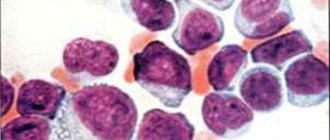
Macrocytic anemia
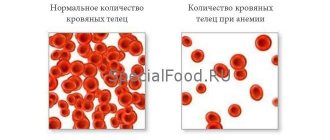
Anemia is a pathological syndrome characterized by a decrease in the level of hemoglobin in the blood, causing dysfunction of the hematopoietic system and oxygen transport, and the development of hypoxia.
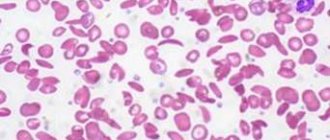
Macrocytic anemia is characterized by an abnormal increase in red blood cells (macrocytosis) to a volume of ≥ 100 fL/cell (femtoliters per cell) when the norm is 80–100 fL. Due to the increase in size of red blood cells, their concentration in the blood decreases, which leads to a decrease in hemoglobin content. As a result, the blood does not receive the required amount of oxygen.
The degree of saturation of red blood cells with hemoglobin is determined by the color index of blood cells. Normally it is 0.86–1.1. Based on deviations from the coefficient, pathology is divided into 2 types:
- Macrocytic hypochromic anemia - color index below 0.86. It is divided into thalassemia and iron deficiency anemia.
- Hyperchromic macrocytic anemia – color index more than 1.1. It is divided into myelodysplastic syndrome, folate deficiency and vitamin B12 deficiency anemia.
Normochromic anemia is also distinguished, in which CP is within normal limits, but there are other pathological processes in the body, for example, bleeding, bone marrow disease, extramarrow tumor, etc. This category includes posthemorrhagic and hemolytic anemia.
Metabolism of iron in the body
Iron is one of the most important trace elements in the human body due to its participation in a wide range of metabolic processes, including oxygen transport, DNA synthesis and electron transport across the cell membrane. Iron homeostasis in the body is maintained by carefully regulating the absorption of iron in the proximal small intestine to compensate for its losses during metabolic processes. A long-term imbalance of iron in the body leads to the development of either hemosiderosis (increased iron concentration) or iron deficiency anemia.
Iron deficiency anemia can be caused by both a decrease in iron absorption in the intestine and an increased rate of loss of the microelement. A decrease in adsorption often occurs due to a decrease in the amount of absorbable iron consumed. Blood loss (hemorrhage) is the most common cause of increased iron loss from the body, but hemoglobinuria with intravascular hemolysis can also cause iron deficiency anemia. Iron malabsorption is a relatively rare cause of anemia associated with severe damage to the small intestine (celiac disease, inflammatory bowel disease, regional enteritis) or with postoperative complications of gastrointestinal surgery.
There are 3 different mechanisms for iron uptake in the proximal intestine. The first mechanism is intended for iron, which is part of the heme, the second - for ferric iron, the third - for divalent iron. In the Russian Federation and Europe, a third of the total iron consumed by the body is heme iron, but the remaining two-thirds enters the body through consumed hemoglobin. Iron in heme is in an unchelated state and is precipitated by many other compounds (phosphates, tannates, oxalates) contained in foods, which interferes with its absorption in the intestine. The solubility and availability of heme for absorption is maintained by the degradation products of globin, which is destroyed by pancreatic enzymes. Heme and non-heme iron are absorbed by enterocytes non-competitively.
Heme enters the cell in the form of intact metalloporphyrin, most likely by a vesicular transport mechanism. Heme is destroyed inside the enterocyte by heme oxygenase, followed by the release of iron. The resulting free heme iron penetrates competitively with non-heme iron through the basolateral wall of the enterocyte to bind to transferrin in the blood plasma.
Trivalent iron uses a different mechanism for penetration into cells than divalent iron. This was demonstrated through competitive inhibition studies using anti-divalet metal transporter-1 (DMT-1) and anti-beta3 integrin blocking antibodies, as well as in transfection model experiments. These studies showed that ferric iron uses beta3-integrin and mobilferrin to enter the cell, and ferrous iron uses the DMT-1 protein.
The route by which larger amounts of non-heme iron are transported into the body is unknown. Most non-heme iron in the human diet is ferric iron.
There are also a number of auxiliary proteins that are also involved in iron adsorption. The iron transport stimulator, hepcidin, increases the absorption of ferric and ferrous iron and plays an important role in the transfer of iron from the enterocyte to the blood plasma. However, the relationship and exact mechanism of action of these proteins is still not completely clear.
The concentration of iron inside enterocytes is directly dependent on the body's need for iron. When specifically staining the epithelium of the small intestine of patients with iron deficiency, iron was not visualized in the enterocytes. However, in people with sufficient iron levels, the coloring of enterocytes was very pronounced. A change in the concentration of iron ions inside enterocytes can cause an increase in the synthesis of receptors or saturation of iron-binding proteins.
Unlike iron deficiency, with increased erythropoiesis (for example, during hypoxia), iron is quickly adsorbed without changing its concentration in the enterocyte. Endotoxins and cytokines change adsorption by a different mechanism, which may be explained by the balance of hepcidin and erythropoietin activity.
Most of the iron that is delivered to the body's cells is bound to transferrin. Transferrin uses two mechanisms to penetrate membranes: the classical transferrin receptor pathway (high affinity, low efficiency) and the receptor-independent pathway (low affinity, high efficiency).
In the classical transferrin pathway, the iron-transferrin complex enters cells along with the endosome. Subsequent acidification of the endosome leads to the release of iron from the protein complex and its entry into the cell cytoplasm. Apotransferrin is then transported from the endosome to the plasma for recycling. The exact mechanism by which iron is delivered into cells via a receptor-independent pathway is not fully understood. Body cells can also deliver iron through mobilferrin and the DMT-1 protein. Their functions in the absence of iron-saturated transferrin are not entirely clear. However, their presence in these cells suggests that they may be involved in the intracellular regulation of iron homeostasis.
Causes of macrocytic anemia
The disease usually develops due to a deficiency of vitamin B12 (cyanocobalamin) or B9 (folic acid).
A deficiency of these compounds often occurs with an unbalanced diet. Vegetarianism is a risk factor, since cyanocobalamin is found mainly in products of animal origin.
In some people, vitamins enter the body, but are not absorbed. Possible causes of this pathology:
- autoimmune disorders;
- inflammatory diseases of the small and large intestines;
- intestinal malabsorption;
- malignant formations;
- excessive alcohol consumption;
- addiction.
Acute and chronic macrocytic anemia is possible against the background of the following diseases:
- hypothyroidism;
- metabolic disorders;
- diseases of the liver (for example, cirrhosis) and spleen;
- leukemia;
- rare metabolic disorders;
- acquired sideroblastic anemia.
The risk group includes people with a hereditary predisposition, alcoholism, smokers, pregnant women and patients taking medications that disrupt DNA synthesis (antitumor drugs and immunosuppressants).
Due to their origin, macrocytosis is conventionally divided into two large groups:
- Megaloblastic - occurs with vitamin deficiency.
- Non-megaloblastic - develops against the background of various diseases and clinical conditions that are not fully understood. Usually these are disorders associated with an increase in the area of the red blood cell membrane, which is characteristic of chronic pathologies of the liver and spleen.
Epidemiology
According to the Ministry of Health of Italy, Belgium, Germany and Spain, the annual incidence of iron deficiency anemia is 7.2-13.9 per 1000 inhabitants per year. Increased incidence is observed in women, young and elderly people, patients with gastrointestinal disorders, pregnant women and women with menometrorrhagia, and people taking aspirin or antacids. In countries where meat is not a major part of the diet, iron deficiency anemia is observed 6-8 times more often than in European countries. This occurs even despite equivalent total iron in the diet, since heme iron from meat products is better absorbed than non-heme iron. In a study of children and adolescents from Sudan and Nepal, iron deficiency anemia was found in 2/3 of those included in the study. Moreover, in some regions, intestinal parasites significantly worsen the course of iron deficiency anemia due to constant blood loss in the gastrointestinal tract.
Symptoms
At the initial stage of the disease there are no symptoms. They develop gradually as normal red blood cells decrease in the blood. Regardless of the form, the main manifestations of the pathology are the same:
- general weakness;
- fast fatiguability;
- frequent headaches;
- dizziness;
- sleep disorders;
- noise in ears;
- flashing “flies” before the eyes;
- cardiopalmus;
- shortness of breath, especially during physical activity;
- loss of appetite;
- pale skin;
- decreased concentration;
- depression;
- fainting.
With B12 deficiency, the following disorders are often observed: memory loss, tingling or numbness in the hands, muscle weakness, gait instability, paresthesia, difficulty walking.
With megaloblastic anemia, glossitis, indigestion, and anorexia may occur.
A comprehensive study taking into account the quantitative and qualitative composition of cells, as well as biochemical blood parameters, which allows us to identify signs of iron deficiency anemia.
The research results are provided with a free doctor’s commentary.
English synonyms
Iron deficiency anemia screening.
Research method
Colorimetric photometric method, flow cytometry, SLS (sodium lauryl sulfate) method, conductometric method, immunoturbidimetry.
Units
μmol/l (micromoles per liter), *10^9/l (10 in st. 9/l), *10^12/l (10 in st. 12/l), g/l (grams per liter), % (percent), fl (femtoliter), pg (picogram).
What biomaterial can be used for research?
Venous blood.
How to properly prepare for research?
- Eliminate alcohol from your diet 24 hours before the test.
- Do not eat for 12 hours before the test.
- Do not take medications for 24 hours before the test (as agreed with your doctor).
- Avoid physical and emotional stress 30 minutes before the test.
- Do not smoke for 30 minutes before the test.
General information about the study
Anemia is a pathological condition characterized by a decrease in the number of red blood cells and/or hemoglobin in the blood. The causes of anemia are varied and can be closely related to chronic somatic diseases (kidney and liver pathologies, autoimmune, infectious and inflammatory diseases, diabetes, tumors), acute and chronic blood loss, hereditary diseases and malnutrition, lack of vitamins and microelements. They occur when there is insufficient production of red blood cells in the bone marrow (for example, with deficiency of iron, vitamins B12 and B6, bone marrow depletion) or premature and excessive destruction of red blood cells in the bloodstream (for example, with hemolysis).
The most common form of anemia, iron deficiency, is detected in 80-90% of patients who have a reduced number of red blood cells and/or hemoglobin in the blood.
Iron deficiency develops gradually. Initially, a negative iron balance is created, in which the body's needs for it and its losses exceed the amount it receives from food. This may be due to blood loss, pregnancy, breastfeeding, growth spurts during puberty, malabsorption of micronutrients in the gastrointestinal tract, or insufficient intake of foods containing iron, as with vegetarianism.
Normally, iron is absorbed in the small intestine, but in some chronic diseases of the stomach and intestines, malabsorption syndrome occurs with impaired absorption of nutrients and microelements from food. In the blood, iron is transported by the protein transferrin to sites of use or accumulation. With iron deficiency, the amount of free transferrin in the blood increases, and the level of serum iron decreases. Hemoglobin synthesis in the bone marrow occurs less actively, and iron deficiency anemia develops with clinical manifestations of anemia. In a general blood test, small pale colored red blood cells are detected, MHC (average hemoglobin content in an erythrocyte), MCV (average erythrocyte volume), MCHC (average hemoglobin concentration in an erythrocyte), and hemoglobin level and hematocrit decrease. Without treatment, the amount of hemoglobin decreases more and more, the shape of red blood cells changes, and the intensity of cell division in the bone marrow decreases. Due to a decrease in the amount of hemoglobin and red blood cells, the transport of oxygen and carbon dioxide in the tissues is disrupted, symptoms of anemia appear: pale skin, headache, memory impairment, drowsiness, spots before the eyes, dizziness, fainting, rapid heartbeat, decreased blood pressure, pain in the area heart, muscle weakness.
In addition to well-being, negative changes occur in appearance: dry skin (as a result, early wrinkles), brittle nails, hair loss.
Children susceptible to anemia are more likely than healthy peers to suffer from colds.
In addition, with iron deficiency, the patient may experience a disturbance of taste and smell: there is a desire to eat chalk, clay, raw cereals and inhale the smells of acetone, gasoline, and turpentine.
Often people do not pay attention to the main signs of anemia - constant fatigue, paleness, dry skin - considering them an insufficient reason to see a doctor. Of course, such symptoms do not always indicate a disease; it cannot be diagnosed only by their presence. Moreover, you should not start taking iron-containing medications on your own - this can lead to serious complications.
It is important to know what exactly causes anemia. For example, in older people it often results from internal bleeding due to a tumor in the gastrointestinal tract. There is a type of anemia associated with poor absorption of vitamin B12. But in order to identify it, you first need to make sure that there is enough iron in the body and that the red blood cells are saturated with it.
At the same time, simply donating blood for hemoglobin is not enough to fully diagnose anemia. For example, in smokers, the level of hemoglobin and red blood cells is often normal: carbon monoxide contained in cigarettes, combining with hemoglobin, forms the substance carboxyhemoglobin, this form of hemoglobin does not have the ability to carry oxygen, and to compensate for oxygen starvation, the level of hemoglobin increases. It turns out that a comprehensive analysis is needed, reflecting the true reserves of this microelement in the body: determination of the levels of serum iron, transferrin, reticulocytes, general blood test, leukocyte formula.
In addition, it is important that timely diagnosis of anemia makes it possible to identify diseases of which it is the first symptom: some malignant tumors, chronic inflammatory processes, helminthic infestations, etc.
What is the research used for?
- For the diagnosis of iron deficiency anemia.
- For differential diagnosis of anemia.
- For the evaluation of patients at high risk of developing anemia.
- To evaluate the effectiveness of treatment for iron deficiency anemia.
When is the study scheduled?
- For symptoms of iron deficiency in the body (pallor and/or yellowness of the skin, general weakness, fatigue, drowsiness, dizziness, tinnitus, flashing spots before the eyes, fainting, atrophy of the tongue mucosa, changes in the structure of the nails, abnormal taste preferences).
- When the number of red blood cells and/or hemoglobin decreases according to a clinical blood test.
- In the presence of risk factors for the development of anemia (pregnancy, breastfeeding, menstrual irregularities, malnutrition, vegetarianism, diseases of the stomach and intestines with impaired absorption of nutrients, chronic stress).
- When examining children during periods of intensive growth.
- When examining patients with asthenia of unknown origin and severe fatigue.
- When monitoring the effectiveness of the use of drugs containing iron.
What do the results mean?
Reference values
- Serum iron
| Floor | Age | Reference values |
| Female | Less than 24 days | 17.9 - 44.8 µmol/l |
| 24 days – 1 year | 7.2 - 17.9 µmol/l | |
| 1-14 years | 9 - 21.5 µmol/l | |
| More than 14 years | 10.7 - 32.2 µmol/l | |
| Male | Less than 24 days | 17.9 - 44.8 µmol/l |
| 24 days – 1 year | 7.2 - 17.9 µmol/l | |
| 1-14 years | 9 - 21.5 µmol/l | |
| More than 14 years | 12.5 - 32.2 µmol/l |
- Leukocyte formula
- Leukocytes
| Age | Reference values |
| Less than 1 year | 6 - 17.5 *10^9/l |
| 1-2 years | 6 - 17 *10^9/l |
| 2-4 years | 5.5 - 15.5 *10^9/l |
| 4-6 years | 5 - 14.5 *10^9/l |
| 6-10 years | 4.5 - 13.5 *10^9/l |
| 10-16 years | 4.5 - 13 *10^9/l |
| More than 16 years | 4 - 10 *10^9/l |
- Neutrophils
| Age | Reference values |
| Less than 1 year | 1.5 - 8.5 *10^9/l |
| 1-2 years | 1.5 - 8.5 *10^9/l |
| 2-4 years | 1.5 - 8.5 *10^9/l |
| 4-6 years | 1.5 - 8 *10^9/l |
| 6-8 years | 1.5 - 8 *10^9/l |
| 8-10 years | 1.8 - 8 *10^9/l |
| 10-16 years | 1.8 - 8 *10^9/l |
| More than 16 years | 1.8 - 7.7 *10^9/l |
- Neutrophils, %
| Age | Reference values |
| Less than 1 year | 16 — 45 % |
| 1-2 years | 28 — 48 % |
| 2-4 years | 32 — 55 % |
| 4-6 years | 32 — 58 % |
| 6-8 years | 38 — 60 % |
| 8-10 years | 41 — 60 % |
| 10-16 years | 43 — 60 % |
| More than 16 years | 47 — 72 % |
- Complete blood count (without leukocyte formula and ESR)
- Leukocytes
| Age | Reference values |
| Less than 1 year | 6 - 17.5 *10^9/l |
| 1-2 years | 6.6 - 11.2 *10^9/l |
| 2-4 years | 5.5 - 15.5 *10^9/l |
| 4-6 years | 5 - 14.5 *10^9/l |
| 6-10 years | 4.5 - 13.5 *10^9/l |
| 10-16 years | 4.5 - 13 *10^9/l |
| More than 16 years | 4 - 10 *10^9/l |
- Red blood cells
| Floor | Age | Reference values |
| Less than 1 year | 4.1 - 5.3 *10^12/l | |
| 1-5 years | 4 - 4.4 *10^12/l | |
| 5-6 years | 4.1 - 4.5 *10^12/l | |
| 6-7 years | 4 - 4.4 *10^12/l | |
| 7-8 years | 4.2 - 4.6 *10^12/l | |
| 8-9 years | 4.1 - 4.5 *10^12/l | |
| 9-14 years | 4.2 - 4.6 *10^12/l | |
| 14-15 years old | 4.4 - 4.8 *10^12/l | |
| Female | 15-19 years old | 3.5 - 5 *10^12/l |
| More than 19 years | 3.5 - 5.2 *10^12/l | |
| Male | 15-19 years old | 3.9 - 5.6 *10^12/l |
| More than 19 years | 4.2 - 5.3 *10^12/l |
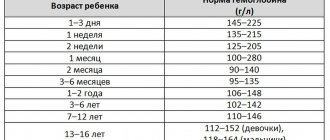
- Hemoglobin
| Age | Floor | Hemoglobin, g / l |
| 134-198 | ||
| 14 days – 1 month. | 107-171 | |
| 1-2 months | 94-130 | |
| 2-4 months | 103-141 | |
| 4-6 months | 111-141 | |
| 6-9 months | 110-140 | |
| 9-12 months | 113-141 | |
| 1-5 years | 110-140 | |
| 5-10 years | 115-145 | |
| 10-12 years | 120-150 | |
| 12-15 years | male | 120-160 |
| female | 115-150 | |
| 15-18 years old | male | 117-166 |
| female | 117-153 | |
| 18-45 years old | male | 132-173 |
| female | 117-155 | |
| 45-65 years | male | 131-172 |
| female | 117-160 | |
| > 65 years old | male | 126-174 |
| female | 117-161 |
- Hematocrit
| Age | Floor | Hematocrit, % |
| 41-65 | ||
| 14 days – 1 month. | 33-55 | |
| 1-2 months | 28-42 | |
| 2-4 months | 32-44 | |
| 4-6 months | 31-41 | |
| 6-9 months | 32-40 | |
| 9-12 months | 33-41 | |
| 1-3 years | 32-40 | |
| 3-6 years | 32-42 | |
| 6-9 years | 33-41 | |
| 9-12 years | 34-43 | |
| 12-15 years | male | 35-45 |
| female | 34-44 | |
| 15-18 years old | male | 37-48 |
| female | 34-44 | |
| 18-45 years old | male | 39-49 |
| female | 35-45 | |
| 45-65 years | male | 39-50 |
| female | 35-47 | |
| > 65 years old | male | 37-51 |
| female | 35-47 |
- Mean erythrocyte volume (MCV)
| Floor | Age | Reference values |
| Less than 1 year | 71 – 112 fl | |
| 1-5 years | 73 – 85 fl | |
| 5-10 years | 75 – 87 fl | |
| 10-12 years | 76 – 94 fl | |
| Female | 12-15 years | 73 – 95 fl |
| 15-18 years old | 78 – 98 fl | |
| 18-45 years old | 81 – 100 fl | |
| 45-65 years | 81 – 101 fl | |
| More than 65 years | 81 – 102 fl | |
| Male | 12-15 years | 77 – 94 fl |
| 15-18 years old | 79 – 95 fl | |
| 18-45 years old | 80 – 99 fl | |
| 45-65 years | 81 – 101 fl | |
| More than 65 years | 81 – 102 fl |
- Average hemoglobin content in erythrocytes (MCH)
| Age | Floor | Reference values |
| 30 - 37 pg | ||
| 14 days - 1 month. | 29 - 36 pg | |
| 1 - 2 months | 27 - 34 pg | |
| 2 - 4 months | 25 - 32 pg | |
| 4 - 6 months | 24 - 30 pg | |
| 6 - 9 months | 25 - 30 pg | |
| 9 - 12 months | 24 - 30 pg | |
| 1 – 3 years | 22 - 30 pg | |
| 36 years | 25 - 31 pg | |
| 6 – 9 years | 25 - 31 pg | |
| 9-15 years | 26 - 32 pg | |
| 15-18 years old | 26 - 34 pg | |
| 18-45 years old | 27 - 34 pg | |
| 45-65 years | 27 - 34 pg | |
| > 65 years old | female | 27 - 35 pg |
| > 65 years old | male | 27 - 34 pg |
- Mean erythrocyte hemoglobin concentration (MCHC)
| Age | Reference values |
| Less than 1 year | 290 – 370 g/l |
| 1-3 years | 280 – 380 g/l |
| 3-12 years | 280 – 360 g/l |
| 12-19 years old | 330 - 340 g/l |
| More than 19 years | 300 – 380 g/l |
- Platelets
| Floor | Age | Reference values |
| Less than 1 year | 214 - 362 *10^9/l | |
| 1-2 years | 208 - 352 *10^9/l | |
| 2-3 years | 209 - 351 *10^9/l | |
| 3-4 years | 196 - 344 *10^9/l | |
| 4-5 years | 208 - 332 *10^9/l | |
| 5-6 years | 220 - 360 *10^9/l | |
| 6-7 years | 205 - 355 *10^9/l | |
| 7-8 years | 205 - 375 *10^9/l | |
| 9-10 years | 177 - 343 *10^9/l | |
| 10-11 years | 211 - 349 *10^9/l | |
| 11-12 years old | 198 - 342 *10^9/l | |
| 12-13 years old | 202 - 338 *10^9/l | |
| 13-14 years old | 192 - 328 *10^9/l | |
| 14-15 years old | 198 - 342 *10^9/l | |
| 15-18 years old | 165 - 396 *10^9/l | |
| 18-45 years old | 159 - 376 *10^9/l | |
| Male | 45-65 years | 156 - 300 *10^9/l |
| Female | 45-65 years | 156 - 351 *10^9/l |
| More than 65 years | 139 - 363 *10^9/l |
- Mean platelet volume (MPV)
| Age | Reference values |
| Less than 10 years | 8.3 - 10.9 fl |
| 10 – 18 years | 8.6 - 12.1 fl |
| 18 – 45 years old | 8.5 - 12.8 fl |
| 45 – 65 years | 8.6 - 12 fl |
| 45 – 65 years | 9 - 12.9 fl |
| More than 65 years | 8.8 - 12.4 fl |
- Reticulocytes
- Reticulocytes,% (RET%)
| Floor | Age | Reference values |
| Female | Less than 2 weeks | 0,15 — 1,5 % |
| 2 weeks – 1 month | 0,45 — 1,4 % | |
| 1-2 months | 0,45 — 2,1 % | |
| 2-6 months | 0,25 — 0,9 % | |
| 6 months – 2 years | 0,2 — 1 % | |
| 2-6 years | 0,2 — 0,7 % | |
| 6-12 years | 0,2 — 1,3 % | |
| 12-18 years old | 0,12 — 2,05 % | |
| Over 18 years old | 0,59 — 2,07 % | |
| Male | Less than 2 weeks | 0,15 — 1,5 % |
| 2 weeks – 1 month | 0,45 — 1,4 % | |
| 1-2 months | 0,45 — 2,1 % | |
| 2-6 months | 0,25 — 0,9 % | |
| 6 months – 2 years | 0,2 — 1 % | |
| 2-6 years | 0,2 — 0,7 % | |
| 6-12 years | 0,2 — 1,3 % | |
| 12-18 years old | 0,24 — 1,7 % | |
| Over 18 years old | 0,67 — 1,92 % |
- Reticulocytes (absolute count, RET#)
| Floor | Reference values |
| Female | 17 - 63.8 *109/l |
| Male | 23 - 70 *109/l |
- Immature reticulocyte fraction (IRF)
| Floor | Reference values |
| Female | 3 — 15,9 % |
| Male | 2,3 — 13,4 % |
- Transferrin
| Age | Reference values |
| Less than 11 years old | 2.03 - 3 g/l |
| More than 11 years | 2 - 3.6 g/l |
Serum iron
Reasons for the increase:
- anemia (aplastic, hemolytic, pernicious), thalassemia;
- polycythemia;
- acute hepatitis, liver damage;
- hemochromatosis;
- hemosiderosis;
- nephritis;
- lead poisoning;
- excessive intake of iron supplements.
Reasons for the decline:
- chronic inflammation;
- Iron-deficiency anemia;
- blood loss;
- burns;
- malignant neoplasms;
- rheumatoid arthritis;
- myocardial infarction;
- nephrosis;
- malabsorption;
- pregnancy;
- uremia.
General blood analysis
1) Red blood cell count
Reasons for the increase:
- erythremia;
- chronic heart and pulmonary failure;
- stress, physical activity, staying at high altitudes;
- Itsenko-Cushing syndrome.
Reasons for the decline:
- anemia of various origins;
- acute and chronic leukemia;
- lymphomas;
- myelodysplastic syndrome;
- radiation sickness;
- renal failure;
- hypothyroidism;
- taking cytostatics.
2) Hemoglobin
Reasons for the increase:
- erythrocytosis;
- polycythemia;
- dehydration;
- burns;
- Cushing's syndrome;
- kidney cysts;
- liver cancer;
- heart failure;
- chronic lung diseases with respiratory failure.
Reasons for the decline:
- anemia of various origins;
- blood loss;
- myelodysplastic syndrome;
- Addison's disease;
- overhydration;
- acute and chronic leukemia, lymphoma;
- multiple myeloma;
- cirrhosis;
- chronic infection;
- hypothyroidism;
- kidney disease;
- pregnancy;
- autoimmune diseases;
- deficiency of vitamins B6, B12.
3) Mean erythrocyte volume (MCV)
Causes of increased (macrocytosis):
- megaloblastic anemia;
- myelodysplastic syndrome;
- liver diseases;
- pregnancy;
- hypothyroidism;
- alcoholism;
- treatment with estrogens, barbiturates.
Reasons for decrease (microcytosis):
- anemia (microspherocetary, iron deficiency, sideroblastic, thalassemia);
- anemia in chronic diseases;
- hypohydration;
- aluminum intoxication.
4) Dispersion of red blood cell distribution by volume (RDW)
Reasons for the increase (anisocytosis):
- anemia (hemolytic, iron deficiency, megaloblastic);
- osteomyelofibrosis.
5) Average hemoglobin content in erythrocytes (MCH)
Causes of increase (hyperchromia):
- megaloblastic anemia;
- cirrhosis of the liver;
- neonatal period.
Reasons for decrease (hypochromia):
- Iron-deficiency anemia;
- thalassemia;
- sideroblastic anemia.
6) Mean erythrocyte hemoglobin concentration (MCHC)
Reasons for the increase:
- megaloblastic anemia;
- microspherocytosis;
- prolonged hypohydration;
- neonatal period.
Reasons for the decrease (absolute hypochromia):
- Iron-deficiency anemia;
- thalassemia;
- sideroblastic anemia;
- hydremia.
7) Hematocrit usually decreases with anemia of various etiologies.
 The number of platelets can be increased in iron deficiency anemia and hemolytic crisis, decreased in aplastic or megaloblastic anemia.
The number of platelets can be increased in iron deficiency anemia and hemolytic crisis, decreased in aplastic or megaloblastic anemia.
9) The number of leukocytes decreases with megaloblastic or aplastic anemia, severe forms of iron deficiency anemia.
Reticulocytes
Reasons for increase (hyperregeneration):
- anemia (hemolytic, acute post-hemorrhagic);
- effective treatment of anemia caused by deficiency of iron, folic acid, vitamins B12 and B6 (on the 6-10th day);
- recovery from the state of bone marrow hypoplasia after cytostatic therapy;
- splenectomy;
- malaria.
Reasons for decrease (hyporegeneration):
- anemia (hypo- or aplastic, megaloblastic);
- anemia of chronic disease;
- myelodysplastic syndrome;
- acute leukemia;
- radiation sickness;
- regeneration crisis in hemolytic anemia;
- kidney pathology;
- taking cytostatics.
Transferrin
Reasons for the increase:
- Iron-deficiency anemia;
- pregnancy;
- taking estrogen.
Reasons for the decline:
- acute inflammation;
- anemia in chronic diseases;
- hypoproteinemia (with chronic infections, chronic kidney diseases, liver diseases, neoplasms, burns and malnutrition);
- genetic defects;
- hemochromatosis;
- oversaturation of the body with iron.
What can influence the result?
- Transfusion of blood and its components.
- Use of intravenous radiopaque agents shortly before the examination.
- Hemodialysis.
- Alcoholic liver disease, acute and chronic inflammatory diseases, neoplasms.
- Taking medications containing iron.
- The use of certain hormonal drugs, as well as other drugs that affect certain test indicators.
Diagnostics
Due to the absence of specific clinical manifestations of the disease, the only way to establish a diagnosis is laboratory tests:
- a general analysis (smear) reveals a low level of hemoglobin, hypo-, normo- or hyperchromia;
- biochemical analysis allows you to determine the presence or absence of folic acid or cyanocobalamin deficiency;
- A peripheral blood smear reveals a high level of red blood cell distribution and macrocytic changes in red blood cells.
Since macrocytic anemia can be caused by various pathologies and diseases (in fact, it is their symptom), a comprehensive diagnosis is carried out to determine the exact cause - ultrasound, CT, MRI, X-ray, endoscopy, gastroscopy and a number of other studies. Quite often, anemia is discovered by chance when examining a patient for another reason.
Treatment of macrocytic anemia
The therapeutic regimen is developed individually for each patient, depending on the severity of the condition and the cause of the pathology.
In mild forms caused by vitamin deficiency, a correction of the diet is sufficient. The diet should contain large amounts of animal protein, vitamins and iron. Patients are prescribed vitamin preparations to treat macrocytic anemia, such as B12 Ankermann (source of cyanocobalamin), Folacin (source of folic acid).
In moderate and severe forms, the patient is hospitalized and vitamins are administered parenterally.
If anemia is a consequence of any disease, appropriate therapy is required.
If the hemoglobin level decreases excessively, red blood cell transfusion is performed.
Complications
Low hemoglobin levels also mean lack of oxygen. Because of this, tachycardia develops and blood pressure rises. If treatment is not started, this can lead to degeneration of tissues and organs, circulatory disorders, myocardial dystrophy with an increase in heart size, and heart failure.
Vitamin B12 deficiency causes various neurological diseases.
If the pathology is caused by bone marrow pathologies, there is a risk of developing leukemia.