General information
Normal lactate levels are low. It increases with a lack of oxygen and disruption of the energy production mechanism. The main amount of energy is produced in mitochondria. This process uses oxygen and glucose. This generation of energy is called aerobic. If this process is disrupted (for example, there is not enough oxygen), energy production occurs anaerobically due to the breakdown of glucose. This produces lactate. If the production of lactic acid exceeds the liver's ability to utilize it, this substance accumulates. In this case, the development of lactic acidosis is possible. In many cases, the body’s compensatory capabilities are sufficient to avoid severe consequences. Sometimes lactic acidosis is not compensated for. In this case, the acid-base balance is disturbed, nausea, weakness, sweating, rapid breathing appear, and coma is possible.
The causes of excess lactate are divided into two groups. The most common is lactic acidosis A. It occurs when blood circulation is slow or oxygen is not being taken up sufficiently by the lungs. This condition can develop with a heart attack, shock from severe blood loss, sepsis, pulmonary edema and in a number of other cases. With type B lactic acidosis, metabolism is impaired and the need for oxygen is increased. It may be caused by kidney or liver disease, leukemia and other diseases.
Clinical significance of blood lactate level in laboratory rapid diagnostics
Torshin V. A., Ph.D., Associate Professor, Department of Biochemistry, RMAPO, Moscow
Lactate metabolism
Since J. Meakins and C. Long described in 1927 the relationship between increased blood lactate levels and the presence of signs of tissue hypoxia in patients with circulatory shock, lactate levels have been assessed as a marker of tissue hypoxia in this group of patients. On the other hand, lactate is a normal end product of glycolysis according to the reaction:
LDH
Pyruvate + NADH + H+ <-> lactate + NAD (1)
Under normal conditions, the ratio lactate: pyruvate = 10:1 is formed. Almost all cells are capable of producing lactate. Tissues with a high metabolic rate (intestines, brain, skeletal muscles, etc.) make the greatest contribution to the daily production of lactate, forming its normal blood level of about 1.3 mmol/l. Normally, the total lactate production is about 1.0 mmol/kg/hour, that is, the daily production per adult individual ranges from 1200 to 1500 mmol. Basal lactate production in vigorously working skeletal muscle can increase tenfold, which has led to interest in blood lactate levels in sports medicine. Against the background of intense physical exercise, the level of blood lactate increases 10-15 times compared to the baseline, reflecting the intensity of the metabolic processes of aerobic and anaerobic glycolysis. The dynamics of growth in lactate levels makes it possible to determine the most promising athletes in sports such as rowing, athletics, cross-country skiing, etc. This increase is completely different from the increase in lactate described by J. Meakins and other authors in critically ill patients.
Lactate is metabolized primarily in the liver through conversion to pyruvate. Therefore, lactate levels depend on pyruvate metabolism. To understand the role of pyruvate, the conversion of pyruvate to acetyl-coenzyme-A under the influence of pyruvate dehydrogenase is important, which is then, in turn, metabolized in the Krebs cycle (tricarboxylic acid cycle) followed by oxidative phosphorylation to form the main universal energy source - adenosine triphosphate (ATP). Pyruvate can also be used to regenerate glucose by conversion to oxaloacetate. In this way, lactate can be converted back into glucose, which can accordingly be metabolized into lactate (the so-called Cori cycle). Reduction of glucose from lactate is an important mechanism for removing lactate from the systemic circulation after prolonged tissue hypoxia (for example, after cardiac arrest). Finally, pyruvate can be converted to alanine and alpha-ketoglutamate. The reversibility of this reaction restores pyruvate, which can be used for oxidation or gluconeogenesis. From Equation 1 we can conclude that even in the case of a normal NAD/NADH ratio and cell pH, lactate levels will increase if there is excessive pyruvate production, impairment of its utilization or conversion to acetyl-coenzyme-A. Pyruvate utilization is impaired by pyruvate dehydrogenase deficiency (inborn errors of metabolism). Dysfunction of the pyruvate dehydrogenase complex can also occur in sepsis, leading to increased levels of pyruvate and lactate in the blood. Clinically, the most significant reason for decreased pyruvate utilization is cellular oxygen deficiency, since both processes: pyruvate oxidation and gluconeogenesis require the presence of oxygen. Therefore, when oxygen is deficient, glucose is converted to a greater extent into lactate, producing only 2 moles of ATP instead of 34 moles when metabolized in the Krebs cycle.
There are three mechanisms for lactate transport across the cell membrane:
- free diffusion of lactic acid;
- exchange for other anions (for example Cl- or HCO3-) facilitates the transport of lactate across the cell membrane;
- transport associated with the transfer of H+ across the membrane; In the presence of a pH gradient across the cell membrane, lactate flux increases.
According to the latter mechanism, acidosis increases lactate uptake by cells (eg, skeletal muscle and cardiomyocytes). In contrast, in alkalemia, lactate is released from the cell, leading to increased lactate levels in the blood. Also, in alkalemia, stimulation of phosphofructose kinase plays a certain role, leading to increased glycolysis and, accordingly, to the production of lactate. Despite the fact that an increase in the level of lactate in the blood is often accompanied by the development of acidosis (so-called lactic acidosis), the production of lactate does not directly lead to the release of H+ ions, since H+ ions are utilized during the production of ATP from ADP or AMP:
ATP -> ADP + Pi + H+ -> AMP + 2Pi + 2H+
The inability of cells to utilize H+ ions generated during ATP hydrolysis is the main reason for the development of metabolic acidosis under hypoxic conditions.
Types of Blood Lactate Disorders
Lactic acidosis as a clinical syndrome was first described by Huckabee in 1961: it is an increase in blood lactate levels due to its overproduction or decreased elimination, or a combination of these factors. Cohen and Woods in 1976 identified four types of lactic acidosis: A, B1, B2, B3.
Type A - the most common in clinical practice, is a consequence of decreased tissue oxygenation, that is, tissue hypoxia (all types of shock, carbon monoxide poisoning, pulmonary edema, acute asphyxia, congestive heart failure, etc.).
Disorders grouped into type B are not accompanied by tissue hypoxia until the terminal stages of the disease.
Type B1 - patients with diseases such as diabetes, liver and kidney diseases, some infections, neoplastic processes, convulsions. For example, in grand mal seizures, lactate levels increase due to both laryngospasm and overproduction of lactate in the muscles. In bacteremia, one of the mechanisms for increasing lactate is damage to the pyruvate dehydrogenase complex by bacterial endotoxin. In leukemia and other neoplastic processes with a chronic increase in blood lactate levels, its decrease is a sign of the effectiveness of cytolytic therapy.
Type B2 is lactic acidosis caused by certain drugs or poisons. Treatment of diabetes with biguanides is accompanied by lactic acidosis due to a decrease in the activity of pyruvate carboxylase, leading to inhibition of gluconeogenesis. The mechanism of lactic acidosis in ethanol poisoning is not entirely clear. The influence of convulsive syndrome, as well as an increase in the NADH/NAD ratio, is expected.
Type B3 - includes fairly rare congenital anomalies associated with impaired mitochondrial pyruvate oxidation.
Lactate measurements are extremely important in the diagnosis of McArdles disease (type 5 glycogen storage disease), which occurs predominantly in men and causes muscle pain and muscle tension after minor physical exertion. With increasing loads, the pain goes away, but muscle necrosis and myoglobinuria develop. Laboratory diagnosis is based on the absence of an increase in lactate levels during physical activity.
Lactate and tissue hypoxia (type A lactic acidosis)
In clinical practice, the level of lactate in the blood is used to monitor the level of tissue hypoxia: the utilization of pyruvate depends on the availability of oxygen and, accordingly, a decrease in oxygen delivery to cells leads to an increase in lactate production and an increase in its level in the blood. Tissue hypoxia is defined as an imbalance between oxygen demand and oxygen delivery (DO2). With a decrease in oxygen delivery, tissues meet the need for it by increasing the extraction of oxygen from arterial blood. This is reflected in an increase in the oxygen extraction index (O2ER) and a decrease in mixed venous blood saturation (SvO2%). Normally, about 25% of the oxygen delivered by arterial blood is extracted from the tissues.
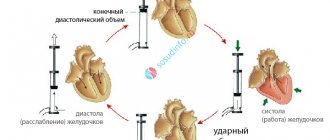
Oxygen delivery to tissues is a function of arterial oxygen content (ctO2) and cardiac output (Qt).
DO2 = ctO2×Qt = (ctHb×SaO2 ×(1-FCOHb-FmetHb) + 0.003×pO2)×Qt (3)
ctHb - hemoglobin concentration
SaO2% - arterial blood saturation
Reducing each component in the equation can result in a decrease in DO2. Typically, a decrease in hemoglobin concentration or saturation level is compensated by an increase in cardiac output so that DO2 remains at the level of requirement and tissue hypoxia does not occur. When compensatory mechanisms fail, DO2 quickly decreases below a critical level, oxygen consumption by tissues decreases, and the level of lactate in the blood increases. This consumption-delivery phenomenon has been demonstrated in experimental studies with reductions in ctHb, SaO2% and Qt.
Shibutani, Komatsu et al. works in 1983 and 1987 showed that the effect of the dependence of consumption on delivery occurs when DO2 decreases below a critical level of 300 ml/min. Since this was a group of cardiac surgical patients with reduced compensatory capabilities, a decrease in DO2 below a critical level led to tissue hypoxia and an increase in lactate levels. In 1990, Vincent et al showed that an increase in oxygen consumption in cardiac surgery patients with elevated lactate occurred only during dobutamine infusion.
The interpretation of elevated lactate levels in septic patients is quite complex. However, in the early phase of septic shock, increased blood lactate levels are associated with the presence of supply-intake dependence and tissue hypoxia.
In the absence of tissue hypoxia, dysfunction of the pyruvate dehydrogenase complex leads to an increase in pyruvate levels. Increased aerobic glycolysis increases intracellular pyruvate levels without the need for increased ATP production. An increase in Na+-K+-ATPase activity in the case of cell normoxia is associated with this mechanism of aerobic lactate production (James JH, Fang CH et al, 1996). The described mechanism is important for understanding the increase in lactate levels in septic patients, as well as in congenital metabolic abnormalities. Protein breakdown results in increased amino acid release, which can lead to increased pyruvate levels during gluconeogenesis. A third mechanism for increased lactate in septic patients in the absence of tissue hypoxia is a decrease in lactate clearance (for example, with decreased regional blood flow and liver dysfunction).
The relationship between lactate level and H+ ion concentration is far from straightforward. A 1996 study by Gutierrez and Wulf found that there was no strong relationship between lactate levels and H+ ion concentrations in septic patients. The authors noted the influence on these relationships of factors such as the presence of renal dysfunction or pCO2 levels due to manipulations with the ventilator.
Summarizing the above, we can conclude that an increase in lactate levels (whether accompanied or not by systemic acidosis) reflects a complex set of metabolic disorders, among which the main elements are an increase in aerobic or anaerobic lactate production and a decrease in its clearance. The significance of these elements is ambiguous in various pathological conditions. There is also no direct correlation with other clinical and laboratory signs of critical illness. All this creates a need to measure blood lactate levels in critically ill patients, as opposed to attempts to derive lactate as a calculated indicator based on other parameters.
Measurement technique and sample type
First described by Gaglio in 1886, measuring lactate levels required 100–200 ml of blood and several days to obtain a result. Broder and Weil, who first used the spectrophotometric method in 1964, significantly reduced the measurement time, which made it possible to assess the dynamics of lactate levels in critically ill patients. Truly revolutionary was the creation of a specific lactate electrode as part of a blood gas analyzer and CBS, which made it possible to obtain lactate in 1-2 minutes of measurement from 100-150 μl of whole blood, along with other parameters for the urgent diagnosis of a critically ill patient. It is important to be able to obtain the parameter from whole blood, and not from plasma or serum, which would require additional time for separation. Arterial blood is most suitable for assessing lactate levels, as well as other urgent diagnostic parameters. However, taking mixed venous blood is also acceptable. A capillary sample can be an alternative only if it is not possible to take an arterial sample. In this case, the rules for sampling must be observed to prevent distortion of the result. The blood sample should be tested immediately if possible. In case of delay, it should be stored in the so-called “ice bath”, that is, cooled to 1-4 C°, which allows several times to reduce the metabolic rate in the whole blood sample.
Clinical significance of blood lactate measurement
Measurement of blood lactate levels should be part of the assessment of any critically ill patient. Blood lactate level as a marker of complex metabolic disorders is a good predictor in intensive practice.
According to Roumen and Redl, published in 1993, lactate turned out to be a better predictor of the development of respiratory distress syndrome and multiple organ failure in patients with polytrauma than the well-known multicomponent scale for assessing the critically ill as APACHE. A decrease in blood lactate levels during intensive therapy turned out to be a good indicator of its adequacy.
Da Silva and Hemneber in a 2000 publication showed the significance and association of parameters such as base deficiency and blood lactate levels measured in a newborn at 30 minutes after birth as prognostic signs of neurological disorders after intrapartum asphyxia. Lactemia less than 5 mmol/L and/or base deficiency less than 10 mmol/L did not lead to neurological complications. Lactate concentrations greater than 9 mmol/L were associated with moderate to severe encephalopathy with a sensitivity of 84% and specificity of 67%.
Timerbaev V. Kh. et al. in a 2005 publication demonstrated the significance of intraoperative dynamics of blood lactate, reflecting changes in tissue perfusion and tissue gas exchange in patients with polytrauma complicated by hemorrhagic shock. The authors used the dynamics of blood lactate as a criterion for the effectiveness of therapy and a predictor of death.
Treatment of HIV infection with nucleoside analogues is often accompanied by lactic acidosis, that is, an increase in lactate levels of more than 5 mmol/l with a parallel decrease in pH < 7.35 (O. Andersen et al, 2003), which is most likely determined by the mitochondrial toxicity of antiretroviral drugs.
Mullner et al in 1997 showed the prognostic significance of blood lactate (100% specificity at a lactate level of 16 mmol/l) in the development of neurological deficits after ventricular fibrillation.
Kellum, in a 1998 publication, emphasizes that lactemia in septic conditions is associated not so much with oxygen deficiency of tissues, but with increased aerobic and anaerobic glycolysis, in parallel with the dissociation of the pyruvate dehydrogenase complex. The author also notes not the diagnostic significance of the lactate level per se, but its significance as a marker of “trouble.”
In a review article by Hameed et al, published in 2003 and devoted to the problems of oxygen delivery in critically ill patients, the authors emphasize the nonspecificity of such traditional signs of shock as heart rate, blood pressure, skin temperature, and diuresis. Even the interpretation of hemodynamic parameters such as pulmonary wedge pressure and cardiac output can be ambiguous as shock progresses. Impaired oxygen delivery to tissues, leading to damage to pyruvate metabolism, will result in lactate accumulation, increased base deficiency, increased anion gap, and decreased systemic pH. These parameters also play a prognostic role in the development of complications such as multiple organ failure, and, ultimately, in predicting the mortality rate. The most informative are direct measurements of oxygen delivery to tissues (DO2) and oxygen consumption by tissues (VO2), carried out with the obligatory measurement of Qt, SaO2%, SvO2%. The significance of regional hypoperfusion in the development of shock has brought such a parameter as pH during gastric tonometry (pHi) to one of the first diagnostic positions. In conclusion, it can be noted that both prognostic and diagnostic possibilities for interpreting blood lactate levels in assessing the efficiency of oxygen transport in critical conditions are far from exhausted. In fact, multicenter, international studies have not yet been conducted that can resolve the controversial issues of assessing tissue hypoxia. Technically, there is an opportunity to improve measurement techniques and create devices for monitoring lactate levels in real time.
Bibliography
- Chelnokov S. B., Pudina N. A. Blood lactate level in newborns born with asphyxia. Materials of the Russian Congress on Pediatric Anesthesiology, Resuscitation and Intensive Care. Moscow, 2001, p. 233.
- Torshin V. A. Blood lactate level as an indicator of STAT analysis. Laboratory. No. 4, 2001, p. 17.
- Bakker J. Increased blood lactate levels: a marker of…? www.bloodgas.org. June, 2003
- Meakins J, Long CNH. Oxygen consumption, oxygen debt and lactic acid in circulatory failure. J Clin Invest 1927; 4:273.
- Gutierrez G, Wulf ME. Lactic acidosis in sepsis: a commentary. Intensive Care Med 1996; 22, 1: 6-16.
- Shibutani K, Komatsu T, Kubal K et al. Critical level of oxygen delivery in anesthetized man. Crit Care Med 1983; 11, 8: 640–43.
- Vincent Jl, Roman A, De Backer D, Kahn RJ. Oxygen uptake/supply dependency. Effects of short-term dobutamine infusion. Am Rev Respir Dis 1990; 142, 1: 2-7.
- Roumen RMH, Redl HR, Schlag G et al. Scoring systems and blood lactate concentration in relation to the development of adult respiratory distress syndrome and multiple organ failure in severely traumatized patients. J Trauma 1993; 35: 349–55.
- Andersen O, Haugaard SB, Jorgensen LT et al. Preanalytical handling of samples for measurement of plasma lactate in HIV patients. Scand J Clin Lab Invest 2003; 63: 449–454.
- Kost GJ, Nguyen TH, Tang Z. Whole-Blood Glucose and Lactate. Arch pathol lab Med 2000; 124: 1128–1134.
- Suen WW, Ridley B, Blakney G, Higgins TN. Comparison of lactate, bilirubin and hemoglobin F concentrations obtained by the ABL700 series blood gas analyzers with laboratory methods. Clinical Biochemistry 2003; 36: 103–107.
- Mullner M, Sterz F, Domanovits et al. The association between blood lactate concentration on admission, duration of cardiac arrest and functional neurological recovery in patients resuscitated from ventricular fibrillation. Intensive Care Med 1997; 23: 1138–1143.
- Kellum JA lactate and pHi: Our continued search for markers of tissue distress. Crit Care Med 1998; 26(11): 1783–1784.
- Hameed SM, Aird WC, Cohn SM Oxygen delivery. Crit Care Med 2003; 31 (12): S658–S667.
- Toffaletti JG Blood Lactate: Biochemistry, Laboratory Methods and Clinical Interpretation. Critical Reviews in Clinical Laboratory Sciences 1991; 28 (4): 253–268.
When is a lactate test necessary?
The test results enable the doctor to determine the reasons for the disruption in the supply of oxygen to the organs and systems of the body. A blood lactate test is used to identify lactic acidosis and hypoxia and assess their severity. The lactate level makes it possible to differentiate the types of metabolic acidosis. For newborns, analysis is prescribed in case of asphyxia. In sports medicine, the study is used to monitor the condition of athletes during intense training.
The test is prescribed in the following cases:
- suspected lack of oxygen (symptoms such as rapid breathing, sweating, pale skin, shortness of breath, sudden weakness);
- impaired clarity of consciousness, fainting, fever, severe headaches, symptoms of meningitis;
- suspicion of diabetes, sepsis, heart or kidney pathologies, shock.
general characteristics
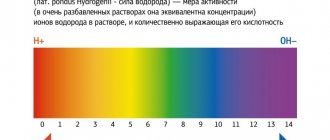
Lactic acid is a product of anaerobic glucose metabolism (glycolysis) and is formed from pyruvate under the action of lactate dehydrogenase when there is insufficient oxygen supply. With sufficient oxygen supply, pyruvate is metabolized in the mitochondria to water and carbon dioxide. The main amount of lactic acid enters the blood from skeletal muscles, brain and red blood cells. Lactate accumulation reduces blood pH and bicarbonate concentrations, thereby leading to metabolic acidosis.
Interpretation of results: norm, reasons for increased performance
Reference values: 0.5-2.2 mmol/liter. The decrease in indicators is not critical. High levels of this substance can indicate various diseases, including life-threatening conditions. For example, an increase in indicators is observed in acute heart failure, leukemia, circulatory disorders, and diabetes. Only a doctor can make an accurate diagnosis and prescribe treatment. Also, an increase in indicators can be caused by emotional or physical stress, alcohol intoxication. Even in the absence of symptoms, if abnormalities are detected, the patient's condition should be monitored by a doctor.
Where does the pain come from?
Then why do muscles hurt the next morning after training? The fact is that under intense loads, the so-called myofibrils are destroyed - thin threads running along the muscle fibers. This is how pieces of dead tissue form in the muscles. The body's immune system finally destroys them and removes them from the body. However, while the destruction process is taking place, free radicals are released in the tissues, and the cells begin to lack water. As a result, pain receptors located on cell membranes are excited, and the person feels pain in the muscles.
Recounting protons
The first and main source of protons in an actively working muscle cell is considered not to be the synthesis, but the breakdown of ATP, the energy of which is used for contractions and relaxations: ATP + water -> ADP + phosphate + proton. Phosphate itself is capable of serving as a buffer system that softens fluctuations in the acidity of the environment (this is how it works in the body), but it is actively involved in new reactions in the cell, and does not solve this problem very effectively.
Another source of protons is the above-mentioned coenzyme NAD+, which during glycolysis reactions loses a proton, turning into NADH. By the way, a significant part of the protons (as well as phosphate and pyruvate) found in the intracellular environment are transported to mitochondria and used for the processes of oxidative phosphorylation that take place there. Thus, mitochondria can also be called a factor in reducing acidity. But when muscles absorb energy with enormous intensity, converting ATP into ADP, this reaction is stronger than anything acting against it.
Rice. 3.
The balance between the production and use of protons in a working muscle cell. The appearance of protons is associated with ATP hydrolysis and glycolysis reactions. They are consumed in the reactions of creatine phosphate and lactate. In addition, protons bind to inorganic phosphate and buffer compounds in the cytoplasm.
How is lactic acid formed in muscles?
Lactic acid is formed during the breakdown of carbohydrates - glycolysis. This is a complex chemical process consisting of several reactions, but we will describe it more primitively. It can be aerobic (with the participation of oxygen) and anaerobic (without the participation of oxygen).
Aerobic glycolysis occurs when energy in the body is produced by the oxygen energy system. This is normal activity or light physical activity of low to moderate intensity. This glycolysis occurs in 2 stages:
- Lactic acid and energy are formed from glucose and ADP molecules
- Lactic acid is neutralized when interacting with oxygen and ADP molecules, as a result of the reaction energy is released, carbon dioxide and water are formed
While the oxygen system is working, lactic acid is not retained in the muscles.
During intense exercise, you need quick energy. The oxygen system is slow, so the lactate energy system comes to the rescue - it works without the participation of oxygen, does not waste time on its transportation, and therefore quickly produces energy. Anaerobic glycolysis occurs: glucose is broken down into lactic acid and energy. Without the participation of oxygen, lactic acid is not neutralized, as in aerobic glycolysis, but accumulates in the muscles in the form of lactate.
Indications
- Diagnosis of circulatory pathologies resulting in tissue hypoxia (insufficient oxygen consumption by tissues);
- Assessing the degree of acidosis and prescribing resuscitation measures;
- Detection of diseases of the cardiovascular system;
- Suspicion of non-insulin-dependent diabetes mellitus;
- Determining the cause of lactic acidosis;
- Assessment of the acid-base status of the body and blood pH;
- Diagnosis of asphyxia (severe oxygen deprivation) and enzymopathy (impaired enzyme activity) in newborns;
- Pathological changes in muscles and tissues;
- Differential diagnosis of myopathies (hereditary muscle diseases).
The analysis is deciphered by specialists: endocrinologist, cardiologist, oncologist, traumatologist, surgeon, therapist, pediatrician, etc.
How to avoid pain
Determining the load
City dwellers move much less than they think.
Calculate how active you are and find out how much you need to move. First
, dose the loads, increasing them gradually and systematically.
If the load is selected correctly, there will be less pain or no pain at all. Be sure to familiarize yourself with the basic principles of effective training to avoid not only discomfort the next day after exercise, but also sports injuries. Secondly
, keep your workouts regular - this will help your muscles get used to constant stress.
Thirdly
, if it was not possible to avoid overtraining, take time for full recovery. Healthy sleep, as well as foods containing antioxidants, help speed up the process of breaking down substances that cause muscle pain.
BIOLOGICAL CONTROL (MONITORING) IN SPORTS TRAINING[edit | edit code]
Source:
Textbook for Universities “Sports Physiology”. Author
: I.I.
Zemtsova Ed.
: Olympic literature, 2010.
The general goal of biological control in sports is to increase the effectiveness of sports training by optimizing physical activity based on an objective assessment of the athlete’s functional readiness.
At different stages of athletes’ training, there are different tasks, in accordance with which the goal and forms of control are determined. In the theory and practice of sports, there are four main types of control: operational, current, staged and in-depth (Volkov, 1996; Biological control..., 1996; Kurochenko, 2005; Levushkin, 2001; Platonov, 1997; Clausen, 1997).
Operational control (urgent)
involves the assessment of operational states - urgent reactions of the athletes’ body to the load during individual training sessions and competitions.
Current control
is aimed at assessing current conditions resulting from physical activity in a series of classes, training or competitive microcycles.
Stage control
allows you to assess the athlete’s condition, which is a consequence of the long-term training effect at certain stages of preparation.
Advanced Control
carried out once a year for a comprehensive assessment of the athlete’s preparedness and state of health.
Indicators used in accordance with a certain type of control must be informative and reliable and comply with:
- specifics of the sport;
- age and qualifications of the subjects;
- orientation of the training process.
In sports associated with the manifestation of endurance (swimming, rowing, cycling, cross-country skiing, middle and long distance running, etc.), indicators characterizing the state of the cardiovascular and respiratory systems and metabolic processes are mainly studied. Thanks to them, it is possible to most reliably assess the potential capabilities of athletes in achieving high sports results.
In speed-strength sports, where the main task is the ability to demonstrate short-term muscle tension (sprint running, athletics jumping and throwing, weightlifting, certain disciplines of cycling, speed skating, swimming, etc.), indicators characterizing the state of the nervous system are used as means of control. muscular system, central nervous system, speed-strength components of motor function, which are manifested in specific test exercises.
In sports where sporting achievements are predominantly determined by the activity of analyzers, the mobility of nervous processes that ensure accuracy, regularity of movements in time and space (gymnastics, acrobatics, figure skating, diving, all types of sports games, shooting, etc.), in The control process uses a wide range of indicators. They characterize the accuracy of reproducing the temporal, spatial and power parameters of specific movements, the ability to process information and quickly make decisions, the elasticity of skeletal muscles, joint mobility, coordination capabilities, etc. (Belotserkovsky, 2005; Biological control..., 1996; Brgsyun, 2003; Platonov , 1997).
Increased values (lactic acidosis)
- Pathologies of the cardiovascular system: heart failure, cardiogenic shock (acute left ventricular failure), Raynaud's syndrome (severe vascular disease, spasm of small blood vessels);
- Blood flow disorders and diseases of the circulatory system;
- Non-insulin-dependent diabetes mellitus;
- Increased physical activity (usually among professional athletes);
- Tetany (convulsions due to calcium metabolism disorders);
- Tetanus (a bacterial disease affecting the nervous system);
- Epilepsy (pathology of the nervous system, manifested by convulsive seizures with loss of consciousness);
- Hepatitis (viral inflammation of the liver) in acute form;
- Cirrhosis of the liver (an abnormal change in the structure of the organ tissue);
- Oncological processes: lymphoma (cancer of the lymphatic system), leukemia (blood cancer), etc.;
- Poliomyelitis (highly contagious disease of the nervous system, spinal paralysis);
- Tissue hypoxia (oxygen starvation);
- Hypotension (low blood pressure);
- Heavy bleeding;
- Pulmonary insufficiency, hyperventilation (impaired frequency or depth of breathing).
A temporary increase in lactic acid concentration is possible as a result of:
- deficiency of vitamin B1 in the body;
- long-term regular use of alcohol;
- poisoning with chemical elements: ethanol, salicylates, toxins, methanol, etc.;
- dehydration (dehydration of the body);
- pregnancy (in the 3rd trimester the level of lactic acid may increase slightly);
- taking medications: sodium drug, nitroprusside, adrenaline, metformin, fructose or glucose, propylene glycol, methylprednisolone, phenformin, etc.