Briefly about the treatment method
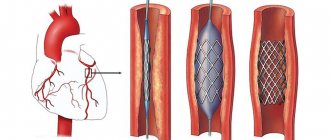
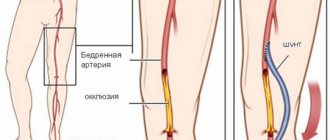
A femoropopliteal bypass is an open surgery to create a bypass for blood flow when there is a blockage in the femoral artery in the leg. The operation is indicated for critical ischemia and the threat of loss of a limb, but sometimes also for limiting intermittent claudication, if the patient does not have enough walking distance for everyday life. The operation consists of connecting a shunt (artificial vessel) to the common femoral artery in the groin area and passing it to the patent area of the popliteal artery above or below the knee joint. Femoropopliteal bypass surgery can be performed using an artificial prosthesis or using the patient's own vein. The operation usually lasts about 60 minutes and is performed under epidural or spinal anesthesia. The operation requires at least two surgical incisions. The efficiency of the operation is high. When used according to indications, the shunt patency is 80% within 5 years.
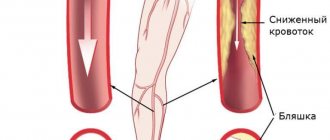
Treatment of critical lower limb ischemia (CLI) is one of the most difficult issues in modern vascular surgery. The incidence of CLI reaches 500–1000 cases per 1 million population per year [29].
To treat a patient with CLI, the doctor chooses one of two options: amputation of the affected limb or an attempt to restore blood flow through it. Currently, from 70 to 90% of amputations in the world are performed due to CLI; in Russia this figure is even higher [8, 9, 22]. When a leg is amputated, the quality of life sharply decreases and its duration decreases [32, 33, 41]. From a surgical point of view, amputation in patients of this category cannot be the operation of choice. With amputation below the knee joint, up to 60% of purulent-necrotic complications develop [38], repeated amputation in this case is required in another 15% of patients [29], and the mortality rate when performed exceeds 30% [12]. The wound after amputation above the knee heals better, but the procedure worsens the patient’s ability to rehabilitate.
The most important task for limb preservation is maintaining blood flow at the minimum required level. This is achieved in three ways [26, 28, 31, 45]:
— direct revascularization ( bypass surgery or percutaneous angioplasty of the great arteries (PCMA)
;
— indirect revascularization: denervating interventions, stimulation of neoangiogenesis, surgical (perforating osteotrepanation, transplantation of the greater omentum to a limb) and therapeutic (introduction of bone marrow mononuclear cells, gene preparations, platelet suspension);
- drug treatment.
A unified tactic for treating such patients has not yet been determined; each of the authors tries to show the most optimal method from his point of view as a “panacea”.
It is generally accepted that the most effective reconstruction option is direct limb revascularization. However, when performing interventions, the doctor is faced with a number of questions about the cost-effectiveness of the procedure, long-term results and the greatest safety for the patient.
Most patients with CLI have severe concomitant diseases, which increase mortality in the immediate postoperative period [21]. The best treatment option must be: minimally invasive, safe, easy to perform and accessible to use.
CLI is a life-threatening condition in which the main goal is to preserve the limb and life of the patient. From our point of view, treatment of CLI can and should be divided into two stages:
— Stage 1: direct limb salvage
the least invasive method with subsequent stabilization of the clinical condition. This stage should provide compensation for blood circulation in the leg. We consider the optimal condition to be the presence of main blood flow within 3 months after reconstruction. This period is sufficient to conduct a thorough examination and correction of concomitant diseases, for example, performing myocardial revascularization and/or reconstruction of brachycephalic arteries (BCA), as well as rehabilitation after these interventions;
— Stage 2: surgical reconstruction aimed at maintaining limb function “indefinitely” for a long time
.
Based on the above, we will analyze treatment options in 2 clinical situations: damage at the level of the femoropopliteal segment and at the level of the popliteal tibial segment. The aortofemoral segment was excluded from this review, since, according to the general opinion, this issue has been resolved in the international community: restoration of adequate flow to the leg is the key to the quality of care and does not depend on the treatment method (bypass operations have similar long-term patency rates compared to PCI) [24 ].
Restoration of blood flow in the femoral-popliteal segment
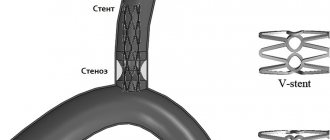
When planning reconstruction of the femoropopliteal segment, the surgeon is faced with a choice of 3 technologies: bypass surgery, PCI, or PCI with stenting. Modern surgical science believes that the most optimal situation for performing PCI is a short (up to 10 cm) stenosis or occlusion of the superficial femoral artery (SFA) [47]. Recently, work has been carried out to assess the safety of PCI in patients with extensive lesions, as well as lesions extending to the popliteal artery (class C and D according to the TASC II classification). The authors demonstrate a high percentage of successful PCI (about 94%) and satisfactory long-term results up to 2 years after the intervention [23]. Such results became possible with the advent of hydrophilic guidewires and subintimal angioplasty techniques. A large meta-analysis [18] demonstrated that primary PCI shows similar long-term results compared with PCI + stenting. The same work noted similar indicators of quality of life in patients of both groups. Other studies have shown that the 2-year patency of the stented segment without damage to the popliteal artery reaches 87%, and with its damage, only 75% of reconstructions work after 1 year [20, 46]. G. Rigatelli et al. [45] suggested using long-term balloon angioplasty with a pressure of 18 atmospheres for 2 minutes with repeated sessions until less than 30% of residual stenoses remain on the angiogram. According to their data, the average duration of dilatation was 5.9±1.9 minutes with the need for stent implantation in 11.9% due to the development of dissection. Primary patency of the reconstruction zone was 86.7% within 2 years, which corresponds to the results with implantation of covered stents. It should be noted that more than 60% of patients in this group had TASC C lesions.
Primary surgical revascularization is accompanied by low mortality and almost 100% effectiveness in saving the patient's limb. Based on literature data [35], it can be argued that the degree of preservation of the limb in the early stages after surgery is the same, regardless of the material used for bypass.
Femoropopliteal bypass (FPB) is currently a standard vascular operation that provides satisfactory results regardless of the extent of the FPA lesion [42]. When assessing long-term results (up to 5 years), it was shown that the patency of the autovenous vein is almost twice as high as when using a prosthesis (75% versus 42%, respectively) [49]. Taking these data into account, when performing reconstruction, it is recommended to use an autovenous shunt from the great saphenous vein [2], however, the results of the effective use of cadaveric allografts in this position have been described [26, 39].
The latter are more often used for infection of a previously installed shunt, but their standard use as biological material will reduce the time of surgery with satisfactory long-term results. The preserved native saphenous vein can be subsequently used for myocardial revascularization.
To improve long-term results, hybrid operations can be used: PCI in the proximal part and BPS with a short bypass from the distal part of the SFA to the popliteal artery (RCA) [43]. Another study [51] demonstrated that this approach reduces the number of postoperative complications by 2 times, but in the long term, limb salvage rates remain similar to surgical revascularization.
The difficulty of reconstructing the femoral-popliteal segment is its dependence on the condition of the tibial arteries. The better the distal channel, the better the results. Considering that about 17% of patients have an unsatisfactory course, it is necessary to think through the tactics of simultaneous treatment in this area of the limb [1].
In our opinion, in case of CLI, stage-by-stage correction of the channel is the most justified. This is due to the systemic reactions of the body to the start of blood flow after prolonged ischemia of the limb [1]. The main task of the surgeon is to select a group of patients in whom, immediately after restoration of blood flow through the SFA, reconstruction of the tibial arteries is necessary due to the high risk of early thrombosis of the reconstruction zone. For these patients, in our opinion, PCI of the tibial arteries should be performed. For patients with satisfactory blood flow, isolated reconstruction of the femoral-popliteal segment with further measures aimed at improving blood flow in the leg is indicated.
We believe that in patients with CLI at stage 1, PCI of the SFA should be performed without stenting. Indications for stenting are cases of intimal dissection and significant residual stenosis. Even with unsatisfactory mid-term results, this approach will allow you to save the limb, begin rehabilitation and continue treatment in conditions of chronic ischemia, using all available methods of correction of the distal bed and/or repeated endovascular interventions. It is also worth considering that PCI can be repeated an “unlimited” number of times to improve long-term results [3].
Restoration of blood flow along the popliteal tibial segment
With atherosclerotic lesions of the arteries below the level of the knee joint, surgeons are faced with the problem of low effectiveness of reconstructive operations. Surgical treatment in this area is associated with frequent limb loss as a result of rapid thrombosis of the shunt [5, 11].
The use of endovascular methods makes it possible to improve the outflow through the arteries of the leg, open occlusions and, importantly, restore blood flow along the plantar arch [7]. The latter is most significant for patients with diabetes, as well as in cases of threat of minor amputation. The problem with PCI is the rapid “restenosis” of the tibial arteries. When blood flow in the lower leg is corrected, the condition of the limb returns to the preoperative level in 48% of those operated on within 1 year [15, 33, 34]. Signs of restenosis during a follow-up examination are found in 2/3 of patients after 3 months [48]. For patients with diabetes, these results are of less importance - the main task in diabetic foot is to eliminate trophic changes (as the main infectious agent) during this period. The indicated 3 months are sufficient to resolve this issue in most patients [7]. Limb preservation in patients with CLI was observed in 96.7% after PCI was performed at the level of the leg [17].
In patients with CLI, amputation is performed due to lack of blood supply to the tissues. The surgeon is faced with a choice: to save the limb, it is necessary to perform angioplasty every 3-6 months, which is not economically justified, or during this period it is necessary to provide an alternative path of blood flow through the lower leg and foot.
The first option has high labor intensity and cost. To perform isolated PCI, it is necessary to improve modern technical support and drug approaches to treatment. The most common practice to improve the results of PCI is the implantation of stents in the area of stenosis after angioplasty, but a large meta-analysis [50] demonstrated the lack of effectiveness of this approach in peripheral arteries.
After peripheral angioplasty, all patients are prescribed dual antiplatelet therapy for 3 months, which is believed to improve long-term results [25]. At the same time, such studies have not been conducted directly for stenting the tibial arteries. Cilostazol may also be a drug that improves the results of PCI at the tibial level. In other vascular territories, it can significantly reduce the incidence of restenosis and amputation [37, 40].
The second way involves a hybrid approach. The use of angiogenesis stimulation seems to be a justified method. To date, two methods have been described: therapeutic angiogenesis - the introduction of bone marrow mononuclear cells, platelet-rich plasma or gene drugs [5, 13, 16, 30], and surgical angiogenesis - through transplantation of the greater omentum [10].
Therapeutic angiogenesis makes it possible to increase the distance of pain-free walking, reduce pain in CLI and reduce the frequency of amputations [6]. At the same time, the method is quite new and scientists still do not have an accurate understanding of the mechanism of action and the frequency of side effects, especially the development of malignant neoplasms. Surgical angiogenesis, as we understand it, is the use of the greater omentum to improve blood supply to the tissues of the leg and foot. Implantation of the omentum on the vascular pedicle has two places of application: closing skin defects and “sprouting” the muscles of the limb with vessels from adipose tissue. The main limitation for the successful use of greater omentum transplantation for ischemia of the lower extremities is the functional state of the regional circulation [14]. Freedom from amputation in patients with CLI for up to 5 years using combined treatment, shunt surgery together with implantation of the greater omentum on the lower leg, reaches 87%, while 42% had no restrictions on the duration of walking due to intermittent claudication [36].
Tactics of revascularization of the lower limb in critical ischemia
The effectiveness of the fight to save a limb in CLI in most cases depends on the condition of the distal vascular bed. With minimal changes in the tibial arteries, any method of reconstruction of the femoral-popliteal segment is suitable. A large study [19] demonstrated that in periods of up to 2 years, limb preservation is similar between BPS and PCI of the SFA; in longer periods, a clear advantage of using an autovein was revealed over other methods. Considering the severity of the patient's condition, PCI is probably the operation of choice with further preparation of the patient for distal bypass surgery.
If the distal flow is unsatisfactory, it is necessary to restore blood flow through the maximum number of tibial arteries using PCI. This allows the surgeon to stabilize the clinical situation and gives time to select a balanced tactic and correct concomitant pathology. Restenosis often develops within 3 to 6 months. During this time, it is necessary to compensate the limb and, if possible, improve the volume of the receiving distal channel. The most justified tactic may be the use of angiogenesis methods. Therapeutic angiogenesis has been poorly studied, so we propose the use of greater omentum transplantation. In our opinion, it needs to be transplanted on a vascular pedicle. The tributary artery can be any distal branch of the deep femoral artery. These branches are rarely affected by atherosclerosis and are located away from the access points to the SFA. We consider an important surgical technique to be the distribution of the mass of the greater omentum in the area of accumulation of the main muscle mass and its passage to the distal parts of the foot.
Based on the analyzed literature, it is not advisable to perform this operation in the acute period of ischemia, since for the best engraftment of the transplanted tissue, compensation of blood flow through the limb is necessary. In our opinion, this particular operation will allow the limb to be preserved for the longest time.
As a possible option, hybrid surgery should be considered: BPS + PCI of the tibial arteries. Among the disadvantages of the method, one can note the relatively short period of restoration of blood flow in the lower leg, the need for monthly monitoring of the patency of the tibial arteries, and repeated PCI in case of significant stenosis. These shortcomings can probably be solved through the use of modern antiplatelet agents.
Advantages of treatment at the ISC
The technique of femoral-popliteal bypass in our clinic has been developed to pinpoint precision. We use this operation most often as an element of hybrid surgery for severe lesions of the arteries of the lower limb. To improve the results of treatment of patients with multi-story lesions, we use a femoropopliteal bypass, through which we then perform angioplasty and stenting of the arteries of the leg. There is no need to perform femoral-popliteal bypass as an independent operation, since with isolated blockage of the femoral artery, patients most often do not have serious complaints.
A special feature of the operation in our clinic is mandatory ultrasound monitoring of blood flow during surgery. If we identify problems with the shunt, we perform X-ray contrast angiography and can use angioplasty to improve the results of the intervention.
Preparing for treatment
Preparation for surgery consists of a special examination to determine the nature of vascular damage:
- Ultrasound of the arteries of the lower extremities
- MSCT of the aorta and arteries of the extremities
General examination, determining concomitant pathology and risks of surgery:
- Echocardiography (ultrasound of the heart)
- Gastroscopy (EGD)
- X-ray of the lungs
- A set of tests for hospitalization
Direct preparation for the intervention:
- Shaving of the surgical field from the groin to the middle third of the leg is carried out on the day of surgery
- Do not eat after seven o'clock on the evening before surgery
- Cleansing enema at night
- Installation of a urinary catheter (directly on the operating table)
Why is the procedure necessary?
This operation is performed in the presence of steno-occlusive lesions of the femoral and iliac arteries. Helps prevent the development of atherosclerotic processes and avoid:
- lack of blood supply to the lower extremities;
- intermittent claudication;
- fatigue of the muscles of the lower leg, thigh and buttocks;
- obliterating atherosclerosis;
- ulcers;
- ischemia;
- gangrene;
- amputation.
Restoring the aorta eliminates pain in the buttock area, and the femoral and iliac arteries – in the lower leg and thigh area. The operation is also a prevention of foot ischemia.
Pain relief during treatment
The operation can be performed under local, spinal or epidural anesthesia. In our clinic, epidural anesthesia is used predominantly, since an epidural catheter allows for effective pain relief in the postoperative period.
The catheter is inserted into the lumbar region using a special needle. After inserting the catheter, a small amount of anesthetic is injected into the epidural space, and the patient talks about his sensations. Gradually, the dose increases and sensitivity in the legs is turned off, and then movement.
During the operation, the doctor connects a tracking monitor to the patient, which measures blood pressure using a cuff on the shoulder and takes a three-lead ECG. In addition, a special sensor is placed on the finger of the hand - a pulse oximeter, which measures the pulse wave and blood oxygen saturation (saturation).
How the treatment method works
The patient is placed on the operating table on his back. A bolster is placed under the knee of the affected leg. The entire leg and groin area is treated with a special antiseptic solution. The foot is placed in a special diaper, the groin area is also covered with a diaper.
Revision of the outflow artery
The incision to evaluate the artery can be in the lower third of the thigh (above the knee) or just below the knee joint, depending on the patency of a particular part of the artery according to the preliminary examination. The length of the incision is usually 7-10 centimeters.
After isolating the artery, the surgeon evaluates its density, the presence of atherosclerotic plaques, and using a two-pincer test, the occupancy of the artery from the lower leg is assessed. If the artery is found suitable for bypass, then it is taken on a holder; if not, then it is isolated in another area.
Revision of the tributary artery and proximal (upper) anastomosis
The next incision is made in the groin area. The common femoral artery should be isolated there, along with its branches - deep and superficial. This section of the artery is where the shunt will be powered from.
If the common femoral artery is suitable as a donor vessel, then it is clamped with vascular clamps. Before this, a heparin solution is administered intravenously. After clamping the artery, it is dissected longitudinally, by 2-3 cm. A vascular prosthesis is sewn into the incision of the artery with a blanket suture. Immediately after it is sewn in, the clamps are removed and the prosthesis is filled with saline solution with heparin.
Carrying out a shunt
The next step is to create a tunnel under the skin to accommodate the vascular graft. We try to place the prosthesis as deep as possible, under the muscles, since in case of infection in the wounds, it does not reach the infected area.
Distal anastomosis (lower anastomosis)
The prosthesis is removed into the lower wound, where a longitudinal incision is also made in the artery, and blood flow from the underlying sections is assessed. The prosthesis is sewn with a vascular suture into the artery wound. All vascular clamps are removed and blood flow starts.
Control study after reconstruction
In our clinic, this is always followed by intraoperative ultrasound of the restored arteries. Blood flow through the shunt and popliteal artery, and blood flow closer to the foot is also assessed. Tests with clamping of the shunt are required to assess how dependent the blood flow in the leg is on this shunt.
If a control ultrasound reveals problems with the patency of the artery in the anastomosis or in the arteries below, high resistance along the shunt, then we perform X-ray angiography to identify possible problems. If vascular narrowing is detected, we perform angioplasty or stenting of the problematic arteries.
Completing the operation
The bleeding of anastomoses is assessed. If no bleeding is observed, then special silicone tubes (drains) are installed in the wounds, which are connected to plastic bulbs. This is necessary to remove fluid accumulation near the anastomoses and control possible bleeding.
The patient's wounds are sealed with special bandages and he is transferred to the department for further observation.
Treatment methods for femoral artery occlusion
With limited occlusion, the body can compensate for the blood circulation of the limb using blood flow through the lateral branches of the arterial system (collateral circulation). In this case, conservative treatment is possible.
With increasing severity of ischemic symptoms, intermittent claudication occurring after less than 100 meters of walking, pain at rest, it is necessary to resort to surgical treatment. Such symptoms mean that circulatory compensation is insufficient, and this threatens the development of ulcerative-necrotic changes, gangrene and loss of limb.
Surgery
In the surgical treatment of occlusion, depending on the area of artery damage, the following are used:
- endarterectomy (removal of atherosclerotic deposits from the lumen of the artery);
- femoropopliteal bypass surgery;
- femoral-tibial bypass (if there is concomitant occlusion of the popliteal artery).
Make an appointment Do not self-medicate. Contact our specialists who will correctly diagnose and prescribe treatment.
Rate how useful the material was
thank you for rating
Possible complications during treatment
Femoropopliteal bypass surgery is a well-established operation and complications are rare. However, sometimes they still happen. We divided them into several groups.
Complications during surgery:
- Bleeding is very rare, more often with severe damage to the arterial wall by calcification.
- Damage to the deep veins is an extremely rare complication associated with holding a holder under the artery with a pronounced adhesive process. Leads to massive venous bleeding.
- Damage to nearby nerves - with a good knowledge of anatomy, practically does not occur. As a result, there may be a decrease in the sensitivity of the skin on the leg.
- Damage to the lymphatic vessels - can be observed with an incorrect approach to the vascular bundle in the groin area, or with repeated interventions through scar tissue.
General complications:
- Hemorrhagic shock - can occur when there is bleeding during surgery.
- Myocardial infarction is a rare complication observed in patients with initial severe coronary artery disease.
Postoperative complications:
- Bleeding in the postoperative period occurs most often on the first day after surgery, which is why drainage is installed for control.
- Lymphorrhea or lymphocele is the leakage of lymphatic fluid from a postoperative wound or its accumulation in the subcutaneous tissue. Correction requires puncture of fluid accumulations or careful dressings. Gradually goes away on its own.
- Lymphatic edema - there may be an increase in the volume of the leg by several centimeters. It goes away within 2-3 months.
- Suppuration of the prosthesis in the late postoperative period can occur due to poor wound healing and when an infection occurs on the vascular prosthesis. The complication is very dangerous and is fraught with additional complications. If detected, it requires removal of the vascular prosthesis and repeated vascular surgery to restore blood flow.
Color duplex scanning after bypass surgery on the arteries of the lower extremities
Ultrasound scanner HS70
Accurate and confident diagnosis.
Multifunctional ultrasound system for conducting studies with expert diagnostic accuracy.
Introduction
Modern clinical guidelines for the diagnosis and treatment of steno-occlusive diseases of the aorta and arteries of the lower extremities consider color duplex scanning as an effective method in assessing the nature, localization, extent and extent of damage in each segment of the arterial bed (class of indications I, level of evidence B) [1-4 ]. During the dynamic monitoring of patients who have undergone bypass surgery on the arteries of the lower extremities, ultrasound examination of blood vessels is the method of choice for diagnosing bypass stenosis and complications of reconstructive operations [5]. Numerous studies have shown that without preventive surgery, the risk of thrombosis of a stenotic bypass is about 25% [6-8], and regular monitoring using duplex scanning allows you to clarify or determine the indications for preventive interventions, including angioplasty or replacement of a fragment of the bypass vessel [4].
Types of bypass operations
Bypass operations, depending on the level of proximal (central) and distal anastomoses, are divided into aorto-femoral (aorto-bifemoral) bypass, iliofemoral, femoro-popliteal (above or below the knee joint gap), femoral-posterior tibial bypass, etc. As a bypass vessel (transplant), an autovenous vein and prostheses (synthetic or biological) can be used, which are anastomosed with the arteries according to the “end to end” (Fig. 1, 2) or “end to side” type (Fig. 3-5 ) [9,10].
Rice. 1.
Femoro-popliteal bypass with a combined prosthesis, end-to-end anastomosis.
Longitudinal section, arrow 1—the junction of the synthetic Dacron prosthesis (arrow 2) and the biological prosthesis (bovine internal mammary artery, arrow 3). The location of the intermediate anastomosis is above the level of the knee joint gap (the level of the upper edge of the patella).
Rice. 2.
Iliofemoral bypass with a synthetic Dacron prosthesis, proximal end-to-end anastomosis.
A)
Angiogram. Longitudinal section, arrow 1 - the junction of the common iliac artery (arrow 2) with the prosthesis (arrow 3).
b)
Echogram. Longitudinal section, arrow 1 - the junction of the common iliac artery (arrow 2) with the prosthesis (arrow 3).
Rice. 3.
Aorto-femoral bypass with a synthetic Dacron prosthesis, proximal anastomosis with the aorta in an “end-to-side” manner.
Longitudinal section, arrow 1 - aorta, arrow 2 - prosthesis, arrow 3 - lumen between the aorta and the prosthesis.
Rice. 4.
Iliofemoral bypass with a synthetic Dacron prosthesis, distal end-to-side anastomosis with the common femoral artery.
A)
Longitudinal section at the level of the distal anastomosis. Arrow 1 - wall of the synthetic prosthesis, arrow 2 - segment of the superficial femoral artery, arrow 3 - segment of the deep femoral artery, arrow 4 - posterior wall of the common femoral artery.
b)
The cross section is 3-5 mm above the level of the distal anastomosis (the level of the cross section is shown by a dot in figure “a”). Arrow 1 - prosthesis, arrow 2 - common femoral artery.
V)
Cross section at the level of the distal anastomosis. Arrow 1 - anterior wall of the prosthesis, arrow 2 - posterior wall of the common femoral artery.
Rice. 5.
Femoro-popliteal bypass with an autovenous vein, distal anastomosis with the popliteal artery according to the “end to side” type.
Longitudinal section. Arrow 1 - autovenous, arrow 2 - popliteal artery.
During reconstructive operations on the arteries of the femoral-popliteal and tibial segments, autovenous bypass (in the in situ position or reversed great saphenous vein) is characterized by a lower incidence of bypass thrombosis in the long-term period compared to bypass with a prosthesis [4, 11-12] (Fig. 5-7 ).
Rice. 6.
Stenosis of the femoral-popliteal autovenous shunt is more than 70%, the highest risk group. Stenosis outside the anastomotic zone - upper 1/3 of the bypass (middle 1/3 of the thigh).
A)
Dopplerogram of blood flow from the shunt 3-5 cm above the stenosis zone (Vps - 83 cm/s).
b)
Dopplerogram of blood flow from the area of stenosis (Vps - 430 cm/s).
V)
Dopplerogram of blood flow 8-10 cm distal to the zone of stenosis (Vps - 35 cm/s).
G)
Dopplerogram of blood flow from the shunt 2-3 cm above the level of the distal anastomosis (Vps - 25 cm/s).
Rice. 7.
Femoropopliteal autovenous shunt without signs of stenosis, low risk of shunt thrombosis.
A)
The area of the proximal anastomosis, longitudinal section, there is no filling defect in the CD mode.
b)
Dopplerogram of blood flow from the proximal anastomosis area (Vps - 125 cm/s).
V)
Dopplerogram of blood flow from the middle 1/3 of the shunt (Vps - 76 cm/s).
G)
Dopplerogram of blood flow from the shunt 2-3 cm above the level of the distal anastomosis (Vps - 80 cm/s).
Aortofemoral shunts have the longest effect [13-14]. For aortofemoral bypass surgery, synthetic prostheses are used, the material of which can be fluorolavsan or dacron with a ribbed structure (see Fig. 2-4), polytetrafluoroethylene or polyurethane [4, 10] with a smooth inner surface (Fig. 8).
Rice. 8.
Aortofemoral bypass with a synthetic prosthesis made of polytetrafluoroethylene, proximal anastomosis.
Arrow 1 - aorta, arrow 2 - prosthesis.
A number of studies have shown the high efficiency of operations using biological prostheses [15-16], which are specially treated umbilical veins or vessels of animals (cattle, pigs). Biological prostheses do not differ in structure from the native artery or vein (Fig. 9). However, biological prostheses, compared to autovenous and synthetic ones, can be more susceptible to aneurysmal expansion and subsequent thrombus formation (Fig. 10).
Rice. 9.
Femoro-popliteal bypass with a biological prosthesis (internal thoracic artery of a bull), middle 1/3 of the prosthesis.
Arrow 1 - biological prosthesis, arrow 2 - anechoic liquid formation (exudate) in the paraprosthetic space, an indirect sign of inflammation.
Rice. 10.
Aneurysmal dilatation and parietal thrombosis of a biological prosthesis.
The middle 1/3 of the femoral-popliteal bypass is shown, the prosthesis is unevenly expanded. Arrow 1—functioning lumen of the prosthesis, arrow 2—thrombomass at the level of maximum aneurysmal dilatation.
In some cases, a combination of prostheses is used as a bypass vessel (a synthetic prosthesis is connected to an autovenous vein or synthetic and biological prostheses are connected to each other) (see Fig. 1). If reconstruction of the femoropopliteal segment is performed after correction of a lesion in the aortoiliac segment, then the best results are observed when an anastomosis is formed with the branches of the prosthesis in the area of the distal anastomosis [4].
Duplex scanning frequency
Patients who have undergone autovenous bypass grafting should be periodically (at least during the first 2 years after surgery) examined by duplex scanning with measurement of peak systolic blood flow velocity (Vps) and calculation of the blood flow velocity ratio throughout the entire length of the shunt (indication class I, level evidence C) [4]. Duplex scanning examination is recommended in the first month after surgery. If autovenous graft stenosis is not detected in the first month (approximately 80% of grafts), observation is recommended at 6-month intervals during the first year, and then annually after the first year after autovenous grafting [10, 17]. Observation of prosthetic reconstructions, as after autovenous reconstructions, is recommended at 6-month intervals [17]. However, one of the key clinical recommendations, the Trans-Atlantic Inter-Society Consensus (TASC II), notes the need only for regular clinical examination and measurement of the brachial-ankle pressure index [18].
Basic Duplex Scanning Tasks
Due to the fact that shunt thrombosis is accompanied by a low probability of limb salvage, the primary objectives of dynamic ultrasound examination of vessels after bypass surgery are the detection of ultrasound signs of an increased risk of developing shunt thrombosis and early diagnosis of shunt thrombosis.
Diagnosis of shunt thrombosis is not difficult. The conclusion about thrombosis is made based on the detection of structures in the lumen of the shunt in B-mode, in the absence of blood flow in the shunt according to color and pulsed wave Doppler modes (Fig. 10, 11), as well as based on the characteristics of blood flow below the area of the distal anastomosis.
Rice. eleven.
Thrombosis (occlusion) of the shunt.
A)
Middle 1/3 of femoral-popliteal autovenous shunt. In the lumen of the shunt, heterogeneous, predominantly hyperechoic structures are visualized (arrow), blood flow is not recorded in the color flow mode.
b)
Femoro-popliteal bypass with a polytetrafluoroethylene prosthesis. Middle 1/3 of the prosthesis, anechoic structures in the prosthesis (arrow), blood flow is not recorded in the color flow mode.
Indirect signs of inflammation (exudate) in the projection of the prosthesis and infection of the prosthesis discovered during ultrasound examination (see Fig. 9) are unfavorable prognostic factors, accompanied by a high risk of shunt thrombosis in the early postoperative period.
The most significant and common precursors of shunt thrombosis are stenoses of the central and/or distal anastomoses, stenoses of shunts outside the anastomotic zones, as well as a decrease in the speed of blood flow through the shunt [10] (Fig. 6, 12, 13). The role of duplex scanning after autovenous bypass surgery has been studied in detail. It has been established that about 20% of infrainguinal autovenous shunts have residual postoperative stenosis or stenosis develops during the first year after reconstruction [6, 10]. Stenoses are localized both in the area of anastomosis and in any other segment of the shunt. For artificial or biological prostheses, stenotic damage to the prosthesis outside the anastomotic zones is less typical compared to autovenous shunts [10] (see Fig. 6).
Rice. 12.
Iliofemoral bypass with a synthetic Dacron prosthesis, stenosis in the area of the distal anastomosis 50-75%, high-risk group.
A)
Dopplerogram of blood flow from the shunt 3-5 cm above the level of the distal anastomosis (Vps - 34 cm/s).
b)
Dopplerogram of blood flow from the area of stenosis (Vps - 292 cm/s).
V)
Cross-section of the superficial femoral artery in the area of the distal anastomosis, planimetric assessment of the degree of stenosis “by diameter”, stenosis 59%.
G)
Main blood flow in the superficial femoral artery in the middle 1/3 of the thigh (Vps - 111 cm/s). Normal blood flow characteristics below the distal anastomosis exclude stenosis greater than 75% and confirm the results of the planimetric “diameter” assessment.
Rice. 13.
Iliofemoral bypass with a synthetic fluorolavsan prosthesis, low-velocity blood flow in the prosthesis.
Dopplerogram of blood flow from the middle 1/3 of the prosthesis (Vps - 30 cm/s), significant narrowing of the proximal and distal segments of the vascular bed were not detected.
In the protocol for ultrasound examination of the aorta and arteries of the lower extremities after bypass operations, special attention is paid to the description of structural and anatomical changes in the afferent vessels and the characteristics of blood flow in them, the characteristics of blood flow and assessment of the condition of the proximal, distal anastomoses and the bypass vessel along its entire length, as well as the description of the structural and anatomical changes in the efferent vessels and the characteristics of blood flow in them [10].
When studying a bypass vessel, emphasis is placed on analyzing Vps and calculating the ratio of the peak blood flow velocity in the zone of its local increase (in the area of stenosis) to the peak blood flow velocity recorded proximal to the stenosis (Vps-ratio). In addition, the ultrasound examination protocol requires the most complete description of the planimetric characteristics of the stenotic segment of the shunt, and in the case of autovenous shunting, an assessment of the cross-sectional diameter of the shunt vein above and below the stenosis, the extent of the stenosis, and a clear indication of the anatomical localization of the stenosis.
Doppler gradation of the degree of stenosis and the risk group for shunt thrombosis
There is a Doppler gradation of the degree of stenosis of autovenous shunts, which is based on the assessment of Vps in the area of stenosis and Vps-ratio [19], developed based on a comparison of duplex scanning data with angiography results (Table).
Table.
Dopplerographic criteria for the degree of narrowing of the autovenous shunt.
| Stenosis degree,% | Vps in the area of stenosis, cm/s | Vps-ratio, c.u. |
| 20-50 | 1,5-2,4 | |
| 50-75 | > 180 | 2,5-4,0 |
| > 75 | > 300 | > 4,0 |
However, the greatest predictive efficiency is in assessing the degree of risk of thrombosis of an autovenous shunt based on a combination of indicators such as Vps in the area of stenosis, Vps-ratio, average Vps in the shunt (the average value of the peak systolic blood flow velocity from 3-4 equidistant zones of the shunt outside the area of stenosis) and Ankle-brachial pressure index (ABP). Low blood flow through the shunt increases the risk of thrombosis [20]. The risk of thrombosis of an autovenous shunt is lower compared to an artificial prosthesis [10], and prophylactic administration of anticoagulant and antiplatelet therapy reduces the risk of thrombosis of low-speed shunts [6-7].
The highest risk group (I degree) with a threat of thrombosis in the near future includes shunts that are stenotic by more than 70% with Vps in the stenosis zone > 300 cm/s, Vps-ratio > 3.5 a.u., with low-velocity blood flow in shunt (average Vps
The group of high risk of thrombosis (grade II) includes shunts that are stenotic by more than 70% with Vps in the stenotic area > 300 cm/s, Vps-ratio > 3.5 a.u., with normal blood flow velocity along the shunt outside the area of stenosis (average Vps > 45 cm/s) and a decrease in LID (compared to the previous assessment) by less than 0.15 c.u.
Groups of the highest and highest risk of thrombosis (grades I and II) require revision of the shunt and surgical restoration of its patency. The probability of progression of stenosis or development of thrombosis in these groups within 3-6 months is 40-50%. For the highest risk group (grade I), immediate restoration of shunt patency is recommended, while for the high-risk group (grade II), planned restoration is possible within 1-2 weeks.
The group of moderate risk of thrombosis (III degree) includes shunts that are stenotic by 50-70% with Vps in the stenosis zone of 180-300 cm/s, Vps-ratio > 2 a.u., with normal blood flow velocity along the shunt outside the area of stenosis ( average Vps > 45 cm/s) and a decrease in LID (compared to the previous assessment) by less than 0.15 c.u.
The group of low risk of thrombosis (grade IV) includes shunts that are not stenotic or stenotic 45 cm/s) and a decrease in LID (compared to the previous assessment) by less than 0.15 cu. (see Fig. 7).
In risk groups of grades III and IV, shunt lesions detected in the first 3 months. from the moment of bypass, in 20-30% of cases they regress, in 10-20% of cases they remain stable, and in 40-50% of cases they progress to stenosis of more than 70%.
For the moderate-risk group, follow-up monitoring is recommended at intervals of 4-6 weeks to confirm or rule out progression of stenosis. During a dynamic study, the progression of stenosis is confirmed by an increase in Vps in the area of stenosis, a decrease in the average value of Vps along the shunt outside the area of stenosis, and a decrease in LID > 0.15 a.u. and, accordingly, transition to a high or highest risk group [10].
Compared with autovenous bypass, studies on the role of duplex scanning in monitoring prosthetic bypasses and determining the indications for surgery on modified prostheses are not so numerous. However, it has been shown that after prosthetic bypass surgery, the inclusion of duplex scanning in the monitoring program and the performance of reconstructive intervention on the bypass vessels based on its results is accompanied by a 5-year patency of the shunts of about 88% and exceeds those indicators without dynamic duplex monitoring [7]. In femorofemoral prosthetic bypass surgery, ultrasound precursors of shunt thrombosis are Vps in the afferent iliac artery of more than 300 cm/s, the presence of stenosis of the prosthesis with a local increase in Vps of more than 300 cm/s and the average value of Vps in the prosthesis of less than 60 cm/s [7]. There is no detailed Doppler gradation of the degree of stenosis and the degree of risk of thrombosis for synthetic and biological prostheses. In this regard, it is permissible to use ultrasound criteria for the degree of stenosis and the degree of risk of thrombosis of autovenous shunts for synthetic and biological prostheses (Fig. 12).
An average Vps value in the prosthesis of less than 45 cm/s can be used as a prognostic criterion for the risk of prosthetic shunt thrombosis [8]. However, the low blood flow velocity through the shunt (Fig. 13) first of all requires a thorough examination of the proximal and distal segments of the vascular bed in order to exclude/confirm significant narrowings. The independent meaning of low blood flow velocity through the shunt has not been fully determined, but requires the following indication in the ultrasound examination protocol: “a decrease in the speed of blood flow through the shunt.”
One of the predisposing factors to the development of thrombosis is kinking (torsion) of the shunt/prosthesis (Fig. 14), accompanied by a narrowing of the functioning lumen and local disturbances of blood flow in the form of an increase in Vps.
Rice. 14.
Aortofemoral bypass with a synthetic prosthesis made of Dacron, bending of the prosthesis.
A)
The longitudinal section of the prosthesis is 2-4 cm below the level of the proximal anastomosis, the arrow shows the bend of the prosthesis.
b)
Dopplerogram of blood flow from the inflection area, Vps locally increased to 150 cm/s.
Diagnosis of complications of bypass surgery
Duplex scanning is highly accurate in diagnosing such complications of bypass operations as vascular aneurysm in the area of proximal (central) and/or distal anastomoses (Fig. 15), prosthetic aneurysm along the length (more typical for biological prostheses, Fig. 10), diagnosis of pulsating hematoma (Fig. 16), false aneurysm in the area of proximal or distal anastomosis (Fig. 17), as well as in the diagnosis of arteriovenous fistulas (Fig. 18, 19).
Rice. 15.
Iliofemoral bypass with a synthetic Dacron prosthesis, aneurysm in the area of the distal anastomosis.
A)
Dopplerogram of blood flow from the shunt 1-2 cm above the level of the distal anastomosis (Vps - 120 cm/s).
b)
Zone of distal anastomosis, arrow 1 - prosthesis, arrow 2 - dilated common femoral artery (diameter - 17 mm).
V)
The main blood flow in the superficial femoral artery is 1-2 cm below the level of the distal anastomosis (Vps - 70 cm/s).
Rice. 16.
Pulsating hematoma in the ilioinguinal region. The study was conducted on the first day after surgery.
A)
Longitudinal section. Arrow 1 - hematoma cavity, arrow 2 - common femoral artery, arrow 3 - canal between the common femoral artery and the hematoma cavity, canal diameter - 3-3.5 mm.
b)
Dopplerogram of blood flow from the canal, Vps locally increased to 320 cm/s.
Rice. 17.
False aneurysm in the area of the distal anastomosis of the iliofemoral shunt. The study was conducted 3 months after surgery.
A)
Longitudinal section. Arrow 1 - aneurysmal sac, arrow 2 - wide base of the aneurysmal sac, arrow 3 - synthetic prosthesis, arrow 4 - superficial femoral artery.
b)
Dopplerogram from the shunt in front of the area of the aneurysmal sac, main blood flow, Vps - 84 cm/s.
V)
Dopplerogram from the middle 1/3 of the superficial femoral artery, distal to the aneurysmal sac, main blood flow, Vps - 103 cm/s.
Rice. 18.
Arteriovenous fistula between the superficial femoral artery and the superficial femoral vein, upper 1/3 of the thigh. Condition after thrombectomy from the superficial femoral artery.
A)
Dopplerogram of blood flow from the fistula area, fistula diameter is 2.5-3 mm. Arrow 1 - superficial femoral artery, arrow 2 - superficial femoral vein.
b)
Dopplerogram from the superficial femoral vein 3-5 cm distal to the fistula, the blood flow is pseudoarterial, Vps - 45 cm/s.
Rice. 19.
Arteriovenous fistula after autovenous shunting in situ in the lower 1/3 of the thigh. Study 1 day after bypass surgery.
A)
Dopplerogram of blood flow from an autovenous shunt 4-5 cm above the fistula (inflow of the autovenous vein), middle 1/3 of the thigh, the nature of the blood flow is main-altered (low-resistant), Vps - 120-130 cm/s, RI - 0.8 a.u.
b)
Dopplerogram from a fistula (inflow of the autovenous vein), the nature of the blood flow is arterial, Vps - 95 cm/s.
Conclusion
Obviously, duplex scanning is the primary diagnostic procedure for suspected shunt dysfunction at any time from the moment of reconstructive surgery. The results of a duplex scan performed by an experienced specialist can be considered as an indication for angiographic examination [9] or for preventive surgery on bypass vessels [4].
Literature
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Guidelines for the Management of Patients With Peripheral Arterial Disease (Lower Extremity, Renal, Mesenteric, and Abdominal Aortic): A Collaborative Report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) // J Am Coll Cardiol. 2006. N 47. P. e1-e192.
- Layden J., Michaels J., Bermingham S., et al. Diagnosis and management of lower limb peripheral arterial disease: summary of NICE guidance // BMJ. 2011. N 345. P. e4947.
- Rooke TW, Hirsch AT, Misra S, et al. ACCF/ AHA Focused Update of the Guideline for the Management of Patients With Peripheral Artery Disease (Updating the 2005 Guideline) // J Am Coll Cardiol. 2011. V. 58. N 19. P. 2020-2045.
- National recommendations for the management of patients with diseases of the arteries of the lower extremities (Russian Society of Angiologists and Vascular Surgeons, Association of Cardiovascular Surgeons of Russia, Russian Scientific Society of X-ray Endovascular Surgeons and Interventional Radiologists, All-Russian Scientific Society in Cardiologists, Associate Phlebologists of Russia) // Angiology and Vascular Surgery. 2013. T. 19. Appendix 2. P. 2-67.
- Tendera M., Aboyans V., Bartelink ML, et al. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC) // Eur Heart J. 2011. V. 32. N 22. P. 2851-2906.
- Tinder CN, Chavanpun JP, Bandyk DF, et al. Efficacy of duplex ultrasound surveillance after infrainguinal vein bypass may be enhanced by identification of characteristics predictive of graft stenosis development // J Vasc Surg. 2008. V. 48. P. 613-618.
- Stone PA, Armstrong PA, Bandyk DF, et al. Duplex ultrasound criteria for femoral-femoral bypass revision // J Vasc Surg. 2006. V. 44. P. 496-502.
- Brumberg SR, Back MR, Armstrong PA, et al. The relative importance of graft surveillance and warfarin therapy in infrainguinal prosthetic bypass failure // J Vasc Surg. 2007. V. 46. P. 1160-1166.
- Haimovici's vascular surgery. 6th edition / edited by Ascher Å., Veith FJ, Gloviczki P., et al. —Blackwell Publishing. 2012. 1317 pp.
- Rutherford's vascular surgery. 6th edition / edited by Cronenwett JL, Johnston KW - Elsevier Health Sciences. 2014. 2688 pp.
- Bradbury AW, Adam DJ, Bell J., et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: Analysis of amputation free and overall survival by treatment received // Journal of Vascular Surgery. 2010. V. 51. P. 18S-31S.
- Conte MS Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) and the (hoped for) dawn of evidence-based treatment for advanced limb ischemia // Journal of Vascular Surgery. 2010. V. 51. P. 69S-75S.
- Szilagyi DE, Elliott JP, Smith RF, et al. A thirty-year survey of the reconstructive surgical treatment of aortoiliac occlusive disease // J Vasc Surg. 1986. V. 3. N 3. P. 421-436.
- Nevelsteen A., Wouters L., Suy R., et al. Aortofemoral Dacron reconstruction for aorto-iliac occlusive disease: a 25-year survey // Eur J Vasc Surg. 1991. V. 5. P. 179-186.
- Barbarash L.S., Ivanov S.V., Zhuravleva I.Yu. and others. 12 years of experience in the use of bioprostheses for the replacement of infrainguinal arteries // Angiology and Vascular Surgery. 2006. T. 3. No. 12. P. 91-97.
- Zhuravleva I.Yu., Kudryavtseva Yu.A., Ivanov S.V. and others. Ways and prospects for improving infrainguinal arterial bioprostheses // Pathology of blood circulation and cardiac surgery. 2005. No. 1. P. 78-83.
- Mohler ER, Gornik HL, Gerhard-Herman M., et al. ACCF/ACR/AIUM/ASE/ASN/ICAVL/SCAI/ SCCT/SIR/SVM/SVS 2012 appropriate use criteria for peripheral vascular ultrasound and physiological testing, part 1: arterial ultrasound and physiological testing // J Am Coll Cardiol. 2012. V. 60. P. 242-276.
- Norgren L., Hiatt WR, Dormandy JA, et al. TASC II Working Group: Inter-Society consensus for the management of peripheral arterial disease (TASC II) // J Vasc Surg. 2007. V. 45. P. S5-S67.
- Jaff M., Mohler E., Roman M., et al. Guidelines for noninvasive vascular laboratory testing: a report from the American Society of Echocardiography and the Society for Vascular Medicine and Biology // Vasc Med. 2006. V. 11. N 3. P. 183-200.
- Bandyk D., Cato R., Towne J. A low velocity predicts failure of femoropoliteal and femorotibial bypass grafts // Surgery. 1985. V. 98. P. 799-809.
Ultrasound scanner HS70
Accurate and confident diagnosis.
Multifunctional ultrasound system for conducting studies with expert diagnostic accuracy.
Prognosis after treatment method
If the operation is performed according to strict indications, then its results are very good. More than 80% of femoral-popliteal shunts function within 5 years. If the closure of this shunt gradually develops, then the body compensates for blood flow bypassing this segment and the patient may not feel that the shunt has stopped working.
Secondary complications after femoral-popliteal bypass surgery develop in 10% of patients. Most often this is early thrombosis of the shunt. This outcome occurs when the operating surgeon underestimates the condition of the vascular bed, or when antithrombotic drugs are not taken. To prevent this complication, the condition of the shunt is regularly monitored using ultrasound.
Early thrombosis of the shunt can lead to the development of critical ischemia or gangrene. Timely re-intervention allows you to save the leg and prevent amputation.
Observation program after treatment method
Effective and long-term operation of the femoral-popliteal shunt is impossible without a detailed assessment of its performance. Most often, ultrasound angioscanning of the arteries of the operated limb is performed.
The first examination after discharge should be carried out one month after the operation. The condition of the wounds on the thigh, the presence of swelling and sensitivity disorders, the patient’s range of motion, and the distance of pain-free walking are assessed. Ultrasound of the arteries assesses the condition of the anastomoses and the speed of blood flow through the arteries in the foot.
Follow-up examinations are recommended every 6 months after surgery. A year later, it is advisable to perform MSCT of the arteries of the lower extremities with contrast. Or this study may be ordered by your vascular surgeon based on the results of a periodic examination.