Local therapist
Burnatskaya
Svetlana Nikolaevna
33 years of experience
Local therapist, occupational pathologist. Member of the Russian Scientific Medical Society of Therapists
Make an appointment
Agranulocytosis is a severe pathological condition. With it, there is a critical decrease in granulocytes in the blood. Granulocytes are an important fraction of the leukocyte series (neutrophils, eosinophils and basophils). At the same time, the concentration of basophils and eosinophils in the blood in the normal state is a small percentage, so changing their number does not have a significant effect. Thus, it can be argued that it is the decrease in the concentration of neutrophils that leads to the development of blood agranulocytosis. Hence the second name of the pathology – neutropenia.
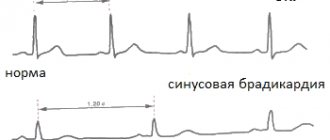
Experts raise the question of agranulocytosis in a patient when the leukocytes in the blood decrease below 1×109/l and granulocytes below 0.75×109/l. Since leukocytes and granucolites perform a protective function against pathogens, a decrease in their concentration reduces the body’s ability to resist infections and viruses. The development of agranulocytosis is almost always accompanied by the development of various infectious processes, these can be ulcerative stomatitis or tonsillitis, pneumonia, hemorrhagic manifestations, etc.
Depending on the concentration of granulocytes, the severity of peripheral blood pathology is determined. The lower their concentration, the more severe the degree; there are three degrees: mild, moderate and severe. In severe cases, the granulocyte concentration is below 0.4 × 109/l.
In women, agranulocytosis is more common. In men, this disease is diagnosed on average 2-3 times less often. In most cases, the manifestation of pathology occurs after the age of 40 years.
Symptoms and signs
Since the pathology has several forms, the symptoms of agranulocytosis of different types differ. The immune type is characterized by acute agranulocytosis with the following symptoms:
- severe weakness and sweating;
- body temperature up to 39-40°C;
- pallor;
- the appearance of infectious stomatitis;
- inflammation of the pharynx and tonsils;
- inflammation of the gums;
- sore throat and spasm of the chewing muscles.
Myelotoxic and autoimmune types progress gradually and also gradually develop symptoms:
- hemorrhagic symptoms in the form of nosebleeds, bleeding gums, etc.;
- presence of blood in the urine;
- the occurrence of bruises, hematomas;
- abdominal pain and bloating, diarrhea if the intestines are affected;
- possible occurrence of chest pain when breathing.
Structure
Lymphocytes
There are large (from 10 to 18 microns), medium (from 6.5 to 10 microns) and small (from 4.5 to 6.5 microns) forms. In human blood, predominantly small lymphocytes circulate, the size of which does not exceed 7-10 microns in diameter. Large lymphocytes are found in the blood only in newborns. In adults, large cells are localized in organs (liver, lungs, kidneys) and play the role of natural killers.
Their structure is such that most of the cell is occupied by a dark, bean-shaped or rounded nucleus. The lymphocyte contains a small amount of light blue cytoplasm, which contains lysosomes (organelles containing enzymes). The cytoplasm is usually visible as a rim around the nucleus. Lymphocytes often look like purple balls due to the fact that the cytoplasm seems to merge with the nucleus.
Monocytes
These are the largest white cells, their diameter ranges from 12 to 20 microns. The reddish-violet nucleus can have different shapes: bean-shaped, horseshoe-shaped, butterfly-shaped, mushroom-shaped, etc. It occupies an equal or greater part of the cell than the cytoplasm.
The cytoplasm is light blue or smoky, lighter near the nucleus, and more intensely colored at the periphery. It contains very small lysosomes, which are usually located closer to the nucleus. The cytoplasm may contain vacuoles, vesicles, pigment granules, and phagocytosed cells.
Causes
The occurrence of agranulocytosis can have different causes, depending on which the pathology is classified.
Types of illness
Myelotoxic agranulocytosis is the result of the influence of various unfavorable factors on the red bone marrow. Under their influence, deep depression occurs in the hematopoietic process responsible for the production of granulocytes. Unfavorable factors should be understood as internal diseases of the body and external adverse effects. Examples of external influences: poisoning with substances and poisons that suppress hematopoiesis (mercury, arsenic, benzene, etc.), radiation exposure, taking myelotoxic drugs. Among the diseases that most often cause agranulocytosis, experts identify acute leukemia, sarcoma and metastasis to the red bone marrow, and chronic myeloid leukemia.
Immune agranulocytosis is characterized by the destruction of granulocytes in the blood due to the occurrence of pathological immune reactions in the body. This type is further divided into the following forms of agranulocytosis.
- Autoimmune - occurs in most cases against the background of connective tissue diseases. These include, for example, rheumatoid arthritis or systemic lupus erythematosus.
- Hapten agranulocytosis or drug-induced agranulocytosis develops with the participation of haptens in immune reactions. Haptens themselves are harmless substances, but under certain situations and reactions they can cause the destruction of granulocytes. The role of haptens most often are drugs that the patient takes as part of the treatment of various diseases. Hence the second name of the pathology – “medicinal”.
- Genuine - this type is assigned to the patient in cases where diagnostics have not revealed the reasons for the decrease in granulocytes in the bloodstream.
Agranulocytes: their characteristics, properties and participation in the immune response
Speaking about scientists of the 19th and early 20th centuries, I am invariably amazed at how vast the areas of their research interests and aspirations were. Nevertheless, each of the greats of this world had certain merits, thanks to which humanity especially benefited and stepped forward in understanding certain laws of nature. And so, when it comes to the great Russian physiologist (immunologist, biologist, embryologist and further on the list) Ilya Mechnikov, every time I involuntarily imagine him as a warrior on a horse, leading an army of macrophages, for it was this man who was the first to realize the role of these cells , inherent in all organisms from echinoderm to vertebrates. Later, in the 1960s, van Furt and Cohn defined the mononuclear phagocyte system as a community of agranular myeloid cells, which, as we now know, play a critical role in tissue renewal, maintaining homeostasis and stimulating and regulating both innate and acquired immunity . In general, the mononuclear phagocyte system includes three types of cells: fully differentiated macrophages and dendritic cells (which, in turn, are also divided into many subpopulations), as well as monocytes.
Actually, monocytes are understood as circulating blood cells that make up about 10% of all leukocytes in human peripheral blood. Monocytes enter the bloodstream from the bone marrow, where they develop from the myeloid progenitor cell common to agranulocytes and granulocytes. Not least because of the short duration of stay of monocytes in the circulating blood (about 3 days in humans), this environment is considered a reservoir of myeloid precursors, ensuring constant renewal of the pool of tissue macrophages and, in particular, short-lived dendritic cells.
Rice. 1. System of mononuclear phagocytes.
Developing macrophages are found in the yolk sac, as determined by both morphological characteristics and the expression of macrophage markers (eg, FMS, CD11b, mannose receptor). Later, when hematopoiesis begins in the fetal liver, it becomes the main source of macrophages, which resemble those observed in the adult body, but the number of macrophages populating the organs of the embryo exceeds that of an adult. Monocytes can play the role of precursors, which, upon differentiation, replenish the pool of tissue macrophages, but still most of them are formed during the local proliferation of precursors rather than as a result of the recruitment of monocytes from the peripheral blood. Monocytes exit the bone marrow into the bloodstream, having the Ly6C+ phenotype, and change subsequently, giving rise to another phenotypically different subtype (Ly6C-).
Initially, attempts were made to classify monocytes circulating in the blood based on cellular morphology and their specific density. However, these characteristics are quite heterogeneous and do not have the proper specificity, so it is very difficult to distinguish monocytes solely by histological characteristics. The first thoughts that monocytes could probably be divided into subtypes, which are characterized by different physiological characteristics, crept in when the first two subpopulations of monocytes were identified, differing both in morphology and in the differential expression of antigenic markers - CD14 and CD16. Cells with the CD14hi CD16 phenotype are classified as classical monocytes, since high levels of CD14 expression and CD16 expression were characteristic of the first described monocytes. The second major subtype of monocytes is characterized, in addition to the expression of CD14 and CD16 (referred to as CD14+ CD16+), by increased levels of class II major histocompatibility complex molecules and CD32 (also known as FcγRII), and it has been suggested that these cells are similar to mature tissue cells. macrophages. In addition to differences in the degree of expression of key antigenic markers, both monocyte subtypes described above show differences in the expression of chemokine receptors (for example, CD14hi CD16 monocytes express C-C chemokine receptor 5 (CCR5), while CD14+ CD16+ express CCR2). However, when both monocyte subtypes are cultured in the presence of GM-CSF and IL-4, both differentiate into dendritic cells. And in in vitro experiments using a transendothelial migration model, mononuclear cells isolated from peripheral blood during incubation with a monolayer of human umbilical vein endothelial cells on a collagen matrix migrated, bypassing the endothelial barrier, and differentiated into macrophages, which remained in the subendothelial matrix or turned into dendritic cells. cells migrating back through the endothelial cell layer.
Blood monocytes take their origins in the bone marrow, being formed from hematopoietic stem cells through sequential commitment and differentiation of precursors. The first cells from which the differentiation path to monocytes begins are the common myeloid progenitor cell and the granulocyte-macrophage progenitor. In addition, in 2006, specific clonogenic precursors were described, found in the bone marrow, which, unlike granulocyte-macrophage precursors, do not give rise to granulocytic, lymphoid, erythroid or megakaryocyte lineages, but provide the growth of only several subtypes of macrophages and resident dendritic cells . Based on what kind of cells such precursors give rise to, they were called macrophage and dendritic cell precursors (MDP), it’s simple. The bone marrow microenvironment potentiates MDP to differentiate into monocytes, which are subsequently released into the blood.
From an evolutionary point of view, the mammalian acquired immune system represents the crown of all possible immune responses in terms of its complexity and molecular specificity. Unlike cells belonging to the innate immune system and recognizing typical motifs associated with a wide range of pathogens due to receptors encoded by multiple germline genes, cells of the acquired immune system can be provided with an almost inexhaustible variety of antigen-recognition receptors, which occurs due to one of mechanisms of somatic recombination (V(D)J-recombination), which, among other things, is the basis for the existence of immunological memory in cells due to the differentiation, distribution and maintenance of persistence of long-lived antigen-specific lymphocytes. Being direct effectors of the immune system and realizing cytotoxic properties, as well as producing antibodies, lymphocytes at the same time play the role of a key regulator of the immune response, strengthening or weakening it, influencing various types of cells through a positive or negative feedback mechanism.
Let us examine in a little more detail the mechanism of recombination, thanks to which the diversity of antigen-recognition receptors in lymphocytes becomes possible. V(D)J recombination serves as the basis for the diversity of B-cell receptors due to the fact that in developing B-cells the genes encoding the heavy and light chains of immunoglobulins are composed of several different gene segments. B cell development begins in the fetal liver with the differentiation of pluripotent hematopoietic stem cells (HSCs) and subsequently occurs in the bone marrow.
HSCs differentiate into progenitor B cells, which, with the help of the Pax5 transcription factor, reach the stage of mature B cells and acquire the surface marker c-kit, as well as CD19, characteristic of all stages of B lymphocyte differentiation, except plasma cells. It is at the stage of progenitor B cells that somatic DNA recombination occurs at the immunoglobulin heavy chain locus (IgH locus), which consists in the formation of a functional VDJ exon, consisting of V- (variable), D- (diversity) and J- (joining) segments. This exon encodes the V region of the heavy chain of immunoglobulin and, together with the V region of the light chain, forms the antigen-binding region of membrane-bound B-cell receptors. V(D)J-recombination in the IgH locus occurs in 2 stages, for which this DNA section first undergoes modifications of chromatin and only then becomes available for changes: first, at the stage of early progenitor cells in both alleles, D and JH are combined by non-homologous recombination -segments, and then, already in the late progenitors of B cells, the VH segment, located immediately after the above-mentioned sections, becomes available for interaction, and when the locus is reduced, the VH segment joins the already rearranged segments D and J. Of course, a complex process V( D)J-recombination requires the participation of numerous enzymes, among which are RAG1 and RAG2 (Recombination-activating Genes 1 and 2), specific for lymphocytes, as well as enzymes and double DNA break repair systems inherent in all cells, such as Ku70, Ku80, XRCC4, DNA ligase IV, DNA-dependent protein kinases (DNA-PK), Artemis.
RAGs recognize specific recombination signal sequences (RSS) surrounding immunoglobulin gene segments and initiate V(D)J recombination by breaking both DNA strands between the Ig gene segments and the adjacent RSS. The free 3'-OH ends of the segments bind to complementary chains, forming hairpin structures (they are called coding compounds, Coding Joint), and RSS are connected by their free ends and form signal compounds - Signal Joint. The resulting hairpin structures are opened again by the Artemis endonuclease due to the breaking of one of the strands, and after processing, the exonucleolytic properties of Artemis and polymerases ensure the connection of gene segments with each other with the formation of blunt DNA ends. It is important to pay attention to the fact that hairpin structures should not open in the same places (at the level of the same bases) where their association initially occurred, and therefore the nucleotide sequence lying between them can be mirrored, resulting in the formation of palindromic nucleotides ( P-nucleotides).
The variety of possible combinations of segments (in addition to recombinatorial diversity) increases precisely due to P-nucleotides, as well as due to randomly added non-template nucleotides by terminal deoxynucleotidyl transferase. To ensure direct recombination of the V and D/D and J segments, the RSS of the regions involved in the recombination are different. Between the relatively conserved hepta- and nonamer sequences (CACAGTG and ACAAAACC, respectively), found in all RSS, there are spacer sequences, which for the RSS segments V and J are 23 base pairs, and within the RSS, which borders segment D on both sides, the spacers cover 12 base pairs. Thus, due to the presence of spacers, direct interaction between the V and J segments in the IgH locus is prevented, since V(D)J recombination occurs, by and large, almost exclusively between RSSs with spacers of different lengths (rule 12/23).
After rearrangement of the gene segments responsible for the structure of the immunoglobulin heavy chain, its synthesis occurs directly. The resulting heavy chains bind to surrogate light chains consisting of the VpreB and λ-like protein proteins and the signaling molecules Igα and Igβ, together forming the pre-B receptor complex. This complex moves to the cell surface, where it is able to perceive and transmit signals that promote the differentiation of cells into early pre-B lymphocytes and their clonal expansion. In addition, signals conducted through the pre-B complex inhibit recombination at the IgH locus.
Rice. 2. RAG-mediated rearrangements of gene segments.
Sometimes V(D)J recombination is not without errors and it happens that the reading frame is shifted due to additionally built-in P- or N-nucleotides or, conversely, due to their loss. In such cases, the resulting VDJ exon is nonfunctional, and recombination continues in the second allele of the gene. If the second rearrangement fails, then in such a pro-B lymphocyte the signals that allow the cell to live and continue differentiation are not carried out, and its apoptosis is triggered.
But let’s return to normal cells, for which recombination at the IgH locus resulted in the formation of a productive VDJ exon and the synthesis of a heavy chain capable of binding. Expression of the pre-B receptor complex invariably means for such a cell a transition to the stage of an early precursor of B lymphocytes, which is accompanied by the cell losing the c-kit marker and cessation of expression of terminal deoxynucleotidyl transferase. In addition, the activity of RAG1 and RAG2 is temporarily stopped. Instead, early pre-B lymphocytes express CD25 and enter a short proliferative phase, spanning 4-6 cycles of cell division, the purpose of which is to increase the number of pre-B lymphocytes possessing functionally active IgH chains capable of forming complexes with IgL- chains. Such selective clonal expansion of pre-B cells makes a significant contribution to the expansion of the Ig spectrum, since each new precursor cell of the future B lymphocyte has its own L-chain, which is not similar to others, and therefore acquires an Ig receptor with a new one, specificity characteristic only of a given cell.
To be very precise, the clonal expansion of early precursors leads to their differentiation into small resting pre-B lymphocytes, in which, by analogy with rearrangements in the IgH locus, the κ- and λ-loci of immunoglobulin light chains are redeployed; after rearrangement of the IgL gene, the IgL chain is synthesized, which, combining with the IgH chain, becomes part of the B-cell receptor, necessary for the cell to transition to the stage of immature B-lymphocyte. Once the complete B cell receptor is expressed on the cell surface, immature B cells undergo negative selection, during which their receptors are tested for autoaggression. Identified cells with autoaggressive properties can be apoptotically destroyed, or they can get rid of autoreactive receptors, being involved in the process of secondary rearrangement of the Ig loci of their receptors.
So, the tissues and organs of the lymphatic system, being in close relationship with each other, provide the body with protection from foreign agents. Speaking directly about the acquired immune system, the first thing worth mentioning is antigen-presenting cells, which migrate from the site of infection to the nearest regional lymph nodes, where they present microbial antigens to intact T cells. When faced with an antigen “recognized” by antigen-presenting cells, intact T cells begin to actively divide, resulting in the differentiation of both effector T lymphocytes, which are sent to the site of infection and are engaged in eliminating foreign agents, and memory T cells within a few days. which, persisting in the bloodstream, are ready to support the immune response to a secondary infection.
Actually, this is why lymphocytes are usually considered as cells characteristic of lymphoid organs and circulating in the blood, however, we should not forget that there are also resident types of lymphocytes present in non-lymphoid tissues (especially the skin and mucous membranes). Specialized resident tissue lymphocytes include innate lymphoid cells (ILCs), tissue-resident memory T cells (TRMs), and special types of T lymphocytes such as invariant natural killer cells (iNKTs). ), mucosal-associated invariant T cells (MAIT cells), γδ T cells, and CD8aa+ intraepithelial lymphocytes (IELs).
Rice. 3. Tissue and circulating lymphocytes and their functions.
To understand more clearly the role of tissue-resident lymphocytes, let us turn to how these cells differ from those that circulate in the bloodstream. The first thing worth mentioning is their ability to self-renew, regardless of circulating precursors. A significant part of the pool of tissue lymphocytes is concentrated in barrier tissues. Another important property of these cells is a wide range of microbial antigens and signals from other cells of the body, which they are able to recognize and perceive, thereby receiving information about inflammation, the development of the infectious process and tissue damage.
Activation of tissue lymphocytes is carried out using semi-invariant antigen receptors, NK-activating receptors and receptors for alarmins and cytokines. Semi-invariant receptors inherent in iNKT cells resemble pattern recognition receptors and recognize glycolipids with an α-anomeric glycosidic bond, which are often characteristic of pathogenic bacteria and can directly trigger intracellular signaling reactions after binding to these receptors. However, even in the absence of potent microbial antigens, T lymphocytes can be activated by low-affinity endogenous ligands competitively stimulated by cytokines, typically IL-12 and IL-18. This suggests that activation of iNKT does not necessarily accompany infectious processes, but can also be caused in response to metabolic stress, characterized by impaired synthesis and metabolism of glycolipids.
Some subpopulations of intraepithelial lymphocytes are also characterized by a reaction to endogenous ligands associated with disruption of metabolic processes in tissues. And finally, the final feature of tissue-resident lymphocytes is their ability to quickly respond to activating signals, which is expressed in the massive secretion of antimicrobial factors (cytokines, cytolytic molecules, growth factors). Equally important is the expression of certain transcription factors by tissue lymphocytes, for example, PLZF. In experiments on mice, those individuals that were deficient in PLZF expression showed a sharp decrease in the number of iNKT and MAIT cells, and the existing cells showed impaired function and localization.
Rice. 4. Strengthening the immune response by tissue-resident lymphocytes.
(A) Helminthic infection causes the release of IL-33 and IL-25 from dying epithelial cells from chemosensitive hair cells. These cytokines induce innate immune response lymphoid cells (ILC2s) to secrete IL-13, which causes goblet and hair cell hyperplasia. (B) Activated by the presented antigen, CD8+ TRMs produce interferon gamma, which puts tissue cells on alert and attracts circulating memory cells. (C) NKTs produce cytokines and, having been previously activated by Kupffer cells, themselves activate CD40L, which in turn presents CD1d-linked glycolipids found in bacterial pathogens.
Rice. 5. Generalized idea of the effector functions of tissue-resident lymphocytes.
This concludes our short review of agranulocytes. Their complex relationships and multifaceted contributions to the development of immune responses, of course, still raise many questions in your mind, to which I hope you will immediately go looking for answers.
Sources:
- Xiying Fan, Alexander Y. Rudensky Hallmarks of Tissue-Resident Lymphocytes, Cell, 2016
- Siamon Gordon, Philip R. Taylor Monocyte and macrophage heterogeneity, Nature Reviews Immunology, 2005
- Simon Yona, Steffen Jung Monocytes: subsets, origins, fates and functions, Current opinion in hematology, 2010
- Bassing, C. H., W. Swat, et al. The mechanism and regulation of chromosomal V(D)J recombination, Cell, 2002
Risk factors
The risk group primarily includes patients who are frequently exposed to severe infections, as well as unusual, rare infections. Another risk group is patients who, as part of the treatment of other diseases, receive drugs or therapy (for example, radiation therapy), which can cause the development of agranulocytosis. Patients at risk should be especially attentive to their well-being and pay attention to alarm bells. Accurate laboratory diagnostics are required to confirm the diagnosis.
Causes of agranulocytosis
- Ionizing radiation and radiation therapy, chemicals (benzene), insecticides.
- Drugs can cause agranulocytosis as a result of direct inhibition of hematopoiesis (cytostatics, valproic acid, carbamazepine, beta-lactam antibiotics) or by acting as haptens (gold preparations, antithyroid drugs, etc.).
- Autoimmune diseases (eg, lupus erythematosus, autoimmune thyroiditis).
- Viral infections (caused by Epstein-Barr virus, cytomegalovirus, yellow fever, viral hepatitis) are usually accompanied by moderate neutropenia, but in some cases agranulocytosis may develop.
- Severe generalized infections (both bacterial and viral).
- Emaciation.
- Genetic disorders.
Diagnosis of agranulocytosis in patients
If the patient is at risk or corresponding symptoms appear without other visible causes, agranulocytosis is diagnosed using the following methods.
- A general blood test is the very first and most important test that allows you to determine the concentration of granulocytes and leukocytes in the bloodstream.
- Antibody test - prescribed to identify autoimmune agranulocytosis, allows you to determine antineutrophil antibodies.
- Myelogram - allows you to determine the decrease in myelokaryocytes and other indicators of the condition of peripheral blood.
If necessary, when diagnosing agranulocytosis, other research methods are prescribed to determine the degree of damage to organs and systems caused by this pathology. In particular, the following may be prescribed:
- X-rays of the lungs;
- visiting a dentist;
- blood chemistry;
- blood sterility tests;
- consultation with an otolaryngologist.
Treatment
Once a diagnosis is made and the type of pathology is verified, treatment should begin immediately. In most cases, the patient requires hospitalization in the hematology department. This is necessary to create aseptic conditions. In the isolation ward, quartz treatment is carried out according to a schedule, and visits to the patient are also limited. Such measures are necessary to prevent the occurrence of additional infectious complications.
The specialist prescribes a comprehensive treatment for agranulocytosis to the patient, and it begins with eliminating the factors under the influence of which the development of this pathological condition began. The set of measures for the treatment of agranulocytosis includes:
- elimination of negative impact factors, including refusal to take myelotoxic drugs, various chemicals, etc.
- treatment and prevention of infectious diseases;
- enhanced oral care;
- steroid therapy;
- administration of immunoglobulin (intravenously);
- leukocyte transfusion;
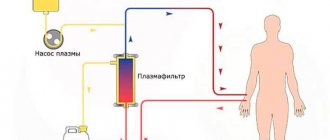
- plasmapheresis, etc.
The range of therapeutic measures depends on the type of disease, its degree, as well as the degree of other pathologies present in the body. In each case, the treatment complex is prescribed individually.