Obstetricians-gynecologists begin to listen to the baby’s heart during the period of its intrauterine development. Before birth, a baby's heart murmur cannot be detected. Doctors focus on the sonority of tones and the frequency of contractions, indicators of “good health” of the fetus, and the uncomplicated course of pregnancy in the expectant mother.
After birth, on the first day, the newborn is examined by a special pediatrician in the maternity hospital. Large perinatal centers have neonatologists. These are pediatricians specially trained to diagnose and treat early childhood pathologies and congenital diseases. They conduct the first auscultation of the baby, and if noise is detected, they are required to establish the cause.
Features of auscultation in children
The therapist gets “scared” when listening to the child. The auscultatory picture is the same as in an adult, but audibility is several times stronger. Especially in infants.
- The baby's chest wall is much thinner, all sounds are transmitted very clearly.
- A newborn needs to be able to listen to a scream. They try to carry out examinations of an infant after feeding, so that he is in a good mood and has the opportunity to listen to the heart in silence.
- Older children have to be distracted by their mothers.
- There are always toys in the offices of local pediatricians; it should be warm.
- The doctor tries to warm the head of the phonendoscope in his hands so as not to frighten the patient by touching a cold object.
This is how you have to explain yourself to a little person
In assessing tones, it is important that in children and adolescents, unlike adults, the third additional tone is heard more often. It does not indicate ventricular pathology, is better audible after exercise and gives a “gallop rhythm”.
Why does noise occur in children?
You can read about the causes and possible mechanisms of the formation of heart murmur in a child here.
Most often, murmurs in a child’s heart are associated with growth processes and are functional.
Newborns often have a slight systolic murmur due to the transition of the heart to independent circulation and the gradual occlusion of the ductus arteriosus.
Functional murmur occurs in children of different ages and adolescents due to a discrepancy in the growth rate of muscle tissue and valve leaflets. It intensifies after exercise, is systolic in nature, and disappears with age. At the same time, the child grows and develops normally, does not lag behind his peers, and is fully involved in physical education.
As body temperature rises, noise is caused by increased blood flow velocity
It is possible to listen to a systolic murmur during acute childhood infections, against a background of high temperature. In this case, the doctor must monitor residual effects during the recovery period. Intoxication and the effects of infectious agents on the myocardium often cause myocarditis with shortness of breath, tachycardia, and weakness. Therefore, such manifestations cannot be excluded from diagnosis.
From 1 to 1.5% of newborns are born with organic changes (defects). Auscultation indicates congenital pathology from the first day of life.
The cause of noise in organic defects is:
- narrowing of the openings between the atria and ventricles, at the exit to the aorta and pulmonary artery;
- insufficient fixation of the valves when they close, the formation of a gap for the return flow of blood;
- the presence of interatrial and interventricular defects in the septum;
- additional communication between the aorta and pulmonary artery along the patent ductus botallus;
- several combined defects in tetralogy of Fallot and other rare defects.
In adolescents, you should be especially attentive to any newly detected murmurs. This is the only way to promptly diagnose the onset of rheumatic carditis with the formation of acquired heart disease.
Treatment and diagnostic tactics for the management of newborns with congenital heart disease at the preoperative stage
The key point in improving the management of newborns with congenital heart disease is a clear division of the main directions of therapy in different groups of patients:
1. Etiotropic and pathogenetically based therapy is considered adequate.
2. Possible therapy - aimed at relieving any syndrome and not having life-threatening side effects.
3. Potentially dangerous - this is therapy that leads to a deterioration of the condition and a threat to life.
For the treatment of ductus-dependent pulmonary circulation, adequate therapy is: lack of oxygen therapy, intravenous administration of prostaglandins, correction of the acid-base state. Therapy with the purpose of correction or no therapy is considered possible. Insufflation or mechanical ventilation with oxygen will be potentially dangerous for such patients. Today it is known that two factors play a role in the mechanism of arterial flow closure: an increase in blood oxygen saturation after the newborn’s first breath and a progressive decrease in the level of maternal prostaglandins depending on the gestational age.
For defects with large left-right shunts, therapy with cardiotonics and diuretics is considered adequate; possible - by any of the above means; potentially dangerous - vasodilators, increasing the volume of discharge and exacerbating pulmonary hypervolemia, thereby reducing systemic output.
In table Table 2 groups the main approaches to the treatment of various groups of congenital heart disease (L.M. Mirolyubov, Yu.B. Kalinicheva, 2005).
Auscultatory picture of common congenital defects in children
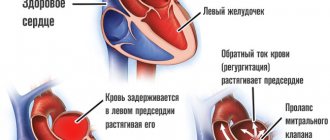
Organic heart murmurs always indicate pathology and require further diagnosis and a decision on conservative or surgical treatment of the child.
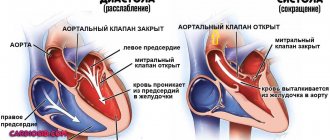
- A patent ductus bollus is manifested in an infant by a typical murmur in the second intercostal space to the left of the sternum. It has a rough systolic prolonged character (“machine noise”), weakens during inspiration.
- With narrowing of the pulmonary artery, stenosis of the aortic isthmus, a noise is also heard to the left of the sternum, but it is carried out in the third intercostal space, on the carotid arteries, in the interscapular space.
- Defects of the interatrial and interventricular septa are characterized by systolic and diastolic uniform murmurs along the left edge of the sternum at the level of the attachment of the third and fourth ribs, carried to the apex, but not audible on the vessels.
Screening technologies for detecting congenital heart defects in newborns
- Karpova Anna Lvovna
- Bockeria Ekaterina Leonidovna
- Tatyana Nikitichna Nikolaeva
- Evgeniy Markovich Spivak
- Mostovoy Alexey Valerievich
- Alexandra Vladimirovna Marasina
Summary
The review provides information on methods for early detection of congenital heart defects in newborns, which are used in world practice as screening technologies. The relevance and importance of screening for congenital heart defects in the early neonatal period as one of the ways to reduce infant mortality is shown.
Particular attention is paid to targeted clinical examination of newborns, measurement of blood pressure in order to identify cardiac pathology, and emphasis is placed on the need to mandatory supplement these clinical examination methods in the early neonatal period with screening of blood oxygen saturation.
The most detailed information is given on screening blood oxygen saturation in newborns, on the advantages and disadvantages of the method, on its sensitivity and specificity, on the advantages of later pulse oximetry in newborns in order to identify critical heart defects, data on false-positive and false-negative results and ways to reduce them are analyzed. .
Key words: newborns, oxygen saturation, congenital heart defects, blood pressure, peripheral vascular pulsation
Neonatology: news, opinions, training. 2021. No. 2. P. 40-49.
Congenital malformations (CDM) are one of the main causes of death of children in the first year of life, occupying 2nd place in the structure of infant mortality [1]. Among them, the most common are congenital heart defects (CHD), which in our country in 2014 in the structure of congenital defects amounted to 44.4% (in 2013 - 42.1%, in 2012 - 41.5%). According to Rosstat, in 2014, 1,393 children died from congenital heart disease in the first year of life, which accounted for 45.3% of losses from congenital heart disease; the infant mortality rate corresponded to 7.3 per 10 thousand live births [2]. The incidence of congenital heart disease varies widely and ranges from 2-4 to 14-15 per 1000 newborns (average 8-14 per 1000) [3, 4]. According to TE Roberts et al. (2012), in the UK the incidence of congenital heart disease is 4-10 per 1000 live births [5], in Italy - 8-10 per 1000 [6].
The modern level of diagnosis and treatment (primarily we are talking about surgical methods) in most cases allows saving the lives of children with congenital heart disease. Among children in the first year of life who underwent surgery for congenital heart disease, newborns accounted for 34.3% in 2014 (in 2013 - 32.7%; in 2012 - about 33.1%; in 2011 - 34.3%). - 29.6%) [2]. This primarily concerns “critical heart defects.” To save the life of a newborn in such cases, emergency surgical interventions must be performed in the first hours or days after birth, so the presence of cardiac pathology is extremely important to determine as early as possible.
The concept of “critical heart disease” is used to refer to congenital heart disease accompanied by the development of critical conditions in the next few hours or days after birth. The incidence of the discussed congenital heart defects in the first 28 days of life ranges from 20 to 30% [4, 7]. The critical condition of a newborn with congenital heart disease is characterized by an acute deficit of cardiac output, rapid progression of heart failure, oxygen starvation of tissues with the development of decompensated metabolic acidosis and dysfunction of vital organs [4].
Critical congenital heart defects may not be clinically apparent immediately after birth, but as the patent ductus arteriosus (PDA) closes, there is a progressive deterioration in the baby's condition. Mortality in such cases is higher than with other variants of congenital heart disease. More than 70% of children with critical congenital heart disease can be saved with accurate early diagnosis, adequate intensive care and timely surgical intervention [4].
However, unfortunately, until now every 4th critical congenital heart disease is diagnosed only after discharge from the maternity hospital [6]. So, in New Zealand in 2006-2010. the frequency of late diagnosis was 20% of cases with critical congenital heart disease and 51% with non-critical congenital heart disease [8].
This is primarily due to the fact that often with severe congenital heart disease, clinical symptoms in the early neonatal period can be extremely sparse, which significantly complicates diagnosis based solely on clinical examination. Visible cyanosis may be absent; a heart murmur, as a rule, does not provide an objective characteristic of the severity of damage to the cardiovascular system in newborns. According to C. Lundsgaard et al., children with moderate hypoxemia and arterial oxygen saturation of 80-95% will not have visible cyanosis of the skin and mucous membranes [9]. M. H. Lees et al. found that for newborns with a hemoglobin level of 200 g/l, cyanosis will be visible only when arterial oxygen saturation is less than 80%; at a hemoglobin concentration of 100 g/l, the saturation to visualize cyanosis should be less than 60% [10]. Often, with critical congenital heart disease in the early neonatal period, there is either no heart murmur, or it may appear much later, only after the resistance in the pulmonary circulation has decreased.
In order to improve outcomes and more timely diagnosis of congenital heart disease, the practice of measuring blood oxygen saturation (SpO2) in newborns in the early neonatal period has been well established around the world [5, 8, 11, 12]. The possibility of using pulse oximetry as a screening test was first confirmed more than 10 years ago, and since then data on more than 370 thousand children examined have already been published. Pulse oximetry is the measurement of the degree of blood oxygenation - the degree of saturation of hemoglobin with oxygen [4, 13], therefore the main principle of this technology is based on identifying hypoxemia, which is characteristic of critical congenital heart disease in newborns.
The American Academy of Pediatrics (AAP) recommends pulse oximetry, characterizing it as a simple, non-invasive and painless technique for assessing SpO2 in order to early identify a group of newborns who are subject to in-depth cardiac examination, in particular echocardiography (EchoCG) [14-16]. The effectiveness of pulse oximetry as a method for detecting congenital heart disease in newborns is confirmed by numerous studies conducted in different countries of the world [6, 17-21]. In table Figure 1 presents an analysis of the sensitivity and specificity of the method according to data from different authors [22].
SpO2 screening, according to a number of authors, led to a significant reduction in the mortality of newborns from congenital heart disease [29], since early diagnosis made it possible to timely identify more than 90% of critical congenital heart disease, including hypoplasia of the left heart, in 85% of cases it was possible to timely detect transposition of the main vessels (TMS) [8].
Considering the published results of a large number of studies and clear evidence of the effectiveness of the discussed screening strategy in neonatology, in many countries around the world, pulse oximetry has been recommended for inclusion in the screening program as an additional method for more timely detection of critical congenital heart disease in newborns in obstetric hospitals, during home births, as well as in neonatal intensive care units [11, 30, 31]. Currently, SpO2 screening is being increasingly introduced into domestic practice [32, 33].
In order to obtain an objective result of SpO2 screening, its technology requires compliance with certain requirements. SpO2 screening is carried out for all newborns in any of the neonatal departments, with the exception of those children who have previously (prenatally or postnatally) been diagnosed with congenital heart disease. Screening is performed by a doctor, nurse or the child’s parents under the supervision of medical workers. The recommendations indicate the need to obtain parental consent for screening, and this issue is usually resolved before birth [5].
During the study, it is very important to observe a number of conditions: screening is optimally performed on the 2nd day of life (decompensation of the child’s condition when the ductus arteriosus closes at a later date can lead to false results), compliance with the temperature regime is very important (in particular, the legs should be evenly warm), the assessment of indicators is carried out in the presence of a stable continuous pulse curve (for at least 3 minutes, provided there are no artifacts) [6], it is necessary to ensure the reliability of the child’s pulse rate monitored by a pulse oximeter (using palpation or auscultation) [11]. The measurement duration, according to TE Roberts et al., is 6.9 minutes (minimum -1 minute, maximum - 30 minutes, average 5 minutes) [5]. On average, according to A.L. Karpova et al., in “conditionally healthy” newborns, SpO2 in the first 12-24 hours of life in all limbs is 97.47+2.03% [34], according to BM Levesque et al. - 97.3+1.3% [35].
It is considered optimal to carry out dual-zone pulse oximetry, when SpO2 is measured on the right arm and on either leg (in the areas of blood supply above and below the ductus arteriosus), since it allows identifying not only potentially cyanotic congenital heart disease, but also congenital heart disease with ductus-dependent systemic circulation by detecting differences in saturation values blood oxygen levels between the upper and lower extremities, with lower levels in the legs [35]. On average, according to TR Hoke et al., the difference in SpO2 between the upper and lower extremities in healthy newborns, as a rule, was no more than 1% [21], with critical congenital heart disease - more than 3%. Adding an assessment of the SpO2 gradient, according to A. de WahL GraneLLi et al., can significantly increase the sensitivity of the test [18].
Based on the results of the obtained measurements of blood oxygen saturation, their interpretation is carried out (Fig. 1).
There are two possible options: positive (the child requires further examination) and negative (there is no evidence of critical congenital heart disease). The test is considered positive if any of the SpO2 measurements is less than 90%; SpO2 = 90-95% on the arm and leg (i.e., signs of potentially cyanotic congenital heart disease were detected); the difference in SpO2 on the arm and leg is more than 3% (there are signs of ductus-dependent blood circulation). The test is negative and does not need to be repeated if the SpO2 is more than 95% with a difference of less than 3% in the arms and legs [11].
Any newborn with positive test results requires a comprehensive examination to identify the causes of hypoxemia, since pulse oximetry helps to identify not only congenital heart disease, but also other secondary causes of hypoxemia [36], so the attending physician should be notified of a positive test result within 10 minutes. In the absence of other causes of hypoxemia, congenital heart disease should be excluded as soon as possible, therefore, in the next 60 minutes after the test and notification of the doctor, an urgent on-site echocardiography is indicated. If a critical congenital heart disease is detected, in all cases an urgent consultation with a cardiologist and cardiac surgeon and transfer to a cardiac surgery hospital are indicated as soon as possible. If the patient is not transportable, it is necessary to resolve the issue of palliative intervention on site. Verification of critical ductus-dependent congenital heart disease means the need to begin infusion of E1 prostaglandins
That is why, before introducing blood oxygen saturation screening in newborns in the clinic, it is necessary to resolve the issue of further clarifying the diagnosis for children with positive results. This may include on-site echocardiography, remote consultation using telemedicine, or transporting the child to a cardiac surgery hospital.
It is also important to note that it is currently considered optimal to perform a later SpO2 measurement (after 24 hours of life), as this reduces the number of false positive results. False-positive screening results are those in which a critical congenital heart defect was not identified during echocardiography due to a positive test. In table 1 shows the results of a number of studies in which the false-positive rate was less than 1% when SpO2 measurements were performed after 24 hours of life [11, 12, 34].
Thus, the implementation of blood oxygen saturation screening in newborns in different countries of the world has significantly improved the identification of critical congenital heart disease. However, at the same time, it turned out that screening for blood oxygen saturation can also be accompanied by false negative results. False-negative results are those in which the test was regarded as negative, but during echocardiography a critical congenital heart disease is detected. First of all, this diagnostic method turned out to be not quite sufficient for detecting malformations of the aorta (coarctation of the aorta, interruption of the aortic arch), since routine dual-zone pulse oximetry for the pathology under discussion often does not reveal a decrease in saturation in the lower extremities.
Thus, in a study by T. Riede et al. describe congenital heart defects missed during SpO2 screening, among which aortic malformations predominate [19]. JJ Lhost et al. recorded one false-negative result when coarctation of the aorta in combination with a ventricular septal defect was missed [30]. In a study by E. Ozalkaya et al. It has been shown that pulse oximetry screening in newborns in the first 24-48 hours after birth often misses coarctation of the aorta, the detection of which using this method does not exceed 25% [37]. L. C. Johnson et al. also recorded one false-negative screening in a child with a rupture of the aortic arch [28].
Coarctation of the aorta is a congenital segmental narrowing that can be localized at any level and accounts for from 7 [38] to 8% [2] in the structure of all congenital heart defects. In relation to the PDA, coarctation of the aorta is divided into: narrowing proximal to the origin of the PDA - “preductal” coarctation of the aorta; narrowing at the level of the origin of the PDA - “juxtaductal” coarctation of the aorta; narrowing distal to the origin of the PDA is “postductal” coarctation of the aorta.
In the vast majority of cases, coarctation is located in the area from the left subclavian artery to the PDA (“preductal” coarctation of the aorta). In such a situation, the perfusion of the lower half of the body directly depends on the functioning PDA (a variant of critical congenital heart disease), therefore, according to pulse oximetry between the right arm and either leg, a SpO2 gradient of more than 3% can be determined, pulsation in the femoral arteries can be reduced, and blood pressure will also be lower on the legs compared to the arms. At the same time, we should not forget that with a large PDA, the clinical picture of aortic coarctation may be blurred, i.e. pulsation in peripheral arteries and blood pressure may be almost normal. However, dual-zone pulse oximetry in this situation is highly likely to reveal a SpO2 gradient.
“Juxtaductal” and “postductal” coarctation of the aorta is much less common. Hemodynamics in these types of aortic coarctation is characterized by a significant increase in blood pressure to the site of narrowing and a decrease below it, therefore, pulsation in the femoral arteries in such a situation will be sharply weakened until it is absent, and blood pressure will be significantly reduced (by 10 mm Hg or more ) on the legs compared to the arms, while differences in SpO2 between the right arm and either leg may not be detectable.
Thus, in order to reduce the frequency of false-negative results, not only the isolated conduct of dual-zone pulse oximetry, but also a mandatory targeted clinical examination of any newborn child, including not only a search for signs of cardiac and respiratory failure, but also a comparative analysis of pulsation in the peripheral arteries [19] is of greatest relevance. , i.e.:
■ visual examination: assessment of general condition, color of skin and mucous membranes, frequency of respiratory movements;
■ assessment of pulsation in the radial and femoral arteries, and in the case of asymmetry, determination of blood pressure in both arms and both legs;
■ auscultation: frequency and rhythm of heart contractions, the presence of heart murmurs, splitting and accents of heart sounds.
It is advisable to assess pulsation in newborns on the radial and femoral arteries. In children, the determination of pulsation in the hands is usually assessed in the cubital fossa. At this point, the brachial artery ( a .
brachialis )
passes into the radial artery (
a . radialis [
39]. To avoid mistakes, we deliberately draw the doctor’s attention to this fact and in the future, when we talk about determining pulsation in the peripheral arteries in the arms of children in the first year of life, we mean the radial artery.
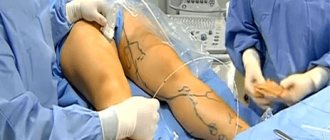
To assess pulsation on the radial arteries on the right and left, it is necessary to simultaneously place the first fingers of both hands of the researcher on the back sides of the forearms, closer to the medial epicondyles of the humerus, clasping the child’s upper limbs in the area of the elbow joints and forearms with the other fingers so as to straighten the child’s arms at the elbows joints and keep them straight during the study; press the artery to the radius and feel the pulse (Fig. 2).
To assess pulsation in the femoral arteries, it is necessary to place either the thumbs or the second and third fingers of the hands in the area of the inguinal folds parallel to the child’s body on the right and left, and feel the pulse (Fig. 3). In addition, it is also necessary to evaluate the pulse simultaneously in the right radial and left femoral arteries (Fig. 4). When assessing pulsation, it is necessary to evaluate the symmetry of the pulse, rhythm, filling, tension, and frequency. In case of a weak pulse or its asymmetry, it is necessary to perform an oscillometric measurement of blood pressure in all extremities.
The issue of routine screening measurement of blood pressure in all newborns in the arms and legs along with dual-zone pulse oximetry remains currently controversial. Thus, N. Patankar et al. recommend that all newborns between 24 and 48 hours after birth (after closure of the PDA) measure SpO2 together with blood pressure in all four limbs in order to increase the sensitivity of screening for cardiac pathology [38]. At the same time, according to KL Boelke et al., routine blood pressure measurement increases the frequency of false-positive screening results for cardiac pathology in newborns, thereby increasing the need for echocardiography, which leads to an increase in the cost of the project, but the diagnostic value of the method does not increase [ 40].
It is also important to note that a reduction in false-negative SpO2 screening results, as well as a generally improved detection of congenital heart disease in newborns, is achieved by repeated measurement of blood oxygen saturation before discharge from the maternity hospital in combination with a targeted clinical examination [23, 26-28, 37, 41] .
Conclusion
Thus, screening for congenital heart defects in the early neonatal period is one of the important ways to reduce infant mortality. The addition of pulse oximetry as an additional method of examining “conditionally healthy newborns” significantly reduces the frequency of missed congenital heart diseases. At the same time, screening of blood oxygen saturation does not exclude a thorough targeted medical examination of the newborn for cardiac pathology, since pulse oximetry in rare cases does not reveal critical heart defects, which primarily include aortic developmental anomalies.
The addition of routine blood pressure measurement to screening for congenital heart disease in the early neonatal period in all newborns has not yet received convincing evidence of its effectiveness and urgent need. However, this issue requires further research aimed at assessing the sensitivity and specificity of the method in relation to more timely detection of congenital heart disease in combination with pulse oximetry. Measurement of blood pressure in all extremities in the event of detection of weak or asymmetrical pulsation in the peripheral arteries should be carried out without fail and immediately immediately after identifying symptoms suspicious of congenital heart disease.
Newborns with suspected congenital heart disease according to screening studies (targeted clinical examination, pulse oximetry, blood pressure measurement in the arms and legs) cannot be discharged home from the neonatal department without verification of the diagnosis, i.e. without echocardiography. Newborns with detected congenital heart disease cannot be discharged home without consulting a cardiologist and deciding on the tactics for further observation.
The team of authors expresses gratitude to A.V. Marasina for the original drawings presented in the article.
LITERATURE
1. Starodubov V.I., Sukhanova L.P. Reproductive problems of demographic development of Russia. M.: Healthcare Manager, 2012. 320 p.
2. Bockeria L.A., Gudkova R.G. Cardiovascular surgery. year 2014. Diseases and congenital anomalies of the circulatory system. M.: NTsSSKh im. A.N. Bakuleva, 2014. 220 p.
3. Volodin N.N. (ed.) Neonatology: national guide. M.: GEOTAR-Media, 2009. 848 p.
4. Neonatal screening to identify critical congenital heart defects: guidelines (No. 12) / compiled by: M.A. Shkolnikova, E.L. Bockeria, E.A. Degtyareva, V.N. Ilyin, E.S. Sharykin. M.: M-Art, 2012. 36 p.
5. Roberts TE, Barton PM, Auguste PE, Middleton LJ et al. Pulse oximetry as a screening test for congenital heart defects in newborn infants: a cost-effectiveness analysis // Arch. Dis. Child. 2012. Vol. 97, N 3. P. 221-226. doi: 10.1136/archdischild-2011-300564.
6. Zuppa A.A., Riccardi R., Catenazzi P., D'Andrea V. et al. Clinical examination and pulse oximetry as screening for congenital heart disease in low-risk newborn // J. Matern. Fetal Neonatal Med. 2015. Vol. 28. P. 7-11.
7. Senatorova A.S., Gonchar M.A., Pugacheva E.A. The role of pulse oximetry as a screening method for detecting cardiac pathology in newborns. Modern cardiology and cardiac surgery - the path from problems to solutions // Materials of a scientific-practical conference with international participation, dedicated to the memory of cardiac surgeon L.N. Sidorenko (Sudak, October 7-8, 2013). Sudak, 2013. P. 246.
8. Eckersley L., Sadler L., Parry E., Finucane K. et al. Timing of diagnosis affects mortality in critical congenital heart disease // Arch. Dis. Child. 2015 Jun 30. pii: archdischild-2014-307691. doi: 10.1136/archdischild-2014-307691.
9. Lundsgaard C., Van Slyke DD Cyanosis // Medicine. 1923. Vol. 2. P. 1-76.
10. Lees MH Cyanosis of the newborn infant: recognition and clinical evaluation // J. Pediatr. 1970. Vol. 77. P. 484-498.
11. Kemper AR, Mahle WT, Martin GR, Cooley WC et al. Strategies for implementing screening for critical congenital heart disease // Pediatrics. 2011. Vol. 128. P. 1259-1267.
12. Mahle WT, Newburger JW, Matherne GP, Smith FC et al. Role of pulse oximetry in examining newborns for congenital heart disease: a scientific statement from the AHA and AAP // Pediatrics. 2009. Vol. 124. P. 823-836.
13. Antonov A.G., Ryndin A.Yu. Transcutaneous blood gas monitoring: clinical guide / ed. E.N. Baibarina. M., 2010. 24 p.
14. Bickler PE, Feiner JR, Severinghaus JW Effects of skin pigmentation on pulse oximeter accuracy at low saturation // Anesthesiology. 2005. Vol. 102. P. 715-719.
15. Mahle WT, Martin GR, Beekman RH III, Morrow WR et al. Endorsement of health and human services recommendation for pulse oximetry screening for critical congenital heart disease // Pediatrics. 2012. Vol. 129. P. 190-193.
16. Ramjattan K., Allen PJ Pulse oximetry screening for critical congenital heart disease in the newborn // Pediatr. Nurs. 2013. Vol. 39, N 5. P. 250-253.
17. Reich JD, Miller S, Brogdon B, Casatelli J et al. The use of pulse oximetry to detect congenital heart disease // J. Pediatr. 2003. Vol. 142. P. 268-272.
18. De Wahl Granelli A., Wennergren M., Sandberg K. et al. Impact of pulse-oximetry screening on the detection of duct-dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns // BMJ. 2009. Vol. 338. P. a3037.
19. Riede FT, Worner C, Dahnert I, Mockel A et al. Effectiveness of neonatal pulse oximetry screening for detection of critical congenital heart disease in daily clinical routine: results from a prospective multicenter study // Eur. J. Pediatr. 2010. Vol. 169, N 8. P. 975-981.
20. Zhao Q., Ma X., Ge X., Liu F. et al. The neonatal congenital heart disease screening group. pulse oximetry with clinical assessment to screen for congenital heart disease in neonates in China: a prospective study // Lancet. 2014. Vol. 384. P. 747-754.
21. Hoke TR, Donohue PK, Bawa PK, Mitchell RD et al. Oxygen saturation as screening test for critical congenital heart disease: a preliminary study // Pediatr. Cardiol. 2002. Vol. 23. P. 403-409.
22. Karpova A.L., Bockeria E.L., Nikolaeva T.N., Spivak E.M. and others. Screening of blood oxygen saturation as a method for identifying congenital heart defects in newborns: modern approaches, problems, opinions // Children's diseases of the heart and blood vessels. 2015. No. 4. pp. 29-37.
23. Richmond S., Reay G., Abu Harb M. Routine pulse oximetry in the asymptomatic newborn // Arch Dis. Child. Fetal Neonatal Ed. 2002. Vol. 87. P. 83-88.
24. De Wahl Granelli A., Mellander M., Sunnegardh J., Sandberg K. et al. Screening for duct-dependent congenital heart disease with pulse oximetry: a critical evaluation of strategies to maximize sensitivity // Acta Paediatr. 2005. Vol. 94. P. 1590-1596.
25. Meberg A., Brugmann-Pieper S., Due R. Jr., Eskedal L. et al. First day of life pulse oximetry screening to detect congenital heart defects // J. Pediatr. 2008. Vol. 152. P. 761-765.
26. Sendelbach DM, Lai S, Jackson GJ, Fixler D, et al. Pulse oximetry (POx) screening of term and late preterm neonates at 4 hours postnatal (PN) to detect cyanotic congenital heart disease (CCHD). Presented at: Pediatric Academic Societics. Honolulu, Hawaii, May 2-6, 2008. Abstract E-PAS2008:5896.2.
27. Ewer AK Evidence for CCHD screening and its practical application using pulse oximetry // Early Hum. Dev. 2014. Vol. 90. P. 19-21. doi: 10.1016/S0378-3782(14)50006-0.
28. Johnson LC, Lieberman E., O'Leary E., Geggel RL Prenatal and newborn screening for critical congenital heart disease: findings from a nursery // Pediatrics. 2014. Vol. 134, N 5. P. 916-922. doi:10.1542/peds.2014-1461.
29. Therell B., Lorey F., Eaton R. et al. Impact of expanded newborn screening: United States, 2006 // MMWR Morb. Mortal. Wkly Rep. 2008. Vol. 57, N 37. P. 1012-1015.
30. Lhost JJ, Goetz EM, Belling JD, van Roojen WM et al. Pulse oximetry screening for critical congenital heart disease in planned out-of-hospital births // J. Pediatr. 2014. Vol. 165, N 3. P. 485-489. doi: 10.1016/j.jpeds.2014.05.011.
31. Goetz EM, Magnuson KM, Eickhoff JC, Porte MA et al. Pulse oximetry screening for critical congenital heart disease in the neonatal intensive care unit // J. Perinatol. 2021. Vol. 36. P. 52-56.
32. Karpova A.L., Spivak E.M., Pykhantseva A.N. Diagnostic value of determining the value of oxygen saturation in full-term newborns // Perm Med. magazine 2014. T. 33, No. 5. P. 6-10.
33. Zharkova I.Yu., Ushakova S.A., Petrushina A.D. Regional experience in introducing dual-zone pulse oximetry as a screening test for identifying critical congenital heart defects in newborns // Materials of the VIII All-Russian Educational Congress “Anesthesia and Resuscitation in Obstetrics and Neonatology”. M., 2015. pp. 58-59.
34. Karpova A.L., Spivak E.M., Pykhantseva A.N. Bockeria E.L. and others. Pulse oximetry as a method of early neonatal screening for the presence of critical heart defects in children // Neonatology: news, opinions, training. 2015. No. 4 (10). pp. 68-72.
35. Levesque BM, Pollack P., Griffin BE, Nielsen HC Pulse oximetry: what's normal in the newborn nursery? // Pediatr. Pulmonol. 2000. Vol. 30. P. 406-412.
36. Kemper AR, Knapp AA, Metterville DR, Comeau AM et al. Weighing the evidence for newborn screening for hemoglobin H disease // J. Pediatr. 2011. Vol. 158, N 5. P. 780-783.
37. Ozalkaya E., Akdag A., Sen I., Comert E. et al. Early screening for critical congenital heart defects in asymptomatic newborns in Bursa province // J. Matern. Fetal Neonatal Med. 2015. Vol. 22. P. 1-3.
38. Patankar N., Fernandes N., Kumar K., Manja V., Lakshminrusimha S. Does measurement of four-limb blood pressures at birth improve detection of aortic arch anomalies? // J. Perinatol. 2016. P. 1-5. doi: 10.1038/jp.2015.203 (in press).
39. Atlas of human anatomy: in 4 volumes. T. 3. / Sinelnikov R.D., Sinelnikov Y.R., Sinelnikov A.Ya. M.: Publisher Umerenkov, 2014. 216 p.
40. Boelke KL, Hokanson JS Blood pressure screening for critical congenital heart disease in Neonates // Pediatr. Cardiol. 2014. Vol. 35, N 8. P. 1349-1355.
41. US Preventive Services Task Force. Universal screening for hearing loss in newborns: US Preventive Services Task Force recommendation statement // Pediatrics. 2008. Vol. 122, N 1. P. 143-148.
How to make a diagnosis
The diagnosis takes into account:
- clinical symptoms - cyanosis of the child’s lips and face, shortness of breath when moving, changes in rhythm, trembling of the chest when palpating the heart area, the presence of a visible venous network under the skin, pulsating vessels;
- phonocardiography and ECG - clarify the localization of damage to the heart chambers;
- Ultrasound and Dopplerography - allow you to establish blood flow in different phases of myocardial contraction, direction, volume of return, degree of adaptability of the ventricles and atria.
What is recommended for parents
Listening to a heart murmur in a child should cause concern for parents. It is necessary to bring the diagnosis to full confidence in the causes. If necessary, consult with a cardiac surgeon several times.
Only a specialist can recommend the correct observation and treatment, and plan a specific date for the operation. If the risk of surgical treatment in terms of complications exceeds the possibility of the expected development of decompensation, then parents are informed about this.
The child must be registered at the dispensary and examined at least once a quarter. The baby needs to be protected from infections, colds, and the sufficiency of vitamins in the diet must be monitored. If necessary, the doctor prescribes supportive therapy and exempts the student from physical education.
Functional noise does not require significant restrictions. You should talk to your pediatrician about the possibility of playing sports. Regular physical exercise is not contraindicated.
How healthy a child grows up depends on the adults. Given the current widespread trend towards a reduction in specialized healthcare services, parents should be prepared for paid consultations.
Therapeutic tactics for critical congenital heart disease
IPN with ductus-dependent circulation:
— oxygen therapy is not indicated;
- intravenous titration of prostaglandin E1 at an initial dose of 0.02–0.05 mcg/kg/min; when the effect is achieved, the dose can be reduced to 0.01–0.025 mcg/kg/min;
— correction of acid-base status;
— if mechanical ventilation is necessary, it is carried out with small concentrations of O2;
— temperature comfort;
— sedative therapy;
- infusion therapy with a moderate positive balance
If the leading syndrome is heart failure or its combination with arterial hypoxemia (AH), then to the above it is necessary to add cardiotonic support, diuretics, and transport the patient to the cardiac surgery department when the condition stabilizes during therapy; continue therapy during transportation.
If a ductus-dependent defect is suspected, the diagnosis is made as follows: congenital heart disease (ductus-dependent pulmonary circulation), increasing arterial hypoxemia or congenital hypoxemia (ductus-dependent systemic circulation), circulatory failure (degree).
Making such a diagnosis does not require anatomical verification; it implies the abolition of oxygen and the beginning of titration of a drug of the PGE1 group after establishing the diagnostic group.
Preparations of the PGE1 group have different names depending on the manufacturer: prostin VR, alprostan, vazaprostan. The active ingredient is alprostadil. 1 ampoule contains 100 mcg of alprostadil. An average dose of 0.02–0.05 mcg/kg/min is used.
The effect of the drug appears almost instantly. When the effect is achieved (increasing oxygen saturation to 85–90% or relieving symptoms of heart failure), the dose can be reduced to 0.01–0.025 mcg/kg/min.
The drug has side effects, the most dangerous of which is apnea. When using the drug you need to be prepared for mechanical ventilation. Other side effects: skin flushing, fever, diarrhea, convulsions, which can be relieved by reducing the dose or discontinuing the drug.
Therapy of heart failure with “simple” TMA:
— careful observance/correction of water and electrolyte balance. Volume of liquid + food = 70–80 ml/kg/day;
— maintaining body temperature — 36 °C;
- exclude additional oxygen!
— prostaglandin E1 — 0.02–0.05 mcg/kg/min;
— dopamine, dobutamine — 2–3 mcg/kg/min;
— balloon atriseptostomy according to Rashkind.
Tactics for congenital heart disease combined with obstruction of left ventricular outlet
Treatment of HF in newborns with coarctation of the aorta and critical valvular aortic stenosis is challenging in the presence of isolated defects. It may include the following activities:
- prostaglandin E1;
- diuretics and digoxin - when combined with VSD (short-term!);
- avoiding oxygen inhalation;
- urgent surgery or dilatation of stenosis. The most correct decision is immediate transportation to a cardiac surgery center.
Such patients are transferred to mechanical ventilation in order to reduce the burden of breathing. The goal of intensive care for critical coarctation of the aorta is to maintain adequate coronary and systemic perfusion and therefore includes inotropic support with epinephrine (0.03–0.1 mcg/kg/min) or dopamine (5–10 mcg/kg/min). It is necessary to maintain the water balance very accurately.
Tactics in patients with tetralogy of Fallot
The most serious complication of this congenital heart disease—dyspnea-cyanotic attack—is based on a sharp spasm of the outflow tract of the right ventricle.
To reduce hypoxemia and relieve spasm of the outflow tract of the right ventricle (RV), it is necessary to relieve the child’s anxiety with any hypnotic (except ketamine, which can itself cause spasm of the outflow tract of both ventricles). Insufflation of oxygen through a mask or nasal cannula is also mandatory.
The administration of soda (2–3 ml/kg) is indicated in order to reduce the acidosis that inevitably accompanies hypoxia.
Pathogenetic treatment of a dyspnea-cyanotic attack is the administration of beta-blockers - propranolol intravenously for 5-10 minutes at a dose of 0.02-0.1 mg/kg or esmolol (breviblok, Baxter), which is an ultra-short-acting drug and as a result can be administered not only as a bolus (at a dose of 500 mcg/kg), but also as an infusion (50–200 mcg/kg/min).
Cardiac glycosides and any catecholamines are contraindicated, as they have a beta-mimetic effect and cause spasm of the excretory tract of the pancreas.
If conservative measures are ineffective, transfer of the child to mechanical ventilation and emergency surgery of systemic-pulmonary anastomosis are indicated.