissue with each other and with the external environment.
The action of unfavorable factors can disrupt the clear regulation of vital processes at the subcellular, cellular, and tissue levels, which will lead to specific structural disorders that can be detected using a microscope and the eyes of a specialist. If such occur, we are talking about dystrophy.
Both the cells of parenchymal organs, which perform a strictly defined complex function, and extracellular structures, that is, connective tissue elements, are subject to dystrophic changes. In some cases, dystrophy manifests itself in both places, and the exchange of both proteins and fats with carbohydrates and minerals suffers.
In other words, when talking about a specific type of dystrophy, we must understand that this is not an isolated process that develops on its own. In parallel, other changes may occur in the cells and in the extracellular substance, especially when it comes to systemic connective tissue diseases, hypertension, diabetes, which leave an imprint on the entire body as a whole.
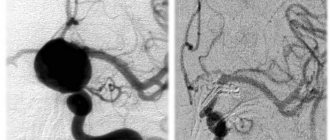
Fig.: hylianosis of the renal vessels
As already mentioned, hyalinosis is a type of stromal vascular dystrophy that occurs within fibrous tissue. To better understand the essence of this disorder, you need to remember a little what connective tissue consists of and which of its elements can become a source of pathological changes.
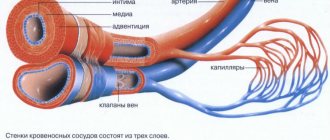
In a simplified way, connective tissue can be represented as a complex consisting of cells, fibers and an extracellular amorphous substrate. The main cells are considered to be fibroblasts, which produce collagen, which forms the fibrous basis of the walls of blood vessels and the ground substance. In addition to collagen and elastic fibers, which are important in the morphogenesis of dystrophy, a significant role is played by glycosaminoglycans, which are also synthesized by fibroblasts and form the main substance in which cells and fibers are immersed.
On the way to hyalinosis, fibrous tissue first undergoes reversible changes - unwinding and partial defibre of collagen, an increase in the concentration in the intercellular space of hyaluronic acid, which attracts water and increases the swelling of the intercellular mass (mucoid swelling), and then irreversible restructuring with destruction of fibers, microcirculation disorder, release of elements blood plasma into tissue. At the stage of pronounced destruction of tissue components, deposition of hyaline-like masses occurs - hyalinosis, ultimately ending in sclerosis.
Thus, the basis of hyalinosis is an increase in the permeability of vascular walls with the release of plasma elements from the vessels and the destruction of connective tissue components with the appearance of complex protein-carbohydrate compounds deposited in the walls of blood vessels and the main substance of the connective tissue.
Hyalinosis is not considered as a separate disease. This is a universal process that reflects a variety of influences and, accordingly, accompanies a variety of pathologies. In rare cases, it can even be regarded as a variant of the norm, but more often it is a structural expression of the disease, which predetermines dysfunction of organs.
Hyalinosis does not appear in the diagnosis, so even the term itself may be unfamiliar to the average person, but its detection in biopsy material or posthumously in organs makes it possible to make a correct diagnosis, determine the stage of the disease, its duration, and explain the symptoms.
How and why does hyalinosis develop?
The protein formed during hyalinosis is a multicomponent combination of plasma proteins, fibrin, immunoglobulins, fats, fragments of destroyed connective tissue fibers, and glycosaminoglycans. The dystrophic process develops against the background of a complex disturbance of metabolic processes, destructive changes, circulatory and nutritional disorders:
- Disintegration and disintegration of collagen and elastin fibers;
- An increase in the permeability of vessel walls with the release of blood proteins into the intercellular space and their infiltration of disintegrated fibers;
- Disorders of microcirculation, metabolism, local immunopathological reactions.
The density of hyaline deposits is due to the presence of chondroitin sulfate in their composition, which normally provides the consistency of cartilage, bones, contained in the sclera, dense fibrous tissue, and in pathology found in foci of degeneration. Chonroitin sulfate is a complex polysaccharide. Due to the significant increase in its concentration during hyalinosis, some sources recommend classifying this dystrophy as a disorder of carbohydrate metabolism, while the classical idea of hyalinosis as a process of protein destruction, accompanied by plasma impregnation, defines it as a group of dysproteinoses.
Hyalinosis accompanies inflammatory and necrotic changes, disorders of microcirculation and vascular permeability, sclerosis, etc., and its causes are considered to be:
- Increased blood pressure in any form of hypertension;
- Diabetes;
- Immune disorders; allergic reactions;
- Inflammatory processes (both local and general) - callous gastric ulcer, inflammation of the appendix, systemic vasculitis, etc.;
- Scarring;
- Collagenosis - rheumatic fever, rheumatoid arthritis, lupus erythematosus, scleroderma, etc.
- Necrotic processes.
Hyalinosis of the splenic capsule and arteries, which is often found in mature and elderly people as a reflection of the blood-depositing function of the organ, is considered as a physiological norm.
Fig.: hylianosis of vessels (left) and capsule (right) of the spleen
HYALINOSIS. LOCALIZATION. MORPHOLOGICAL CHANGES, MORPHOGENESIS. OUTCOMES AND FUNCTIONAL SIGNIFICANCE.
For hyalinosis (from the Greek hyalos
- transparent, glassy), or
hyaline dystrophy,
homogeneous translucent dense masses (hyaline) resembling hyaline cartilage are formed in the connective tissue.
Hyaline is a fibrillar protein. An immunohistochemical study reveals not only plasma proteins and fibrin, but also components of immune complexes (immunoglobulins, complement fractions), and sometimes lipids.
Hyalinosis can develop as a result of various processes:
— plasma impregnation; - fibrinoid swelling (fibrinoid); - sclerosis.
There are:
- vascular hyalinosis; - hyalinosis of the connective tissue itself.
Each of the two types of hyalinosis can be systemic and local in nature.
Vascular hyalinosis . Hyalinosis occurs mainly in small arteries and arterioles. It is preceded by damage to the endothelium, basement membrane and smooth muscle cells of the vessel wall and impregnation of it with blood plasma proteins.
Causes of systemic vascular hyalinosis:
-hypertonic disease; —hypertensive conditions, hypertension (kidney disease, tumors of the endocrine and gonads); —diabetes (diabetic arteriolohyalinosis); —rheumatic diseases; -atherosclerosis.
The leading mechanisms in its development are:
—destruction of fibrous structures; —increased vascular-tissue permeability (plasmorrhagia).
Plasmorrhagia is associated with the impregnation of tissue with plasma proteins and their adsorption on altered fibrous structures, followed by precipitation (gluing) and the formation of protein - hyaline.
Hyalinosis of small arteries and arterioles is systemic in nature, but is most pronounced in the kidneys, brain, retina, pancreas, and skin.
Microscopically, with hyalinosis, arterioles turn into thickened glassy tubes with a sharply narrowed or completely closed lumen.
There are 3 types of vascular hyaline:
1) simple,
arising from slightly changed components of blood plasma (more common in benign hypertension, atherosclerosis and in healthy people);
2) lipohyalin,
containing lipids and beta-lipoproteins (found most often in diabetes mellitus);
3) complex hyaline,
built from immune complexes, fibrin and collapsing structures of the vascular wall (characteristic of diseases with immunopathological disorders, for example, rheumatic diseases).
Local arterial hyalinosis as a physiological phenomenon is observed in the spleen of adults and elderly people, reflecting the functional and morphological characteristics of the spleen as a blood deposition organ.
Exodus. In most cases, it is unfavorable because the process is irreversible. Hyalinosis of small arteries and arterioles leads to atrophy, deformation and shrinkage of the organ (for example, the development of arteriolosclerotic nephrocirrhosis).
Hyalinosis of the connective tissue itself.
Systemic hyalinosis of connective tissue and blood vessels usually develops as a result of fibrinoid swelling, leading to the destruction of collagen and saturation of the tissue with plasma proteins and polysaccharides. This mechanism of development of systemic connective tissue hyalinosis is especially common in diseases with immune disorders (rheumatic diseases).
Local hyalinosis as an outcome of sclerosis develops in scars, fibrous adhesions of serous cavities, the vascular wall in atherosclerosis, involutional sclerosis of the arteries, during the organization of a blood clot, infarction, healing of ulcers, wounds, in capsules, tumor stroma, etc. Hyalinosis in these cases is based on disorders of connective tissue metabolism. A similar mechanism has hyalinosis of necrotic tissues and fibrinous deposits in the pleura, pericardium, etc. Hyalinosis can complete fibrinoid changes in the bottom of a chronic gastric ulcer, in the appendix in appendicitis.
Macroscopic picture. With severe hyalinosis, the fibrous connective tissue becomes dense, cartilaginous, whitish, and translucent.
Microscopic examination. Bundles of collagen fibers lose their fibrillarity and merge into a homogeneous dense cartilage-like mass; cellular elements are compressed and undergo atrophy.
Exodus . In most cases, it is unfavorable due to the irreversibility of the process, but resorption of hyaline masses is also possible. Thus, hyaline in scars - the so-called keloids - can undergo loosening and resorption. Let's reverse hyalinosis of the mammary gland, and the resorption of hyaline masses occurs in conditions of hyperfunction of the glands. Sometimes the hyalinized tissue becomes slimy.
Structural changes in hyalinosis
Based on the localization of characteristic changes, two forms of dysproteinosis are distinguished:
- Vascular hyalinosis;
- Hyalinosis of the connective tissue itself.
Each type is focal and widespread, but more often there is a combination of both vascular and stromal changes, that is, the dystrophic process affects all tissue elements.
Vascular hyalinosis is characteristic of arterial-type vessels and small diameter vessels - arteries and arterioles. Its initial stage is damage to the endothelial lining of the vessel and infiltration of its wall with blood plasma, while changes noticeable to the eye may be absent, and the only “hint” of hyalinosis will be compaction of the tissue or organ.
Hyalinosis of arteries and arterioles is clearly visible during microscopic assessment of the condition of the tissue, and in the advanced stage the vessels are so characteristically altered that the presence of hyalinosis does not raise any doubts even without the use of special staining methods.
stages of artery hylianosis
Microscopically, protein deposits in the early stages are detected under the inner layer of the vascular wall (under the endothelium), from where they begin to compress the middle layer, causing its atrophy. Over time, the entire thickness of the artery wall is replaced by pathological protein, and the vessels become like glass microtubules with thick, compacted walls and a sharply reduced lumen until its complete disappearance.
Hyalinosis of arterioles and small arteries is usually widespread and can be detected in many organs. It is very indicative in the renal parenchyma, brain, dermis, retina, pancreas, adrenal glands, where the described changes unfold against the background of hypertension, diabetes, and immunopathological conditions.
hylianosis of small vessels of the brain
In the kidneys, damage occurs not only to the arterial vessels themselves (1 - in the figure below), but also to the glomeruli (2), which become homogenized, compacted and, accordingly, lose their ability to filter fluid. Hyalinosis keeps pace with sclerosis, which results in nephrosclerosis and cirrhosis of the organ with uremia.
hylianosis of arteries (1) and arterioles (2) of the kidney
The protein deposited in the arterial bed during hyalinosis has a complex and diverse structure, therefore they are distinguished:
- Simple hyaline - consists of close to normal or normal plasma components and is pathognomonic for hypertension, atherosclerosis;
- Complex - contains fibrin, immunoglobulins, degradation products of vascular wall proteins and occurs with systemic disorganization of fibrous tissue;
- Lipohyalin - from the name it is clear that it contains lipids and fat-protein complexes, and is found in the vessels of diabetic patients.
Video: about the process of arteriosclerosis
Hyalinosis in fibrous tissue occurs as a consequence of the previous stages of its disorganization - destruction of collagen to simple components, infiltration of the resulting masses with blood components and carbohydrate polymers. The outcome reveals hyaline deposits in the form of compacted glassy pink deposits in the ground substance.
Microscopic analysis reveals swelling, homogenization of the main substance, and deposits of cartilage-like protein accumulations in the tissue. The cells are compressed and atrophy, the vessels dilate, their walls are saturated with plasma proteins.
The described processes are clearly visible in rheumatic diseases, in long-term gastric ulcers, in appendicitis against the background of a chronic inflammatory reaction, in scarring foci. Sclerosis and hyalinosis accompany each other during scarring, in the glomeruli of the kidneys affected by hypertension, during the formation of adhesions in the serous membranes, atherosclerotic damage to the arteries, fibrosis of thrombotic masses, resolution of foci of necrosis, in the stromal component of neoplasia and capsules of internal organs.
External manifestations of hyalinosis become noticeable with a pronounced degree of dysproteinosis: the density, color, volume of an organ or tissue changes. When the arterial bed of the bloodstream is damaged, hypoxia increases, the production of connective tissue fibers increases, parenchymal elements atrophy and die, the organ is deformed and decreases in volume, becoming dense, lumpy and acquiring a whitish tint.
These changes are clearly visible in arterial hypertension, when hyalinosis of arteries and arterioles is generalized and expressed in the kidneys, retina, brain, adrenal glands and pancreas. Sclerosis and hyalinosis of the kidney against the background of hypertension and diabetes represents the basis for further nephrosclerosis with chronic renal failure.
Local hyalinosis in rheumatism causes compaction, deformation, thickening and shortening of the valve leaflets, fusion between them, forming an acquired defect such as stenosis or insufficiency, leading to chronic heart failure. In scars, this type of dystrophy can result in the formation of a keloid - a dense, painful scar, in which not only fields of dense connective tissue are microscopically detected, but also foci of hyalinosis, which requires surgical care due to pain and cosmetic defects.
In some cases, hyalinosis may not have a harmful effect, reflecting only the process of involution. For example, after lactation, deposits of hyaline are sometimes found in the mammary gland, which do not in any way affect the further function and anatomy of the organ.
Hyalinosis of the corpus luteum of the ovary develops after regression of the corpus luteum of pregnancy, in the white bodies remaining after the once active yellow bodies. These changes are noticeable during menopause, when age-related degeneration and shrinkage of the ovary occurs. Dysproteinosis means involution of the ovary and is detected in the form of microscopically visible deposits of compacted masses of protein in the stroma and arteries, which narrow and become sclerotic.
hylianosis of the arteries and stroma of the ovary
With hyalinosis of the spleen, both the pulp and the vessels can be affected, but this phenomenon is also unlikely to affect the health and well-being of the carrier of dysproteinosis. Impregnation of the capsule with hyaline-like protein is accompanied by its compaction and color change to whitish-pink, which is why pathomorphologists call such a spleen glazed.
Video: example of hylianosis of the splenic capsule
Hyalinosis of the connective tissue itself.
It usually develops as a result of fibrinoid swelling, leading to the destruction of collagen and saturation of the tissue with plasma proteins and polysaccharides.
Microscopic examination. The connective tissue bundles become swollen, they lose their fibrillarity and merge into a homogeneous dense cartilage-like mass; cellular elements are compressed and undergo atrophy. This mechanism of development of systemic connective tissue hyalinosis is especially common in diseases with immune disorders (rheumatic diseases). Hyalinosis can complete fibrinoid changes in the bottom of a chronic gastric ulcer, in the appendix in appendicitis; it is similar to the mechanism of local hyalinosis in the focus of chronic inflammation.
Hyalinosis as an outcome of sclerosis is also mainly local in nature: it develops in scars, fibrous adhesions of serous cavities, the vascular wall in atherosclerosis, involutional sclerosis of the arteries, in the organization of a blood clot, in capsules, tumor stroma, etc. The basis of hyalinosis in these cases There are disorders of connective tissue metabolism. A similar mechanism has hyalinosis of necrotic tissues and fibrinous deposits.
Appearance.
With severe hyalinosis, the appearance of organs changes. Hyalinosis of small arteries and arterioles leads to atrophy, deformation and shrinkage of the organ (for example, the development of arteriolosclerotic nephrocirrhosis).
With hyalinosis of the connective tissue itself, it becomes dense, whitish, translucent (for example, hyalinosis of the heart valves with rheumatic disease).
Exodus.
In most cases, it is unfavorable, but resorption of hyaline masses is also possible. Thus, hyaline in scars - the so-called keloids - can undergo loosening and resorption. Let's reverse hyalinosis of the mammary gland, and the resorption of hyaline masses occurs in conditions of hyperfunction of the glands. Sometimes the hyalinized tissue becomes slimy.
Functional meaning.
Varies depending on the location, degree and prevalence of hyalinosis. Widespread hyalinosis of arterioles can lead to functional failure of the organ (renal failure in arteriolosclerotic nephrocirrhosis). Local hyalinosis (for example, heart valves with heart disease) can also cause functional failure of the organ. But in scars it may not cause any particular distress.
Amyloidosis
Amyloidosis (from the Latin amylum - starch), or amyloid dystrophy, is a stromal-vascular dysproteinosis, accompanied by a profound disruption of protein metabolism, the appearance of abnormal fibrillar protein and the formation of a complex substance - amyloid - in the interstitial tissue and walls of blood vessels.
In 1844, the Viennese pathologist K-Rokitansky described peculiar changes in parenchymal organs, which, in addition to sharp compaction, acquired a waxy, greasy appearance. He called the disease in which such changes in organs occurred “sebaceous disease.” A few years later, R. Virchow showed that these changes are associated with the appearance in the organs of a special substance, which, under the influence of iodine and sulfuric acid, turns blue. Therefore, he called it amyloid, and the “greasy disease” amyloidosis. The protein nature of amyloid was established by M. M. Rudnev together with Klone in 1865.
Chemical composition and physical properties of amyloid. Amyloid is a glycoprotein, the main component of which is fibrillar proteins (F-component). They form fibrils with a characteristic ultramicroscopic structure (Fig. 33). Fibrillar amyloid proteins are heterogeneous. There are 4 types of these proteins, characteristic of certain forms of amyloidosis:
1. AA protein (not associated with immunoglobulins), formed from its serum analogue - the SAA protein;
2. AL protein (associated with immunoglobulins), its precursor is the L-chains (light chains) of immunoglobulins;
3. AF protein, the formation of which mainly involves prealbumin;
4. ASC i-protein, the precursor of which is also prealbumin.
Proteins of amyloid fibrils can be identified using specific sera during immunohistochemical examination, as well as a number of chemical (reactions with potassium permanganate, alkaline guapidine) and physical (autoclaving) reactions.
Fibrillar amyloid proteins, which are produced by cells - amyloidoblasts, are included in complex compounds with blood plasma glucoproteins. This plasma component (P-component) of amyloid is represented by rod-shaped structures (“periodic rods” - see Fig. 33). The fibrillar and plasma components of amyloid have antigenic properties. Amyloid fibrils and the plasma component combine with tissue chondroitin sulfates, and so-called hematogenous additives are added to the resulting complex, among which fibrin and immune complexes are of primary importance. The bonds of proteins and polysaccharides in the amyloid substance are extremely strong, which explains the lack of effect when various enzymes of the body act on amyloid.
Characteristic of amyloid is its red staining with Congo red, methyl (or gentznan) violet; Specific luminescence with thioflavins S or T is characteristic. Amyloid is also detected using a polarizing microscope. It is characterized by dichroism and anisotropy (the birefringence spectrum lies in the range of 540 - 560 nm). These properties allow amyloid to be distinguished from other fibrillar proteins. For macroscopic diagnosis of amyloidosis, the tissue is exposed to a Lugol's solution, and then a 10% sulfuric acid solution; the amyloid becomes blue-violet or dirty green.
The colorful reactions of amyloid, associated with the characteristics of its chemical composition, may vary depending on the form, type and type of amyloidosis. In some cases they are absent, then they speak of achromatic amyloid, or achroamyloid.
The classification of amyloidosis takes into account the following characteristics:
1. possible reason;
2. specificity of amyloid fibril protein;
3. prevalence of amyloidosis;
4. the originality of clinical manifestations due to the predominant damage to certain organs and systems.
Based on the cause, primary (idiopathic), hereditary (genetic, family), secondary (acquired) and senile amyloidosis are distinguished. Primary, hereditary, senile amyloidoses are considered as nosological forms. Secondary amyloid dose, which occurs in certain diseases, is a complication of these diseases, a “second disease”.
Primary (idiopathic) amyloidosis is characterized by: the absence of a previous or concomitant “causal” disease; damage to predominantly mesodermal tissues - the cardiovascular system, striated and smooth muscles, nerves and skin (generalized amyloidosis); tendency to form nodular deposits, inconsistent color reactions of the amyloid substance (negative results are often obtained when staining with Congo red).