Biochemical markers in the diagnosis and evaluation of ACS
- Biochemical markers of myocardial necrosis
- Natriuretic peptides
- Biochemical markers of inflammation
- Biochemical markers of ischemia
NACBLM Guidelines for the Use of Biochemical Markers for the Diagnosis of Myocardial Infarction (2008)
- Biomarkers of myocardial necrosis should be measured in all patients with a clinical picture consistent with ACS.
- If MI is suspected, the clinical picture (history, physical examination) and ECG findings should be assessed in conjunction with biomarker values.
- Cardiac troponin is the marker of choice for diagnosing myocardial infarction. If its measurement is not possible, an acceptable alternative is measuring the QC MV.
- Blood for testing should be collected upon patient admission to the hospital. The timing of subsequent serial specimen collection depends on the clinical circumstances. In most cases, blood should be collected on admission and 6–9 hours later.
- If there is data in the medical history confirming ACS, the following deviations indicate myocardial necrosis, characteristic of MI, if:
- The maximum concentration of cardiac troponin, exceeding the 99th percentile, is detected in at least one case during the first day after the clinical manifestation of ACS.
- The maximum concentration of CK MB exceeds the 99th percentile in 2 consecutive samples.
- In patients presenting within 6 hours of symptom onset, an early marker of myocardial necrosis may be measured in addition to cardiac troponin. The most studied marker for this purpose is myoglobin.
- The question of the specificity of cardiac troponins should not be linked to the question of the mechanism of injury (eg, MI or myocarditis).
- Total CK, CK activity of MB, AST, LDH, GBD are not recommended to be measured as a biomarker in diagnosing MI.
Recommendations for the use of biochemical markers for risk stratification
- Among patients suspected of having ACS, early risk stratification should be performed based on a comprehensive assessment of symptoms, clinical examination, ECG findings, and biomarker measurements.
- Cardiac troponin is the marker of choice for risk stratification and should be measured, if possible, in all patients with suspected ACS. In patients with a clinical picture consistent with ACS, a maximum (peak) concentration greater than the 99th percentile should be considered an indication of an increased risk of death and recurrent ischemic events.
- Blood for testing should be collected upon patient admission to the hospital. The timing of subsequent serial specimen collection depends on the clinical circumstances. In most cases, blood should be collected upon admission and 6–9 hours later.
Source: “Myocardial Infarction Redefined–A Consensus Document of The Joint European Society of Cardiology/American College of Cardiology J. Am. Coll. Cardiol.36, 959–962, 2000 (American Heart Ass., 2008).
Markers of myocardial damage It is now considered proven that the cause of developing myocardial infarction (MI) in more than 80% of cases is coronary artery thrombosis, which usually occurs at the site of an atherosclerotic plaque with a damaged surface. As they die, cardiomyocytes release a huge amount of biologically active substances into the bloodstream, including intracellular enzymes, including AST, CK, LDH, as well as a number of specific proteins, such as myoglobin, troponins Ti I. The determination of some of them is used in clinical practice as markers of myocardial damage (myocardial markers).
Biomarkers of myocardial necrosis
| Biomarker | Molecules mass, D | Specificity for myocardium | Advantages | Flaws | Boost Duration |
| Myoglobin | 18000 | No | High sensitivity and PV (-) | Low specificity for muscle damage and renal failure | ↑ after 1-3 hours and lasts 12-24 hours |
| Heart fatty acid binding protein (h-FABP) | 15000 | + | Early detection of MI | Low specificity for muscle damage and renal failure | ↑ after 1-3 hours and lasts 18-30 hours |
| KK-MB (activity from total KK) | 85000 | +++ | Detects recurrent MI | Low specificity for muscle damage | ↑ after 3-4 hours and lasts 24-36 hours |
| Mass concentration of KK-MV | 85000 | +++ | Early detection of MI | Lack of availability | ↑ after 3-4 hours and lasts 18-30 hours |
| Cardiac troponin I | 37000 | ++++ | Detection of MI up to 14 days, high specificity, indicator for risk ranking | Does not act as an early marker of myocardial necrosis | ↑ after 3-4 hours and lasts 10-14 days |
| Cardiac troponin I | 25000 | ++++ | Detection of MI up to 7 days, high specificity, indicator for risk ranking | Does not act as an early marker of myocardial necrosis | ↑ after 3-4 hours and lasts 4-7 days |
Dynamics of changes in myocardial markers during myocardial infarction MI is a dynamic process, the development of which occurs over time. An increase in the activity of myocardial enzymes and the concentration of myocardial proteins in the blood plasma that accompanies MI is a transient phenomenon and has its own dynamic patterns.
| Parameter | Start ↑ activity, h | Maximum ↑ activity, h | Return to normal, days | Magnification factor, times |
| AST | 4-6 | 24-48 | 4-7 | 2-20 |
| QC | 2-4 | 24-36 | 3-6 | 3-30 |
| KK-MV | 2-4 | 12-18 | 2-3 | up to 8 |
| LDH | 8-10 | 48-72 | 6-15 | up to 8 |
| LDH 1 | 8-10 | 30-72 | 7-20 | up to 8 |
| Myoglobin | 0,5-2 | 6-12 | 0,5-1 | up to 20 |
| Troponin T | 3,5-10 | 12-18 (and 3-5 days) | 7-20 | up to 400 |
The role of myocardial markers in the diagnosis of myocardial infarction Among patients admitted to the hospital with heart pain, only 10-15% have MI. The need to diagnose MI in the early stages is dictated by the fact that thrombolytic therapy in the first 2-6 hours reduces early mortality in patients by an average of 30%; therapy started after 7-12 hours – only by 13%; therapy started after 13-24 hours does not reduce the mortality rate. In 30% of MI cases, ECG changes may be absent or not specific enough to make a diagnosis. It is in these difficult-to-diagnose cases that determination of myocardial markers in the blood can confirm or refute the diagnosis of MI.
The term “ myocardial markers ” implies that the enzymes and proteins used for diagnosis originate from the heart muscle. In reality this is not the case. Almost all of them are contained in other tissues, and an increase in their levels can be the result of not only myocardial damage. For many years, determination of blood activity of AST, CK and its MB isoenzyme and LDH have been used as markers for diagnosing MI. It’s just that other markers were not available to most laboratories. Over the past two decades, numerous clinical studies have been conducted to evaluate the effectiveness and safety of evaluation and treatment of patients with MI. The research results served as the basis for international clinical guidelines for the management of patients with MI. The recommendations indicate that myocardial proteins (troponins T and I) have almost absolute specificity for myocardial tissue, as well as high sensitivity, which makes it possible to detect even microscopic areas of myocardial damage. Troponin testing is mandatory for patients with suspected MI. Cardiac troponins should be determined upon patient admission and again after 6-9 hours. Further studies are carried out after 12-24 hours if the results of previous studies were negative and the clinical suspicion of MI is high. In case of relapse of myocardial infarction, determination of troponin levels is resumed 4-6 hours after the onset of relapse and then again after 6-9 hours. Determination of the level of myoglobin in the blood serum and/or the activity of the MB fraction of CK should be carried out with the recent (less than 6 hours from the onset of acute chest pain) appearance of clinical symptoms (as early markers of MI) and in patients with repeated ischemia after a recent (less than 2- x weeks) MI to detect relapse. In case of recurrent myocardial infarction, the importance of myoglobin and CK-MB studies increases, since troponin levels may remain elevated from the initial episode of myocardial necrosis. Patients with chest pain and troponin T(I) laboratory results above the upper reference limit should be considered to be experiencing “myocardial injury.” They should be hospitalized and intensively monitored to reduce the risk associated with this injury.
Clinical recommendations clearly indicate that the study of the activity of AST, CK, CK-MB, LDH and its isoenzymes should not be used to diagnose MI.
Changes in myocardial markers in other diseases Increased CK activity in the blood is not specific for MI. In some cases, CK is increased in myocarditis and myocardial dystrophies. A significant increase can be observed with traumatic injuries to skeletal muscles and diseases of the muscular system. Thus, with progressive muscular dystrophy, CK activity can increase 50 times. High CK activity is observed in a wide variety of central nervous system disorders: schizophrenia, manic-depressive psychosis, syndromes caused by psychotropic drugs. CK activity increases with hypothyroidism, as well as in the presence of various tumors. An increase in myoglobin concentration is observed when skeletal muscles are damaged, because is contained in them in significant quantities, as well as in cases of thermal burns and secondary toxic myoglobinuria. Non-coronary diseases of the heart muscle (myocarditis, trauma, cardioversion), septic shock may be accompanied by an increase in the level of troponin T, but the dynamics of change characteristic of MI are absent.
Biochemical markers of myocardial damage In ACS without ST segment elevations, cardiac troponins T and I, as markers of myocardial necrosis, due to their greater specificity and reliability, are preferable to the traditionally determined CPK and its CF form. Elevated levels of troponin T or I reflect necrosis of myocardial cells. In the presence of other signs of myocardial ischemia (retrosternal pain, ST segment changes), such an increase should be called MI.
Determination of troponins allows detecting myocardial damage in approximately a third of patients who do not have an increase in CF-CK.
To detect or rule out myocardial injury, repeat blood draws and measurements are necessary within 6 to 12 hours of admission and after any episode of severe chest pain. Changes in the content of various markers of myocardial necrosis over time in relation to a painful attack are presented in Fig. 1. Myoglobin is a relatively early marker, while increases in MB-CPK and troponin appear later. Troponins may remain elevated for 1–2 weeks, making it difficult to diagnose recurrent necrosis in patients with recent MI.
Biochemical markers of myocardial necrosis and changes in their content in the blood after a painful attack
A – early release of myoglobin or isoforms of the CPK MB fraction B – cardiac troponin after “classical” acute MI C – CPK MB fraction after acute MI D – cardiac troponin after a microinfarction
Source : Myocardial Infarction Redefined – A Consensus Document of The Joint European Society of Cardiology/American College of Cardiology Committee for the Redefinition of Myocardial Infarction. JAmerCollCardiol 2000; 36:959–1062
Natriuretic peptides The relationship between NP levels and outcomes in patients with ACS has been proven. After the onset of MI, the concentration of NP increases rapidly and reaches a peak after 24 hours. The peak concentration is proportional to the size of the infarction. With the development of HF in patients with ACS, the second peak of maximum NP concentrations can be detected after 5 days, which reflects unfavorable ventricular remodeling. Elevated NP concentrations indicate a higher likelihood of death or HF, independent of other prognostic factors, including left ventricular ejection fraction. Currently, the close relationship between the severity of cardiac dysfunction (primarily LV) and the content of NPs in plasma has been fully proven, which allows us to recommend determining the concentration of these peptides as a “laboratory test” for CHF. The most fully characterized are the N-terminal atrial NUP (ANP), the brain NUP (BNP), and its predecessor, the N-terminal brain NUP (NT-pro BNP).
Source: Russian national recommendations VNOK and OSSN for the diagnosis and treatment of CHF (second revision), 2007.
Advantages of determining NT–pro BNP over BNP
| BNP | NT-pro BNP |
| Hormonally active | Hormonally inactive |
| After release, it is cleared from the bloodstream T1/2~21 min | After secretion, we recommend determining it in the blood within several days, T1/2~70–120 min |
| The presence of a circadian rhythm, the level changes rapidly depending on the condition and function of the left ventricle, reflects the state of the myocardium at the time of determination | Not subject to circadian rhythms. The cumulative level of NT-pro BNP reflects myocardial function as a whole, correlating with the degree of cardiac dysfunction (the most objective assessment of disease stage and prognosis) |
| Relatively low plasma levels do not allow diagnosing heart failure in the early stages | High plasma levels allow detection of early myocardial dysfunction (diastolic dysfunction) |
| Low stability in plasma | High stability in plasma - up to 7 days at room temperature and 21 days at 4°C |
| BNP level depends on therapy, in particular with Natrecor | Does not depend on the therapy |
Source: National Institute of Neurological Disorders and Stroke Recombinant, 2007. Brain Injury Indication
Diagnostic value of determining the NT- pro BNP
- A highly specific marker of the presence and severity of myocardial dysfunction;
- Reliable predictor of prognosis in heart failure;
- Prognostic criterion for the severity of pathological remodeling of the heart after myocardial infarction;
- Can be used to monitor the therapeutic effect of therapy with angiotensin-converting enzyme inhibitors (ACEIs).
Potential uses of NT– pro BNP
- Development of an individual course of rehabilitation after acute myocardial infarction;
- Monitoring of patients with angina pectoris (parallel determination of Troponin);
- Preoperative monitoring;
- Early diagnosis of heart failure in patients with diabetes and thyroid diseases;
- Identification of cardiotoxic drugs (oncology, psychiatry);
- Use in gynecological and obstetric practice.
Monitoring and treatment of patients with heart failure (HF) by monitoring NT - pro BNP Measurement of NT-pro BNP is recommended for the initial screening of patients with dyspnea and suspected HF before echocardiography examination, but does not replace it for diagnosis and assessment of the condition. However, NT-pro BNP cannot be the only study, but must be interpreted taking into account the clinical picture. Screening using NT-pro BNP is indicated in patients at high risk of HF caused by coronary artery disease, ECG Q-waves, or left bundle branch block. Natriuretic peptide measurement is the gold standard for treatment monitoring and control, facilitating optimal use of standard therapy and reducing adverse clinical outcomes. Targeted therapy for heart failure is aimed at a 30% reduction in NT-pro BNP levels. In the absence of an initial level, the goal of therapy is an NT-pro BNP level below 400 pg/ml. With a normal response to treatment, NT-pro BNP measurements should be carried out in patients with CHF at intervals of 3 months. When clinical signs and/or the NT-pro BNP level alone (increase of more than 30%) indicate decompensation, adjustment of therapy and further repeated measurement of NT-pro BNP at intervals of 1-2 weeks are required. Patients who do not achieve a 30% reduction in NT-pro BNP levels during therapy are at high risk of recurrent HF or death. An NT-pro BNP level of about 5000 pg/ml is a predictor of mortality within 76 days, and at a level of about 1000 pg/ml – within a year.
Dynamics of NT- pro BNP content in patients with ACS According to the GUSTO-IV Study, conducted in patients with ACS without ST segment elevation (n = 6806), acute ischemia provokes the production of natriuretic peptides in the first 24 hours, and approximately 80 hours after the onset of pain. NT-pro BNP level is normalized. In case of poor prognosis in patients with exacerbation of coronary disease, NT-pro BNP may remain elevated for 12 weeks. A persistent increase, regardless of the level of troponin T, heart rate, creatinine clearance, or ST segment depression, is a predictor of an unfavorable outcome. Measurement of NT-pro BNP levels is used for risk stratification both during the acute phase and for subsequent long-term monitoring. After episodes of ACS, patients with NT-pro BNP levels ≥ 1000 pg/ml are at high risk and should be considered for an early invasive strategy. Patients with low NT-pro BNP levels and normal troponin levels are at low risk and should be considered for conservative therapy. Source: James Stefan Ketal, Circulation 2003;108:275-81
NT-pro BNP in stable coronary artery disease Myocardial ischemia is a potential stimulus for the production of NT-pro BNP, the content of which independently correlates with the severity of coronary artery disease and the size of the ischemic area and is an indicator of the absence or presence of left ventricular (LV) dysfunction. With an NT-pro BNP value of ≤100 pg/ml, the likelihood of a diagnosis of LV dysfunction is significantly reduced, and with an NT-pro BNP ≥ 500 pg/ml, the likelihood of a diagnosis of LV dysfunction increases. The risk associated with an increase in NT-pro BNP in stable CAD is independent of age, gender, ventricular dysfunction, severity of ischemia, renal function and CRP levels. Additional measurements at intervals of 12–18 months are useful to assess prognosis. NT-pro BNP is an important predictor of subsequent HF events and death in stable CAD.
NT-pro BNP in diseases of the heart valves The concentration of NT-pro BNP is increased in patients with lesions of the heart valves: an increase in the level reflects the severity of the disease and symptomatic status. NT-pro BNP levels decrease after valve transplantation in patients with aortic stenosis and after successful valvuloplasty in patients with mitral stenosis. NT-pro BNP provides prognostic information for survival in patients with aortic stenosis and aortic regurgitation.
NT-pro BNP in acute pulmonary embolism and PPH Since BNP and NT-pro BNP levels are elevated not only in LV dysfunction, but also in isolated acute or chronic RV overload, they can be considered biomarkers of “cardiovascular dyspnea”, but only as an indicator of cardiac congestion insufficiency. The level of natriuretic peptides closely correlates with ECG and hemodynamic parameters and pancreatic overload. Assessment of BNP and NT-pro BNP levels is included in the prognostic assessment of patients with pulmonary embolism and PE: Elevated levels of peptides in PLH and PE are markers of increasing mortality. A low level indicates a favorable prognosis.
NT-pro BNP and ECG (heart structure and correlation with function) NT-pro BNP level correlates with ECHCG findings: LVEF impairment, severe diastolic dysfunction, RV volume and overload pressure and valvular damage. The NT-pro BNP level within the “normal” range has a high negative predictive value for excluding systolic dysfunction and controls severe diastolic dysfunction. The combined use of NT-pro BNP levels and echocardiography should be considered when the clinical risk associated with normal NT-pro BNP levels is low.
NT-pro BNP in pediatric congenital heart disease In healthy children, NT-pro BNP levels are elevated at birth, decreasing in the first days of life, and remain relatively stable or slightly decrease throughout childhood and adulthood. In children with heart disease, NT-pro BNP levels are elevated and correlate with the severity of the disease. The NT-pro BNP marker can be used to identify children and adolescents with signs or symptoms of heart disease and to differentiate between cardiac and non-cardiac etiologies. NT-pro BNP level predicts outcome in children and adolescents with chronic DCM and in children undergoing surgery for congenital heart disease.
NT-pro BNP and high-risk groups: diabetes and hypertension NT-pro BNP levels increase with the development of hypertension, cardiac dysfunction, or kidney damage and can be used to screen for LV dysfunction in diabetes to predict silent myocardial ischemia, cardiovascular outcomes, and mortality in patient data. Circulating levels of peptides reflect cardiac structure and function in hypertensive patients and help predict hypertension-related morbidity and mortality.
Clinical biochemical blood test
Blood chemistry
- a laboratory research method that reflects the functional state of organs and systems of the body.
Biochemical blood test indicated
, even if the person has no complaints. By changes in the chemical composition of the blood, it is possible to determine which organ is functioning abnormally, which may indicate the development of the disease and the need for urgent treatment.
Markers of myocardial damage and heart failure:
Myoglobin
- a hemoprotein found in large quantities in skeletal muscle and in small quantities in cardiac muscle. Takes part in tissue respiration. During myocardial infarction, the concentration of myoglobin in the blood increases after 2 hours, but this is a nonspecific marker of myocardial infarction, since the heart muscle contains a small amount of myoglobin. This marker is used in the diagnosis of myocardial infarction in combination with other biochemical tests.
Troponin I
– a protein, a specific marker of damage to the heart muscle, used in the diagnosis of myocardial infarction. An increase in troponin I is observed within 4–6 hours after the attack. This test allows you to diagnose even microscopic areas of myocardial damage.
KFK-MV
– creatine phosphokinase-MB
is an isoenzyme of creatine phosphokinase, characteristic of cardiac muscle tissue.
Determining the activity of CPK-MB- is of great importance in diagnosing myocardial infarction and monitoring the post-infarction state, allowing one to assess the volume of the lesion and the nature of the recovery processes. The diagnosis of acute myocardial infarction is also confirmed by the observation of the characteristic dynamics of the indicator; serial determination of CPK-MB with an interval of 3 hours over a 6-9 hour period with nonspecific ECG changes is more informative than a single measurement. The level of CPK-MB can be measured both in weight terms and in activity units. Currently, for the diagnosis of myocardial infarction, it is preferable to determine not the activity, but the mass of CPK-MB.
To adequately assess the relationship between the concentration of CPK-MB and the total activity of creatine phosphokinase, the calculated relative index RI = CPK-MB (µg/l) / CPK total was introduced. (U/l) x 100 (%). Damage to the heart muscle is characterized by RI > 2.5 - 3%.
Heart failure marker ProBNP
is a precursor of brain natriuretic peptide - BNP (BNP - brain natriuretic peptide).
The name “brain” is due to the fact that it was first identified in the brain of animals. In humans, the main source of ProBNP is the ventricular myocardium and is released in response to stimulation of ventricular cardiomyocytes, such as myocardial distension in heart failure. ProBNP is cleaved into two fragments: the active hormone BNP and N - the terminal inactive peptide NT - proBNP
.
Unlike BNP, NT-proBNP
has a longer half-life, better in vitro stability, less biological variability and higher blood concentrations.
The listed features make this indicator convenient for use as a biochemical marker of chronic heart failure. Determining the level of NT - proBNP
in blood plasma helps to assess the severity of chronic heart failure, predict the further development of the disease, and also evaluate the effect of therapy.
Negative predictive value of the test greater than 95% - that is, normal NT-proBNP
with a high probability allows to exclude heart failure (for example, in cases of shortness of breath due to a sharp exacerbation of chronic obstructive pulmonary disease, or edema not associated with heart failure).
It should be noted that NT-proBNP
should not be used as the sole criterion.
LABORATORY DIAGNOSTICS OF MYOCARDIAL INFARCTION: MODERN REQUIREMENTS D.S.Benevo…
Home \ Publications \ LABORATORY DIAGNOSTICS OF MYOCARDIAL INFARCTION: MODERN REQUIREMENTS D.S. Benevolensky
LABORATORY DIAGNOSTICS OF MYOCARDIAL INFARCTION: MODERN REQUIREMENTS D.S. Benevolensky Representative office (Denmark)
Summary : Laboratory methods today play a key role in the diagnosis of myocardial infarction.
However, all currently existing biomarkers of cardiomyocyte necrosis are not ideal. Troponins I and T are the most sensitive and specific. Myoglobin is the least specific, but it appears in the blood more quickly after a heart attack. The third recommended biomarker, creatine kinase MB, has intermediate characteristics. To accurately determine the upper limit of normal for biomarkers, the analytical method must have high sensitivity and specificity. As a result of manufacturers using different sets of antibodies in immunochemical reactions and the lack of clear standardization, biomarker concentrations measured on different analyzers often do not match. Each analyzer has its own reference ranges. This applies primarily to troponin. In cardiology, speed of diagnosis is very important, so testing directly at the patient’s bedside is becoming increasingly important. Some modern analyzers, such as the Radiometer AQT90 FLEX , make it very easy to obtain high-quality biomarker measurement results.
Key words : myocardial infarction, diagnosis, troponin, myoglobin, creatine kinase
MB.
Today, biochemical markers play a key role in the accurate diagnosis of myocardial infarction, and, very importantly, in assessing the risk of an unfavorable outcome and choosing the most adequate treatment method. For the first time, a laboratory method for diagnosing myocardial infarction (measuring the activity of aspartate aminotransferase in the blood) was used more than 50 years ago [5]. Since then, the role of laboratory methods in cardiology has constantly increased. There has been a significant shift from measuring aspartate aminotransferase and lactate dehydrogenase activity to the three biomarkers commonly used today: myoglobin, creatine kinase MB (CK-MB), and troponins I and T. Initially, laboratory results were considered only as an adjunct to the clinical examination and ECG. But in 2000 and then in 2007, the most authoritative American, European and international heart associations decided that the diagnosis of myocardial infarction was directly related to an increase in the level of biomarkers (preferably troponin) in the blood [12]. All other methods of clinical and functional examination are intended only to confirm the ischemic nature of the observed myocardial necrosis. The published document emphasizes the key role of biomarkers in diagnosis: “The term myocardial infarction should be used when there is evidence of myocardial necrosis in the presence of a clinical picture consistent with myocardial ischemia.” Moreover, data on myocardial necrosis is understood as “detection of an increase and/or decrease in the level of cardiac biomarkers (preferably troponin) with at least one value exceeding the upper reference limit,” and at least one of the following is considered sufficient evidence of ischemia: • The presence of symptoms ischemia; • ECG changes indicating the appearance of ischemia (the appearance of ST-T changes or the appearance of a left bundle branch block); • Development of a pathological Q wave on the ECG; • Radiation findings indicating loss of viable myocardium or the appearance of local wall motion abnormalities. Why has the measurement of troponin levels taken center stage in laboratory diagnostics? Table 1 summarizes the properties of an ideal biomarker for myocardial infarction.
Table 1. Properties of an ideal biomarker for myocardial necrosis [3].
| · Absolute specificity for the myocardium : The biomarker should not be present in any other tissues of the body. · Specificity for irreversible damage : The biomarker must distinguish reversible (ischemia) from irreversible damage (necrosis). · Rapid release into the blood : The biomarker should quickly release into the blood after necrosis. Biomarkers with lower molecular weight tend to appear more quickly in the blood. Soluble cytoplasmic biomarkers are faster than structural ones. · High sensitivity : The biomarker must be contained in the myocardium in high concentration, and completely absent in the blood, both normally and in any pathology, except for myocardial necrosis. Biomarker release during necrosis must be powerful. · Sustained increase in level : For a reliable measurement, the biomarker level must remain elevated for hours or days after necrosis. · Predictable elimination : Elimination kinetics should be predictable and independent of concomitant diseases such as renal failure or liver damage. · Complete release : Necrotic myocytes should be completely cleared of the biomarker. The amount of biomarker in the blood should be proportional to the degree of necrosis (infarct size). · Measurement by accessible methods : The nature of the biomarker should allow the use of an accessible, reliable, fast, accurate and economical measurement method. |
To date, none of the existing biomarkers meets all of the above criteria. Troponins I and T are closest to the ideal. Their main advantage is their unique specificity for the myocardium. Troponins are proteins of the muscle contractile apparatus. They are part of the troponin-tropomyosin complex of thin myofibrils. This complex regulates the interaction of the contractile proteins actin and myosin and, thereby, ensures a change in muscle contraction or relaxation. There are three types of troponin - C, I and T. All of them are small proteins with a molecular weight of 20–40 thousand. Troponin T binds the remaining troponins and tropomyosin into a single complex. In relaxed muscle, troponin I prevents the interaction of the myosin head with actin and prevents contraction. Excitation of the cardiomyocyte leads to an increase in the intracellular concentration of Ca2+ ions, which bind to troponin C. The conformation of the entire complex changes, and actin is freed from the inhibitory influence of troponin I - muscle contraction occurs. Troponin C is one of the very conserved proteins. Thus, troponin C from human skeletal muscles differs from troponin C from bovine heart by only one amino acid [10]. In other words, the troponins of the heart and slow skeletal muscles are almost identical. In contrast, the cardiac isoforms of troponins I and T are expressed only in the heart, whereas the isoforms of the analogous fast and slow muscle troponins in skeletal muscle are products of other genes. Being part of the contractile apparatus, troponins I and T are present in high concentrations in myocardial cells. In the blood of healthy people, the concentration of these proteins is extremely low, but in the case of myocardial infarction it increases tenfold (Fig. 1), and the increased concentration persists for several days, in some cases up to one, and for troponin T, even two weeks. According to the general opinion of experts, the concentration of troponins in the blood increases only with irreversible destruction (necrosis) of cardiomyocytes, although the causes of necrosis may be different. Unfortunately, troponins are structural proteins, and they do not appear in the blood immediately, usually 4-6 hours after a heart attack. True, the increasing sensitivity of modern tests is increasingly reducing this time. The kinetics of troponin elimination is ambiguous and depends on renal function. Troponins I and T are contained in the myocardium in equimolar quantities, they are both unique to the heart, and although these are different proteins, from the point of view of diagnosing myocardial infarction, both troponins I and T are completely equivalent, and all international recommendations do not distinguish between them. If it is not possible to measure troponin concentration, then international recommendations suggest as an alternative to measure the level of creatine kinase MB (by mass, not by activity!) [7]. Until recently, this indicator was the “gold” standard in the laboratory diagnosis of myocardial infarction due to its relative specificity for the myocardium. Creatine kinase MB is an enzyme that catalyzes the transfer of high-energy phosphate from creatine phosphate to ATP and is involved in the transport of energy from mitochondria to the contractile apparatus. For intensive and continuous work, the heart needs a constant flow of energy, so this enzyme is contained in large quantities in cardiomyocytes. Creatine kinase consists of two subunits and has a molecular weight of about 42 thousand. Two types of subunits Mi and B are known, and, accordingly, three isoenzymes MM, BB and MB. CPK-MM predominates in skeletal muscle, and CPK-MB accounts for only 1–3%. In the myocardium, the main isoenzyme is also CPK-MM, but approximately 15% is accounted for by CPK-MB. Therefore, an increase in the level of CPK-MB in the blood is specific (but not absolutely!) for myocardial damage. The activity of the gene encoding the B subunit may be increased, for example, in regenerating skeletal muscle, reducing the specificity of this biomarker. Creatine kinase MB is a less sensitive biomarker than troponin. It has been shown that almost 30% of patients admitted with chest pain, without ST-segment elevation on the ECG and without an increase in the level of creatine kinase CPK-MB, actually had myocardial infarction, as shown by measuring troponin levels. For patients admitted within 6 hours of the onset of pain, international recommendations include the determination of an early biomarker of myocardial necrosis in addition to cardiac troponin [7]. Myoglobin is the most studied biomarker for this purpose. It is a small heme-containing soluble protein of skeletal muscle and myocardium (mw 17,500). Its main function is the transport of oxygen in the muscles. Due to its high solubility and small size, myogloblin is quickly released when muscles are damaged and is excreted by the kidneys. The main disadvantage of myoglobin as a biomarker is low specificity. A normal myoglobin level helps exclude the diagnosis of myocardial infarction. But its increase may also be associated with various lesions of skeletal muscles. What are the normal concentrations of biomarkers in the blood, and what is considered a proven increase in their level? According to the recommendations of the National Academy of Clinical Biochemistry (USA) and the International Federation of Clinical Chemistry and Laboratory Diagnostics, for each biomarker it is necessary to establish the upper limit of normal values based on a study of a group of healthy people without a history of heart disease [1,2]. For troponins (T and I) and creatine kinase MB, the threshold value for detecting cardiac damage is the 99th percentile of measurements in a group of healthy people. This means that 99% of healthy people have plasma levels of the relevant analyte below this threshold. On the contrary, exceeding this threshold indicates myocardial damage. Moreover, for creatine kinase MB, studies should be conducted separately for men and women, since in men the 99th percentile value is 2–3 times higher than in women. There are also racial differences. For myoglobin concentration, the 97.5th percentile is taken as the normal limit. Ideally, each laboratory should establish its own reference range, but given the complexity of conducting such a study, it is permissible to rely on the figures provided by the manufacturers. In the above definition of myocardial infarction, the words “rise or fall” in the level of a biomarker are very important. That is, ideally, it is necessary to identify a new peak in the concentration of a biomarker in the blood, corresponding to the clinical picture. To do this, the concentration of the biomarker must be measured at least twice, and measured quantitatively. For most patients, blood sampling is indicated on admission and 6–9 hours later [7]. Once the diagnosis of myocardial infarction is confirmed, subsequent measurements of biomarkers of myocardial damage (approximately once a day) allow us to estimate the size of the infarction and assess the risk of complications for a given patient. Accurate assessment of cardiovascular risk is critical to clinical decision making because the benefits, risks, and costs of different treatments must be weighed to determine the best treatment for each individual patient [4]. Of these biomarkers, the most difficult to measure in the blood is the concentration of troponin, since it is normally extremely low. To accurately determine the upper limit of normal (99th percentile), the analytical sensitivity of the measurement method must be sufficient to determine the concentration of troponin in the blood of, if not all, then at least many healthy people. Figure 2 shows the “true” distribution of troponin I concentration in the blood in healthy people and patients with myocardial infarction. The normal limit at the level of the 99th percentile of the distribution of troponin I concentration in healthy people best allows us to distinguish patients from healthy people [2]. If the analysis method has a detection limit (1), then this threshold can be set quite accurately. A method with a detection limit (2) will certainly identify the majority of patients with myocardial infarction, but the true limit of normal remains unknown, and therefore in some patients myocardial damage remains undiagnosed. According to the recommendation of the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC), to reduce the influence of nonspecific factors, analytical sensitivity should be approximately 5 times lower than the clinically significant threshold level [9]. In addition, the measurement error at the threshold level should be quite low [1]. The error is determined by the value of the coefficient of variation (CV), which is equal to: CV = SD/M*100%, where M is the arithmetic mean of the results of measuring a given concentration, and SD is the standard deviation. At the level of the upper limit of the norm, the error should be no more than 10%, otherwise the test will give many false-positive and false-negative results. Unfortunately, the recommendations outlined are not always followed in real life. The official website of the International Federation of Clinical Chemistry and Laboratory Medicine [https://www.ifcc.org/PDF/IFCC_Troponin_Web_Page_Table_of_Assays_Oct_2008.pdf] provides analytical characteristics for 19 tests for troponins I and T from different manufacturers (data as of October 2008). Only 12 of them had a detection limit less than the upper limit of normal, that is, for the remaining tests, the upper limit of normal (99th percentile) was actually not established. With measurement accuracy, the situation is even worse. Even at a level twice the normal limit, the coefficient of variation does not exceed 10% in only eight tests. Thus, analytical quality is a very important and not fully resolved topic that deserves close attention. All currently available methods for determining the concentration of troponin are based on an immunochemical reaction, the so-called “sandwich” analysis. The quality of the result obtained depends on the correct choice of antibodies. The triple complex of troponins I, T and C, entering the blood from destroyed cardiomyocytes, breaks down into free troponin T and a double complex of troponins I and C. These are the main forms of troponins in the blood. In the blood, under the influence of proteases, the cleavage of the terminal fragments of troponin molecules begins (Fig. 3). Therefore, the antibodies used for analysis must interact with the central, most stable part of the troponin molecule. In addition, troponin I can be in phosphorylated/dephosphorylated, as well as oxidized/reduced forms. The binding of the antibodies selected for analysis should not be affected by chemical modification of the troponin I molecule. In addition, the central part of the troponin I molecule is the object of interaction with autoantibodies, which, by blocking the binding of test antibodies, can lead to false-negative test results. The presence of autoantibodies to troponin I is quite common. They were found in 5.5% of people without signs of cardiovascular disease and in 21% of patients with acute coronary syndrome [6]. Attempts by manufacturers to overcome these difficulties and select the best combination of antibodies for analysis lead to the use of antibodies to different epitopes. As a result of manufacturers using different sets of antibodies in immunochemical reactions and the lack of clear standardization, biomarker concentrations measured on different analyzers often do not match. With almost perfect correlation of values obtained by the best measurement methods, the absolute value of concentration can differ by an order of magnitude. Therefore, direct comparison of absolute values is not possible, and normal limits must be determined separately for each analyzer. Although the clinical interpretation of the results, taking into account the relevant reference ranges, is generally consistent for all tests, this situation creates an obvious inconvenience. Here, a thoughtful clinical and laboratory council is needed to develop general rules for assessing the results obtained, for example, on portable analyzers at the patient’s bedside and in the central laboratory, for each specific hospital. Time plays a critical role in the diagnosis and treatment of myocardial infarction. Reperfusion initiated within the first hour after the onset of myocardial infarction was associated with a 1% mortality rate, whereas the same treatment initiated after 6 hours or more resulted in a 10% mortality rate [11]. According to the general opinion of clinicians and laboratory doctors, the time before receiving a response from the laboratory about the concentration of cardiac biomarkers in a blood sample should not exceed 60 minutes [7]. In reality, few places provide such a speed of analysis around the clock. Therefore, the National Academy of Clinical Biochemistry (USA) has given the following clear recommendations [8]: · The laboratory should measure cardiac biomarkers within 1 hour, preferably in 30 minutes or less. The time is calculated from sample collection to reporting of the result. · Institutions unable to consistently provide cardiac biomarker results within approximately 1 hour should use bedside analyzers. Bringing analyzers closer to the patient, that is, moving them from the laboratory to clinical departments, brings with it new problems. First of all, it is necessary to ensure maximum ease of operation with a minimum need for maintenance. The analyzer will be used by clinical department personnel who do not have special laboratory training. Personnel turnover, which often prevents timely additional training of personnel, should also be taken into account. At the same time, it is necessary to ensure laboratory quality of the analysis (high sensitivity and accuracy of measurement) and sufficient productivity. The optimal scheme is to maintain control of laboratory specialists over all instruments located in the departments. Connecting the analyzer to an in-hospital information system that allows remote access from a laboratory computer terminal greatly facilitates such control. An example of an analyzer that most fully meets all these requirements is the new immunofluorescence analyzer AQT90FLEX manufactured (Denmark), shown on the cover of this magazine. This is a fairly compact benchtop device capable of measuring all of the specified biomarkers of myocardial necrosis (troponin I, creatine kinase MB, myoglobin). TroponinT will join them next year. In addition, biomarkers of heart failure (NT-proBNP), coagulation system activation (D-dimer), inflammation (CRP) and pregnancy (β-subunit of human gonadotropin) can be measured. The operator can freely select the necessary parameters, which are measured in parallel. It is important that no pre-treatment of the blood sample is required. To measure, you just need to insert a closed standard vacuum tube with a sample into the analyzer, select parameters and get the result and the closed tube. Contact with blood or waste during the analysis is excluded. All processes are as automated as possible; it is possible to connect the analyzer to information systems. All this makes it easy to use the analyzer for express diagnostics directly in the department. At the same time, the measurement result meets the highest laboratory standards. Doctors will no longer have to clarify the diagnosis by sending a sample to a central laboratory. Table 2 provides data on the analytical quality of measurements of the main biomarkers of myocardial necrosis. Table 2. Analytical measurement quality of the AQT90FLEX analyzer.
| Parameter | Detection limit | Normal limit (99th percentile) | 10 % CV | Range |
| Troponin I | 0.0095 µg/l | ≤ 0.023 µg/l | 0.039 µg/l | 0.010-50 µg/l |
| Kreatinkina MB | 0.53 µg/l | ≤ 7.2 µg/l | < 5µg/l | 2-500 µg/l |
The results of troponin measurements on the AQT90 FLEX correlate almost perfectly with the results of the TnI-Ultra ADVIA Centaur laboratory analyzer (Siemens) and the correlation coefficient R2 = 0.984. Thus, modern requirements for laboratory diagnosis of myocardial infarction are reduced to the rapid and accurate determination of biomarkers (preferably troponin). For this, in addition to laboratory ones, non-laboratory analyzers are also needed, which would be easy to use and would give quantitative results comparable in accuracy to laboratory data. The use of such analyzers directly in emergency departments and intensive care units will improve the quality of medical care in emergency situations. References 1. Apple FS, Jesse RL, Newby LK, Wu AH, Christenson RH. National Academy of Clinical Biochemistry and IFCC Committee for Standardization of Markers of Cardiac Damage Laboratory Medicine Practice Guidelines: Analytical issues for biochemical markers of acute coronary syndromes. Circulation. 2007 Apr 3;115(13): e352-5. 2. Apple FS, Quist HE, Doyle PJ, Otto AP, Murakami MM. Plasma 99th percentile reference limits for cardiac troponin and creatine kinase MB mass for use with European Society of Cardiology/American College of Cardiology consensus recommendations. Clin Chem. 2003 Aug;49(8): 1331-6. 3. Cardiovascular biomarkers: pathophysiology and disease management (edited by David A. Morrow). 2006 Humana Press Inc. p.6. 4. Criteria for Evaluation of Novel Markers of Cardiovascular Risk. A Scientific Statement From the American Heart Association. Circulation. 2009; 119: 2408-2416. 5. Karmen A, Wroblewski F, LaDue JS. Transaminase activity in human blood. J Clin Invest 1954;34:126–133. 6. Kim Pettersson, Susann Eriksson, Saara Wittfooth, Emilia Engström, Markku Nieminen and Juha Sinisalo. Autoantibodies to Cardiac Troponin Associate with Higher Initial Concentrations and Longer Release of Troponin I in Acute Coronary Syndrome Patients. Clinical Chemistry. 2009;55:938-945. 7. Morrow DA, Cannon CP, Jesse RL, Newby LK, Ravkilde J, Storrow AB, Wu AH, Christenson RH, Apple FS, Francis G, Tang W. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: Clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Clin Chem. 2007 Apr; 53(4): 552-74. 8. National Academy of Clinical Biochemistry: Laboratory Medicine Practice Guidelines: Evidence-Based Practice for Point-of-Care Testing. (Editor James H. Nichols) AACCPress. 2006. 9. Panteghini M, Gerhardt W, Apple FS, Dati F, Ravkilde J, Wu AH. Quality specifications for cardiac troponin assays. Clin Chem Lab Med. 2001 Feb;39(2):175-9. 10. Romero-Herrera, A. E.; Castillo, O.; Lehmann, H. : Human skeletal muscle proteins: the primary structure of troponin CJ Molec. Evol. 8: 251-270, 1976. 11. Rosalki SB, Roberts R, Katus HA, Giannitsis E, Ladenson JH, Apple FS. Cardiac biomarkers for detection of myocardial infarction: perspectives from past to present. Clin Chem. 2004 Nov;50(11): 2205-13. 12. Thygesen K, Alpert JS, White HD on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Circulation. 2007 Nov 27; 116(22): 2634-53.
Figure 1. Increase in the level of markers in the blood during myocardial infarction [7].
Figure 2. Detection limit of a good (1) and insufficiently sensitive test (2) in relation to the true normal limit for troponin I concentration.
Figure 3. Factors influencing the choice of antibodies for troponin I detection.
News all
07/06/2021 X Baltic Forum "Current problems of anesthesiology and resuscitation" June 30 - July 3, 2021
06/03/2021 The 7th Conference “Laboratory Diagnostics of Emergency Conditions 2021” took place
05/11/2021 Conference “Laboratory diagnostics of emergency conditions 2021” June 3, Novotel, St. Petersburg
01/22/2021 Seminar for managers of veterinary clinics
04/09/2020 Manufacturers and suppliers called for simplification of registration of medical devices
Journal "Emergency Medicine" 7-8 (38-39) 2011
Diseases of the circulatory system occupy a leading place in the structure of morbidity and overall mortality of the population of most countries of the world and Ukraine in particular. The main causes of cardiac death are acute coronary syndromes, acute heart failure, thromboembolism and life-threatening cardiac arrhythmias. Correct and early diagnosis of acute conditions allows for timely treatment, and determination of the likelihood of complications developing allows for an individual approach to therapy. Biological markers (biomarkers) provide great assistance in implementing this approach. The term “biomarkers” in medicine usually means blood proteins, the concentration of which reflects the presence or severity of a particular pathological condition.
Scope of application of biomarkers for various acute conditions in cardiology:
1. Acute coronary syndrome:
— diagnosis of acute myocardial infarction;
— risk stratification;
— selection of optimal treatment tactics.
2. Acute heart failure:
— identification of patients with heart failure (screening);
— confirmation of the severity of heart failure;
— targeted monitoring of patients with heart failure (HF);
— therapy monitoring and optimization.
3. Pulmonary embolism (PE):
— differential diagnosis;
— risk stratification.
In acute coronary syndrome (ACS), biomarkers are primarily used to diagnose myocardial damage. Currently, a number of markers are used, which is due to the characteristic levels of cardiac specificity for each of them and the characteristics of entry into the blood after damage to cardiomyocytes, which determine differences in the time of reaching diagnostic and peak values, and the duration of marker circulation (Fig. 1).
Myoglobin. A complex muscle protein that binds oxygen carried by hemoglobin to form oxymyoglobin and, thus, provides the working muscle with some supply of oxygen, transferring it to cytochrome oxidase of muscle mitochondria (Fig. 2).
Since myoglobin is a fairly low molecular weight protein, it easily diffuses through the membranes of damaged muscle cells and quickly appears in the peripheral blood when the myocardium is damaged. Myoglobin content during myocardial infarction (MI) increases in the blood serum within 2 hours after the onset of symptoms. It is excreted unchanged in the urine and disappears from the bloodstream within 24 hours from the onset of the disease. The diagnostic level for MI is considered to be an increase in myoglobin concentration to 20 ng/ml/h. The high content of myoglobin in skeletal muscle and the dependence of its concentration on renal function limit its use for diagnosing myocardial infarction.
Factors influencing changes in myoglobin concentration in the blood:
- acute MI;
— cardiovascular surgery;
- damage to skeletal muscles (including when taking statins);
- excessive physical activity;
- progressive muscular dystrophy;
- shock;
- renal failure.
Advantages of using myoglobin: high sensitivity, effective in early diagnosis of MI, useful in excluding myocardial infarction, can be a marker of reperfusion. Disadvantages: low specificity for damage to skeletal muscles, injuries, quickly returns to normal values, which limits the possibilities of late diagnosis.
Clinical recommendations: should not be used as an independent diagnostic marker due to low specificity.
MB-fraction of creatine phosphokinase (MB-CPK) is a heterodimer with a molecular weight of 86 kDa. Among traditional markers, it was the determination of MB-CPK activity that until recently was considered as the gold standard in the biochemical diagnosis of AMI. The MB-CPK isoform in AMI appears in the blood serum 3–4 hours after the onset of symptoms and reaches a diagnostically significant level by 4–6 hours. Elevated levels may persist for up to 48–72 hours. The proportion of MB-CK among total CK, exceeding 5–6%, is a specific sign of myocardial necrosis.
When using MB-CK for diagnosing MI, repeated, dynamic determination of the concentration of this marker in the blood is necessary, since MB-CK has low sensitivity in the early (before 6–8 hours) and late (after 48 hours) periods of MI. Experts from the European Society of Cardiology consider it preferable to calculate the MB-CK index: MB-CK index = (MB-CK x x 100)/total CK.
The MB-CPK index above 3–6% against the background of an increase in total CPK confirms the diagnosis of MI. Currently, MB-KFK is divided into two isoforms: MB1-KFK and MB2-KFK. Normally, the ratio of MB1-CK: MB2-CK = 1: 1. The ratio of MB1-CK: MB2-CK = 1: 1.5 is an early marker of myocardial damage: peak - 2–4 hours; returns to normal - 18–30 hours; a normal ratio of MB1-CPK: MB2-CPK 6 hours after the attack excludes MI. MB2-CPK is a tissue form that, when entering the bloodstream, is converted into MB1-CPC, therefore the ratio of MB2-CPC/MB1-CPC is greater than 1.5 1–2 hours after the onset of pain may indicate the development of myocardial necrosis.
Advantages of the MB-CPK study: fast, cheap, accessible and accurate determination, the ability to diagnose early reinfarction.
Disadvantages: low specificity for damage to skeletal muscles, trauma, after surgery, low sensitivity in early (< 6 hours) or late (> 36 hours) periods from the development of symptoms of acute MI; low sensitivity with minimal myocardial damage (positive troponin test).
Clinical guidelines: has been the standard in the past and currently; acceptable for most clinical situations. It is of greatest importance for the diagnosis of reinfarction.
Troponins T and I. The main structural contractile unit of the myocyte is the sarcomere, which is formed by orderedly arranged thick and thin fibers. Thin ones contain actin fibers and the troponin-tropomyosin complex. The troponin regulatory complex in striated muscle consists of three polypeptides; When diagnosing myocardial infarction, only the content of troponin T and troponin I is determined in the blood (Fig. 3).
For emergency diagnosis of myocardial infarction at the prehospital stage, a qualitative immunological test is most suitable for determining the content of the specific myocardial protein troponin T in the blood. During myocardial infarction, two peaks of increase in its concentration in the blood are observed. The first begins after 2–3 hours, reaches a maximum after 8–10 hours, the second begins after three days. Normalization of the concentration of troponin T in the blood during AMI occurs after 10–14 days. The sensitivity of the test after 3 hours is approximately 60%, after 10 hours it approaches 100%, and the specificity is close to 100%. Using this method, it is possible to diagnose myocardial infarctions with a Q wave, and it is also used for the differential diagnosis of myocardial infarction without a Q wave and acute coronary syndrome without myocardial damage - unstable angina.
Ultrasensitive troponins have become a new problem in the diagnosis of myocardial damage in acute coronary syndromes. Thanks to the use of new approaches (recombinant proteins, nanotechnology), the lower level of determination of cardiac troponins has been reduced by 10–100 times. The developers of such tests call them either ultra sensitive or highly sensitive. What do ultrasensitive tests “feel”? Troponin molecules "leak" from normal myocardium? Cardiomyocyte damage associated only with ischemia? Or the beginning of myonecrosis?
Is elevated ultrasensitive troponin an indicator of ischemia? An increase in hs-cTnI level > 1.3 pg/ml is an independent predictor of ischemia.
Experimental ischemia was induced in 195 individuals with suspected CAD and monitored using single-photon emission computed tomography. Levels of hs-TnT were determined before stress, then after 18 minutes, after 4 and 24 hours. It was shown that an increase in hs-TnT is more likely a consequence of irreversible myocyte death than the result of reversible myocardial ischemia that occurs after exercise or after pharmacological effects.
Among 50 patients with established myocardial infarction without ST-segment elevation and with negative results of standard cTnI tests, 86 and 100% of individuals had a positive us-cTnI test at 0 and 2 hours after the onset of pain, respectively. In individuals without ACS and without MI, the results of both tests were negative. Thus, us-cTnI detects myocardial damage in the majority of patients who are currently classified as having nephrotic syndrome (NS). This, according to the authors, indicates that ischemic retrosternal pain without myocardial damage is a rather rare event. A more sensitive cTn test will increase the positivity rate and allow early diagnosis of cardiac disorders, although it will identify pathologies not associated with ACS. The use of ultrasensitive troponin tests increases the number of both true-positive and false-positive results in the diagnosis of AMI.
Is elevated ultrasensitive troponin a marker of myonecrosis? To confirm or rule out AMI, ultra cTnI should be serially determined, with an increase of 46% or a decrease of 32% as the basis for interpreting the results.
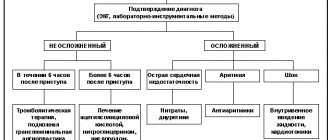
Recommendations for the use of biochemical markers for the diagnosis of myocardial infarction
Class I
1. Biomarkers of myocardial necrosis should be measured in all patients hospitalized with symptoms of acute coronary syndrome (Level of Evidence: C).
2. Clinical features (history, physical examination) and ECG should be used in conjunction with biomarkers in the diagnostic workup of suspected MI (Level of Evidence: C).
3. Cardiac troponin is the preferred marker in the diagnosis of MI. Measuring MB-CPK mass is an acceptable alternative if troponin is not available (Level of Evidence: A).
4. Blood for analysis should be taken upon admission and serially thereafter, depending on the clinical situation. For most patients, on admission and 6–9 hours later (Level of Evidence: C).
5. In the presence of a clinical picture indicative of acute coronary syndrome, further results obtained are considered diagnostic for necrosis accompanying myocardial infarction if (level of evidence C):
a) the maximum cardiac troponin concentration exceeds the 99th percentile value in the reference control group at least once within 24 hours after the clinical event (observation of increases and decreases in levels is useful in determining the time of onset of injury);
b) the maximum concentration of MB-CPK is the 99th percentile of the value in the reference control group, adjusted for the sex of patients, in two consecutive samples (the MB-CPK value should increase and decrease).
Class IIb
1. In patients hospitalized within 6 hours of symptom onset, the use of an early marker of myocardial necrosis in addition to cardiac troponin may be considered. Myoglobin is the best studied marker used for this purpose (level of evidence B).
2. Early detection of myocardial infarction through frequent blood sampling for markers of necrosis may be acceptable if linked to a treatment strategy (Level of Evidence: C).
Class III
1. Total CPK, aspartate aminotransferase (AST), dihydroxybutyrate dehydrogenase and/or lactate dehydrogenase should not be used as biomarkers in the diagnosis of MI (Level of Evidence: C).
2. In patients with diagnostic ECG changes (eg, ST-segment elevations), decisions about diagnosis and treatment should not be delayed while awaiting biomarker results (Level of Evidence: C).
However, new biomarkers of myocardial damage already exist and are even actively used; for some, express diagnostic strips have even been created. New markers for the most part are specific and quickly manifest markers of cardiomyocyte damage: MLC-I - myosin light chain I, DNAse1 - deoxyribonuclease-1, S100A1 - cardiac sensor protein dependent on calcium (cardiac-specific Ca2+- sensor protein), H-FABP - cardiac protein that binds free fatty acids (heart-type fatty-acid binding protein), GP-BB - glycogen phosphorylase BB, carbonic anhydrase III.
New biomarkers: diagnosis of myocardial damage
Cardiac form of fatty acid binding protein. Recently, the attention of researchers has been paid to the cardiac form of fatty acid binding protein (FABP). The first proposal to use BFA as an early marker of MI was made by J. Glatz in 1988. BFA is identical in amino acid sequence to the BFA contained in striated muscle tissue, but is present in skeletal muscles in minimal quantities. The maximum amount of BCFA is found in the myocardium - 0.5 mg/g tissue. The specificity of BSFA is not absolute; a small amount of it is also found outside the myocardium, in particular in the skeletal muscles, and especially in the diaphragm. BFA is also contained in the tissues of the aorta, and it can be assumed that with dissecting aortic aneurysm its content in the blood increases. Small amounts of BCFA circulate in the blood of healthy people; in women, the level of BASF is statistically significantly lower than in men (0.7 versus 1.2 μg/l), which is apparently due to differences in muscle mass. The kinetics of the content of BSFA in the blood of patients with MI is similar to the kinetics of myoglobin. Its content during MI increases during the first 3 hours after the onset of symptoms and returns to normal levels after 12–14 hours. Despite the fact that the level of BSFA in the myocardium is lower than that of myoglobin (0.5 versus 2.5 mg/kg), the minimum detectable concentration of BSFA is 15 times lower than that of myoglobin (2 versus 32 μg/L). This accounts for the greater sensitivity of BCFA compared to myoglobin in detecting myocardial necrosis. According to F. Ghani et al., the sensitivity of BCFA in diagnosing myocardial infarction was 39%, the specificity was 95%, while the sensitivity of myoglobin did not exceed 28%. However, BFA has not yet been sufficiently studied in certain clinical situations.
Ca2+-ATPase of the sarcoplasmic reticulum. In 2009, L. Ciobanu created an ELISA test system for determining the Ca2+-ATPase of the sarcoplasmic reticulum (SR) as a marker of AMI. In a study using a new diagnostic system of 56 patients with AMI, confirmed by an increase in troponin I and MB-CPK, an increase in the level of Ca2+-ATPase SR was detected in all patients. An increase in the concentration of this marker began approximately 4–6 hours after the onset of symptoms, and normalization occurred within 6 days. The Ca2+-ATPase CP titer correlated with the mass of the affected myocardium, the degree of ST segment elevation, and the presence of risk factors for cardiovascular complications in the patient.
Myosin light chains. Myosin is part of the sarcomere, the main component of the contractile apparatus of skeletal and cardiac muscle. The myosin molecule is heteropolymeric and consists of 2 heavy and 2 light chains (Fig. 6). There are 2 types of myosin light chains (MLC): MLC-1 (molecular weight 27 kDa) and MLC-2 (molecular weight 20 kDa).
The physiological role of LCM is to mediate the interaction between actin and myosin. LCM-1 appears in the blood 3–6 hours after the development of an attack of pain, its high concentrations persist for 4 days, and increased levels of LCM-1 are observed up to 10–14 days. The severity of clinical manifestations, disease prognosis and the size of the infarct zone correlate with the level of LCM-1 in the blood. Changes in the concentration of LCM-1 also correlate with the severity of heart failure and make it possible to identify a risk group among patients with heart failure.
Carbonic anhydrase III. Soluble protein is determined in large quantities only in skeletal muscles (type I muscle fibers), in small quantities in smooth muscles and myoepithelial cells. An increase in carbonic anhydrase III levels is observed in neuromuscular diseases and after significant physical activity. Plasma carbonic anhydrase III levels closely correlate with myoglobin release from skeletal muscle, and because carbonic anhydrase III concentrations do not reflect myocardial injury, the myoglobin/carbonic anhydrase III ratio can be used to discriminate between causes of elevated blood myoglobin levels.
Glycogen phosphorylase BB. Glycogen phosphorylase catalyzes the breakdown of glycogen in the sarcoplasmic reticulum. It is a dimeric enzyme with a molecular weight of 18.8 kDa (Fig. 7).
There are 3 isomers of glycogen phosphorylase: BB, found in the brain and heart, MM - in skeletal muscles, LL - in the liver. As a result of glycogen metabolism in ischemic tissue, glycogen phosphorylase BB passes from the sarcoplasmic reticulum to the cytoplasm, and then into the blood through the damaged cell membrane. An increase in the concentration of EV glycogen phosphorylase is observed 2–4 hours after myocardial damage, and a return to normal after 36 hours. A study by J. Mair (1998) demonstrated the high informativeness of this marker in the diagnosis of myocardial damage after myocardial revascularization operations. A limitation of the use of EV glycogen phosphorylase in clinical practice is the lack of commercial kits for its determination.
Risk stratification and selection of optimal treatment tactics
When stratifying the risk of complications in patients with ACS, the most frequently used markers of myocardial damage are troponins, heart failure - proBNP and indicators of the systemic inflammatory process; the most adequate and sensitive is C-reactive protein (CRP) and its highly sensitive component.
The effectiveness in risk stratification of patients with ACS based on markers of myocardial damage has been proven in many studies. The most proven and confirming its prognostic significance is the troponin test (Fig. 8).
The next biomarker, which also effectively predicts the complicated course of ACS, is one of the best indicators of the activity of the inflammatory process in the body - CRH. C-reactive protein (CRP) is a blood plasma protein that belongs to the group of acute phase proteins, the concentration of which increases during inflammation. Plays a protective role by binding the bacterial polysaccharide of Streptococcus pneumoniae. C-reactive protein is used in clinical diagnosis along with erythrocyte sedimentation rate (ESR) as an indicator of inflammation.
The predictive accuracy of this biomarker has been shown in many studies. Our study showed predictive accuracy depending on the severity of the increase in the CRH index. By dividing the levels of increased CR into tertiles, a significant influence of not only increased CR on the prognosis of the course of ACS was shown.
Multimarker approach to risk stratification
Multimarker analysis was the most qualitative and accurate in determining the likelihood of developing complications in ACS. By comparing several markers showing various processes in the body, the most accurate prediction of the course of the disease and its complications was achieved. The combination of a marker of cardiomyocyte damage - troponin, an indicator of the systemic inflammatory process - CRH and an indicator of the presence and severity of heart failure - brain natriuretic peptide (BNP) turned out to be the most accurate predictor of complications of ACS. In the OPUS-TIMI 16 and TACTICS-TIMI 18 studies, increased levels of three diagnostic biomarkers were recorded in patients with ACS: troponin I, CRH and BNP. According to studies, if only one biomarker increased, 30-day mortality increased twofold, but if all three biomarkers were increased, then the risk of death within a month after the development of ACS increased by 6 or up to 13 times. The results of the studies showed the high prognostic value of the multimarker approach in stratifying the risk of an unfavorable course in ACS (Fig. 11).
Other studies have assessed a large number of biomarkers. In 664 patients admitted to emergency departments with ACS, the prognostic significance of the following markers was assessed: cTnT, H-FABP, glycogen phosphorylase BB, NT-proBNP, fibrinogen and D-dimer, hsCRP, MPO (myeloperoxidase), MMP -9 (matrix metalloproteinase-9), PAPP-A, or PBAP-A (plasma protein A associated with pregnancy), sCD40L (soluble fragment of the membrane glycoprotein CD40L). According to the study, the panel of cTnT, BFA and NT-proBNP had the best predictive ability.
NT-proBNP, LP-PLA2, choline in whole blood. Another study followed 432 patients also admitted to the emergency department with ACS. The prognostic significance of cTnI was assessed; the panel of cTnT, BFA and NT-proBNP, hsCRP, PlGF, or PlGF (placental growth factor), LP-PLA2 (lipoprotein-associated phospholipase A2) had the best prognostic ability.
D-dimer, whole blood choline, plasma choline. Best combination: NT-proBNP, whole blood choline and LP-PLA2.
Risk stratification in acute heart failure
Cystatin C, cTnT, NT-proBNP. Effectively predict the risk of adverse outcomes in acute heart failure. 138 patients.
Risk stratification in acute decompensated chronic heart failure
BNP, cTnI, hs-CRP. 577 patients. The higher the levels of these markers, the higher the risk of 30-day mortality.
Plasma levels of B-type natriuretic peptide for the treatment of patients with chronic heart failure have been used in a number of randomized controlled trials. However, the benefits of this treatment were uncertain. Therefore, a meta-analysis was conducted to examine the overall effect of drug-induced changes in BNP on cardiovascular events in patients with chronic HF.
The results of eight randomized controlled trials with a total of 1726 patients and a median duration of 16 months were included in the meta-analysis. There was a lower risk of all-cause mortality (relative risk (RR) 0.76; 95% confidence interval (CI) 0.63–0.91; P = 0.003) in the BNP-guided group compared with the study group. In the subgroup of patients younger than 75 years, all-cause mortality was also significantly lower in the BNP group (HR, 0.52; 95% CI, 0.33 to 0.82; P = 0.005). However, there was no reduction in mortality in patients 75 years of age or older (HR, 0.94; 95% CI, 0.71 to 1.25; P = 0.70). Risk of all-cause hospitalization and hospitalization-free survival were not significantly different between groups (HR 0.82; 95% CI 0.64–1.05; P = 0.12 and HR 1.07; 95% CI 0.85 -1.34; P = 0.58, respectively). The incremental percentage of patients achieving target doses of angiotensin-converting enzyme inhibitors and β-blockers in these studies averaged 21 and 22% in the BNP group and 11.7 and 12.5% in the study group, respectively.
The findings suggest that B-type natriuretic peptide-guided therapy reduces all-cause mortality in patients with chronic HF compared with usual care, especially in patients younger than 75 years (Figure 12).
Pulmonary embolism
Most patients with venous thrombosis experience endogenous fibrinolysis, which causes the destruction of a certain amount of fibrin with the formation of a breakdown product of cross-linked fibrin - D-dimer.
An increase in the concentration of D-dimer to more than 500 μg/l indicates spontaneous activation of the fibrinolytic system of the blood in response to massive thrombus formation in the venous system. This indicator has high sensitivity (95–96%) and negative predictive value (95–99%), but low specificity (about 40–50%).
Currently, D-dimer is a fundamental biomarker and is included in all differential diagnosis schemes for pulmonary embolism (Fig. 13). D-dimer is used in the diagnosis of small branch pulmonary embolism and, less commonly, submassive embolism. However, where computed tomography is not available, the determination of D-dimer can be used to exclude PE, since a negative test categorically excludes the development of PE, and a positive test suggests further examination of a patient with suspected PE, because an increase in its level can also be observed in other thrombotic conditions. conditions (sepsis, acute myocardial infarction, malignant neoplasms, inflammation, after surgery, etc.).
When evaluating patients with pulmonary embolism to identify those at high risk, levels of troponin (Tn) or fatty acid binding protein and brain natriuretic peptide (BNP) should be measured. Due to myocardial ischemia of the pancreas, an increase in the levels of TnI and TnT, which are sensitive markers of irreversibly damaged cardiomyocytes, is detected in the blood. An increase in Tn levels closely correlates with the severity of pancreatic dysfunction, increased mortality, complications and recurrences of pulmonary embolism. Another important marker of poor prognosis is brain natriuretic peptide (BNP). In response to overstretching of the pancreatic cardiomyocytes, due to its overload and dilatation, within a few hours the prohormone BNP (proBNP) begins to be produced in the pancreatic myocardium, which induces the appearance of biologically active BNP and the final part of proBNP (NT-proBNP). An increase in the blood level of these biomarkers is a predictor of RV dysfunction, the development of right ventricular failure and increased mortality.
Thus, the necessity and prevalence of using biomarkers of various pathological processes occurring in the body during cardiac diseases is quite clearly visible. We see how great the contribution of biomarker research is to the diagnosis and stratification of the risk of complications in our patients, which leads to correction of therapy and the use of more aggressive treatment methods. Biomarkers in cardiology play a huge role in determining both tactics and treatment strategies for a given patient. The correct use of biomarkers in medicine not only saves the doctor from errors in diagnosing any disease, but also leads to the correct attitude towards an individual patient for the correct selection of groups of drugs needed by the patient, preventing complications of the disease and aggressive enough treatment so as not to harm, but help the patient.