Every person should know their blood pH. Acidification of the blood leads to illness and poor health. Severe alkalization - loose skin, dry and brittle hair, nails.
Every liquid has its own level of acid-base balance, including human blood. If there is a disorder in the functioning of the body or a malfunction of a specific system or organ, a PH blood test is done.
- The term blood pH is the level of hydrogen content in the body and general acidity. If the balance of alkalis is maintained, then all systems and organs function normally.
- The acid-base balance is in a normal state if the liver, lungs and kidneys function well and harmoniously. These are real “compensators” that remove harmful substances from the body.
- Therefore, every person should monitor their blood PH level to prevent the development of serious diseases.
Blood PH level of a healthy person: normal
PH level of the blood of a healthy person: normal
The level of alkaline indicator in the blood is the basis for doctors to prescribe treatment if they have large deviations from the norm. Thanks to these indicators, it is possible to monitor the condition of the body, and if malfunctions occur in the functioning of organs or systems, such an analysis should be done.
The normal PH level of the blood of a healthy person is no less than 7.35 and no higher than 7.45. All indicators that differ from the norm to a lesser or greater extent are deviations that are incompatible with life and require urgent medical intervention.
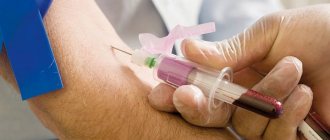
Blood pH tests
There are two main types of tests that doctors can use to determine the pH of your blood: an arterial blood gas test and an electrolyte test.
Knowing the pH of the blood can help a doctor figure out whether a patient has an acid-base disorder.
Doctors can also use these tests to monitor blood pH levels, identify and treat any underlying causes, and help people who are critically ill.
Arterial blood gas tests are usually performed in the hospital. They measure acidity, oxygen and carbon dioxide levels in the blood. The doctor takes a small amount of blood, often from the wrist. He then sends this sample to a laboratory for analysis.
Electrolyte tests may be part of routine first aid, or a doctor may perform them when a patient is seriously ill. The test measures the levels of salts and minerals, such as bicarbonate, that are present in the blood. The doctor will usually take blood from a vein in the arm.
The results of these tests can help your doctor determine what is causing certain symptoms and whether the body's regulatory systems are working properly.
Blood pH level during acidosis
Blood PH level during acidosis
If the acidity in the body is normal, then the PH readings will be at the level of 7.4 units. If this indicator decreases significantly, a diagnosis of “acidosis” is made. The pH level of the blood during acidosis is 7.0 or less.
Mild acidosis does not manifest itself in any way. But, if the indicators decrease to critical limits, which can only be recorded in laboratory conditions, then the person experiences the following symptoms:
- lack of oxygen;
- a feeling of shock at the primary stage of many diseases - diabetes and others;
- nausea;
- vomiting or urge to vomit;
- breathing problems.
Acidification of the body occurs for the following main reasons:
- nervous tension;
- obesity;
- against the background of cardiovascular diseases;
- when eating sweet and meat foods in large quantities.
When a severe form of acidosis is detected, it is necessary to establish the causes of this disease. You should definitely consult a doctor who will correctly prescribe treatment, diet and tell you about all the consequences if you do not control your blood PH level.
Symptoms of changes in blood pH
If the blood pH is outside the healthy range, he may begin to experience certain symptoms.
The symptoms people experience will depend on whether their blood becomes more acidic or less acidic.
Some symptoms of acidosis include:
- headache
- confusion
- fatigue
- lethargy and drowsiness
- cough and shortness of breath
- uneven or rapid heartbeat
- upset stomach or nausea
- muscle cramps or weakness
- loss of consciousness and coma
Symptoms of alkalosis include:
- confusion and confusion
- trembling hands
- numbness or tingling in the legs, arms, or face
- muscle cramps
- vomiting or nausea
- coma
Blood pH level in alkalosis
Blood PH level during alkalosis
Alkalosis, unlike acidosis, appears immediately as soon as the blood PH readings become above 7.45. When the body becomes strongly alkalized, the skin becomes flabby and dry. A person takes on the ugly appearance of a “dried wooden knot.”
The blood pH level during alkalosis is normalized if the causes that caused this deviation are eliminated. The treatment process can begin with breathing exercises. This will help saturate the blood with carbon dioxide and oxygen compounds in the correct proportion. Read more about acidification and alkalization of the body in this article.
Important: Do not self-medicate! It may be dangerous. Never make even rough diagnoses for yourself or your loved ones.
Blood pH level in cancer: comparison
Blood PH level in cancer: comparison
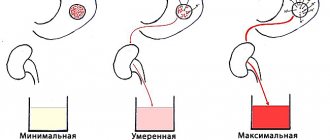
A person's blood PH level fluctuates throughout life. But there are critical indicators when chronic diseases and even cancer can occur. It is bad for the human body when the body is highly acidified, that is, PH values are below 7.45 units, and when a sharp alkalization occurs. If the indicator is below 6.0 units, then the alarm should be sounded.
Blood pH level in cancer is below 6.0. With such indicators, a person has a poor complexion, pale lips, no blush, hair and nails break. We can say that a person has a sick appearance.
Remember: Only a doctor should make a diagnosis! Don't do anything on your own. If you have any doubts about your health, get examined and take the necessary tests. You can only sound the alarm in a timely manner if you feel unwell or other symptoms that interfere with your normal life.
Only a doctor should compare blood pH levels in cancer. He will be able to correctly prescribe treatment and take emergency measures that will be a salvation for your health.
Acidity (pH)
Acidity
(lat.
aciditas
) - a characteristic of the activity of hydrogen ions in solutions and liquids. In medicine, the acidity of biological fluids (blood, urine, gastric juice and others) is a diagnostically important parameter of the patient’s health status. In gastroenterology, for the correct diagnosis of a number of diseases, for example, the esophagus and stomach, a one-time or even average acidity value is not significant. Most often, it is important to understand the dynamics of changes in acidity during the day (night acidity often differs from daytime) in several zones of the organ. Sometimes it is important to know the change in acidity as a reaction to certain irritants and stimulants.
Content
- pH value
- Some misconceptions
- pH value for some foods and water
- Acidity and Digestive Enzymes
- Acidity of saliva and oral cavity
- Acidity of the secretion of the pharynx and larynx
- Acidity in the esophagus
- Acidity in the stomach. High and low acidity
- Acidity in the intestines
- Stool acidity
- Blood acidity
- Acidity of urine
- Acidity of the vagina, cervical canal and uterine cavity
- Publications for healthcare professionals addressing the issue of acidity in the female genital organs
- Sperm acidity
- Skin acidity
- Acidity of other human biological fluids
- Publications for healthcare professionals addressing the issue of acidity in the digestive system
- Measures and standards of acidity
pH value
In solutions, inorganic substances: salts, acids and alkalis are separated into their constituent ions. In this case, hydrogen ions H+ are carriers of acidic properties, and OH− ions are carriers of alkaline properties. In highly dilute solutions, the acidic and alkaline properties depend on the concentrations of H+ and OH− ions. In ordinary solutions, acidic and alkaline properties depend on the activities of the aH and aOH ions, that is, on the same concentrations, but adjusted for the activity coefficient γ, which is determined experimentally. For aqueous solutions, the equilibrium equation applies: аН × аОН = Кw, where Кw is a constant, the ionic product of water (Кw = 10−14 at a water temperature of 22 °C). From this equation it follows that the activity of hydrogen ions H+ and the activity of OH− ions are interconnected. Danish biochemist S.P.L. In 1909, Sørensen proposed to show the hydrogen pH
, equal by definition to the decimal logarithm of the activity of hydrogen ions, taken with a minus (Rapoport S.I. et al.):
pH = - log (a H)
.
Based on the fact that in a neutral environment aH = aON and from the equality for pure water at 22 °C: aH× aON = Kw = 10−14, we obtain that the acidity of pure water at 22 °C (that is, neutral acidity) = 7 units pH.
Solutions and liquids with respect to their acidity are considered:
- neutral at pH = 7
- acidic at pH < 7
- alkaline at pH > 7
Some misconceptions
If one of the patients says that he has “zero acidity,” then this is nothing more than a turn of phrase, meaning, most likely, that he has a neutral acidity value (pH = 7).
In the human body, the acidity value cannot be less than 0.86 pH. It is also a common misconception that acidity values can only range from 0 to 14 pH. In technology, the acidity indicator can be negative or greater than 20. When talking about the acidity of an organ, it is important to understand that acidity can often differ significantly in different parts of the organ. The acidity of the contents in the lumen of the organ and the acidity on the surface of the mucous membrane of the organ are also often not the same. It is typical for the mucous membrane of the body of the stomach that the acidity on the surface of the mucus facing the lumen of the stomach is 1.2–1.5 pH, and on the side of the mucus facing the epithelium it is neutral (7.0 pH).
pH value for some foods and water
The table below shows the acidity values of some common foods and pure water at different temperatures:
| Product | Acidity, units pH |
| Lemon juice | 2,1 |
| Wine | 3,5 |
| Tomato juice | 4,1 |
| Orange juice | 4,2 |
| Black coffee | 5,0 |
| Pure water at 100 °C | 6,13 |
| Pure water at 50 °C | 6,63 |
| Fresh milk | 6,68 |
| Pure water at 22 °C | 7,0 |
| Pure water at 0°C | 7,48 |
Acidity and Digestive Enzymes
Many processes in the body are impossible without the participation of special proteins - enzymes, which catalyze chemical reactions in the body without undergoing chemical transformations.
The digestive process is not possible without the participation of a variety of digestive enzymes, which break down various organic food molecules and act only in a narrow range of acidity (different for each enzyme). The most important proteolytic enzymes (breaking down food proteins) of gastric juice: pepsin, gastrixin and chymosin (rennin) are produced in an inactive form - in the form of proenzymes and are later activated by hydrochloric acid of gastric juice. Pepsin is most active in a strongly acidic environment, with a pH of 1 to 2, gastrixin has maximum activity at pH 3.0–3.5, chymosin, which breaks down milk proteins into insoluble casein protein, has maximum activity at pH 3.0–3.5 . Proteolytic enzymes secreted by the pancreas and “acting” in the duodenum: trypsin has an optimum action in a slightly alkaline environment, at pH 7.8–8.0; chymotrypsin, which is close to it in functionality, is most active in an environment with an acidity of up to 8.2. The maximum activity of carboxypeptidases A and B is 7.5 pH. Similar maximum values are found for other enzymes that perform digestive functions in the slightly alkaline environment of the intestine.
Reduced or increased acidity relative to the norm in the stomach or duodenum, thus, leads to a significant decrease in the activity of certain enzymes or even their exclusion from the digestive process, and, as a consequence, to digestive problems.
Acidity of saliva and oral cavity
The acidity of saliva depends on the rate of salivation.
Typically, the acidity of mixed human saliva is 6.8–7.4 pH, but with high salivation rates it reaches 7.8 pH. The acidity of the saliva of the parotid glands is 5.81 pH, of the submandibular glands - 6.39 pH. In children, on average, the acidity of mixed saliva is 7.32 pH, in adults - 6.40 pH (Rimarchuk G.V. et al.).
Acid gastroesophageal and pharyngolaryngeal refluxes reaching the oral cavity play a leading role in the occurrence of oral pathology. As a result of the ingress of hydrochloric acid, the acidity of mixed saliva decreases below 7.0 pH. Saliva, normally enriched with calcium, phosphates, containing carbonates, sodium, potassium, magnesium and having alkaline properties, at low pH, especially at values of 6.2–6.0, leads to focal demineralization of tooth enamel with the appearance of erosions of hard dental tissues and the formation of cavities in them - caries (Novikova V.P., Shabanov A.M.).
The acidity of dental plaque depends on the condition of the hard tissues of the teeth. Being neutral in healthy teeth, it shifts to the acidic side, depending on the degree of development of caries and the age of adolescents. In 12-year-old adolescents with the initial stage of caries (precaries), the acidity of dental plaque is 6.96 ± 0.1 pH, in 12–13-year-old adolescents with average caries, the acidity of dental plaque is from 6.63 to 6.74 pH, in 16 -year-old adolescents with superficial and medium caries, the acidity of dental plaque is, respectively, 6.43 ± 0.1 pH and 6.32 ± 0.1 pH (Krivonogova L.B.).
Acidity of the secretion of the pharynx and larynx
The acidity of the secretion of the pharynx and larynx in healthy people and patients with chronic laryngitis and pharyngolaryngeal reflux is different (A.V. Lunev):
| Groups of surveyed | pH measurement location | |
| Pharynx , units pH | Larynx , units pH | |
| Healthy faces | 6,5 | 6,5 |
| Patients with chronic laryngitis without GERD | 5,6 | 5,6 |
| Patients with pharyngolaryngeal reflux | 4,9 | 4,9 |
| Patients with GERD without pharyngolaryngeal reflux | 5,2 | 5,2 |
The average pH value of the secretion of the pharynx and larynx within the groups does not have significant differences. In patients, when compared with a group of healthy individuals, the acidity of the secretions of the pharynx and larynx is higher (i.e., the pH is lower).
Acidity in the esophagus
Normal acidity in the esophagus is 6.0–7.0 pH. In addition to food and consumed liquid, the esophagus periodically receives saliva, which has neutral and slightly alkaline acidity, as well as refluxate, which is thrown from the stomach by gastroesophageal reflux. More often, refluxate has an acidity corresponding to the acidity of the stomach. Therefore, while gastric refluxate is in the esophagus, the acidity of the latter increases, the pH value decreases to 1.5–2. If there are relatively few gastroesophageal refluxes, they are considered physiological and do not affect the condition of the esophagus. Otherwise, refluxate may irritate the esophageal epithelium and develop gastroesophageal reflux disease.
The figure above shows a graph of acidity in the esophagus of a healthy person, obtained using intragastric pH-metry (Rapoport S.I.). The graph clearly shows gastroesophageal refluxes - sharp decreases in acidity to 2-3 pH, which in this case are physiological.
Acidity in the stomach. High and low acidity
The maximum observed acidity in the stomach is 0.86 pH, which corresponds to an acid production of 160 mmol/l.
The minimum acidity in the stomach is 8.3 pH, which corresponds to the acidity of a saturated solution of HCO3- ions. Normal acidity in the lumen of the body of the stomach on an empty stomach is 1.5–2.0 pH. The acidity on the surface of the epithelial layer facing the lumen of the stomach is 1.5–2.0 pH. The acidity in the depths of the epithelial layer of the stomach is about 7.0 pH. Normal acidity in the antrum of the stomach is 1.3–7.4 pH. The cause of many diseases of the digestive tract is an imbalance in the processes of acid production and acid neutralization. Long-term hypersecretion of hydrochloric acid or lack of acid neutralization, and, as a consequence, increased acidity in the stomach and/or duodenum, causes so-called acid-dependent diseases. Currently, these include: peptic ulcer of the stomach and duodenum, gastroesophageal reflux disease (GERD), erosive and ulcerative lesions of the stomach and duodenum while taking aspirin or non-steroidal anti-inflammatory drugs (NSAIDs), Zollinger-Ellison syndrome, gastritis and gastroduodenitis with high acidity and others.
Reduced acidity is observed with anacid or hypoacid gastritis or gastroduodenitis, as well as with stomach cancer. Gastritis (gastroduodenitis) is called anacid or gastritis (gastroduodenitis) with low acidity if the acidity in the body of the stomach is approximately 5 units or more. pH. The cause of low acidity is often atrophy of parietal cells in the mucous membrane or disturbances in their functions.
Above is a graph of the acidity (daily pH gram) of the body of the stomach of a healthy person (dashed line) and a patient with a duodenal ulcer (solid line). Moments of eating are marked with arrows labeled “Food”. The graph shows the acid-neutralizing effect of food, as well as increased stomach acidity with duodenal ulcer (Yakovenko A.V.).
Acidity in the intestines
Normal acidity in the duodenal bulb is 5.6–7.9 pH.
The acidity in the jejunum and ileum is neutral or slightly alkaline and ranges from 7 to 8 pH. The acidity of small intestine juice is 7.2–7.5 pH. With increased secretion it reaches 8.6 pH. The acidity of the secretion of the duodenal glands is from pH 7 to 8 pH. The acidity of colon juice is 8.5–9.0 pH.
In the lower parts of the colon, pH values of acidity gradually increase, reaching a maximum pH value in the region of the rectosigmoid junction. The following table shows the acidity in the sigmoid and rectum of a healthy person, obtained by targeted endoscopic pH-metry (Churkin I.A.):
| Measuring point | Point number in the figure | Acidity, units pH |
| Proximal sigmoid colon | 7 | 7,9±0,1 |
| Middle sigmoid colon | 6 | 7,9±0,1 |
| Distal sigmoid colon | 5 | 8,7±0,1 |
| Supraampullary rectum | 4 | 8,7±0,1 |
| Upper ampullary rectum | 3 | 8,5±0,1 |
| Mid-ampullary rectum | 2 | 7,7±0,1 |
| Inferior ampullary rectum | 1 | 7,3±0,1 |
Stool acidity
The acidity of the feces of a healthy person eating a mixed diet is determined by the vital activity of the colon microflora and is equal to 6.8–7.6 pH. Stool acidity is considered normal in the range from 6.0 to 8.0 pH. The acidity of meconium (original feces of newborns) is about 6 pH. Deviations from the norm for stool acidity:
- sharply acidic (pH less than 5.5) occurs with fermentative dyspepsia
- acidic (pH from 5.5 to 6.7) may be due to impaired absorption of fatty acids in the small intestine
- alkaline (pH from 8.0 to 8.5) may be due to the rotting of food proteins not digested in the stomach and small intestine and inflammatory exudate as a result of activation of putrefactive microflora and the formation of ammonia and other alkaline components in the large intestine
- sharply alkaline (pH more than 8.5) occurs with putrefactive dyspepsia (colitis)
Blood acidity
The acidity of human arterial blood plasma ranges from 7.37 to 7.43 pH, averaging 7.4 pH.
The acid-base balance in human blood is one of the most stable parameters, maintaining acidic and alkaline components in a certain balance within very narrow limits. Even a small shift from these limits can lead to severe pathology. When shifting to the acidic side, a condition called acidosis occurs, and to the alkaline side, alkolosis occurs. A change in blood acidity above 7.8 pH or below 6.8 pH is incompatible with life. The acidity of venous blood is 7.32–7.42 pH. The acidity of red blood cells is 7.28–7.29 pH.
Acidity of urine
In a healthy person with a normal drinking regime and a balanced diet, the acidity of urine is in the range from 5.0 to 6.0 pH, but can range from 4.5 to 8.0 pH.
The acidity of the urine of a newborn under the age of one month is normal - from 5.0 to 7.0 pH. The acidity of urine increases if a person’s diet is dominated by meat foods rich in proteins. Heavy physical work increases the acidity of urine. A dairy-vegetable diet causes urine to become slightly alkaline. Increased acidity of urine is observed with increased acidity of the stomach. Reduced acidity of gastric juice does not affect the acidity of urine. A change in urine acidity most often corresponds to a change in blood acidity. The acidity of urine changes with many diseases or conditions of the body, so determining the acidity of urine is an important diagnostic factor.
Under normal conditions, in a healthy person, the urine reaction is slightly acidic, the pH fluctuates depending on the diet between 4.5 and 8. With protein-rich foods, urine usually gives an acidic reaction, and with plant foods, an alkaline reaction. When diphosphates are released, a slightly alkaline reaction is observed. Under pathological conditions, urine can also give a strongly alkaline reaction, and in this case it is usually cloudy. With respiratory alkalosis (hyperventilation) or metabolic alkalosis, loss of acids with gastric juice, and a decrease in the amount of potassium in the blood, the urine reaction becomes alkaline. An acidic urine reaction is observed during fasting, severe diarrhea, acidosis, or after ingestion of acidifying drugs (ammonium chloride). Research is carried out only with freshly released urine. The easiest way to conduct research is with blue and red litmus paper. Acidic urine changes the litmus color from blue to red, and alkaline urine changes the litmus color from red to blue. The true reaction of urine is better determined by universal indicator paper with a range from 1-14 (Ishmanov M.Yu. et al.).
Acidity of the vagina, cervical canal and uterine cavity
The normal acidity of a woman's vagina ranges from 3.8 to 4.4 pH and averages 4.0 to 4.2 pH. Vaginal acidity in various diseases:
- cytolytic vaginosis: acidity less than 4.0 pH
- normal microflora: acidity from 4.0 to 4.5 pH
- candidal vaginitis: acidity from 4.0 to 4.5 pH
- Trichomonas colpitis: acidity from 5.0 to 6.0 pH
- bacterial vaginosis: acidity greater than 4.5 pH
- atrophic vaginitis: acidity greater than 6.0 pH
- aerobic vaginitis: acidity greater than 6.5 pH
Lactobacilli (lactobacillus) and, to a lesser extent, other representatives of normal microflora are responsible for maintaining an acidic environment and suppressing the growth of opportunistic microorganisms in the vagina.
In the treatment of many gynecological diseases, restoration of the lactobacilli population and normal acidity comes to the fore. According to Ivshin V.G. et al. (2020) in the area of the external pharynx the environment is acidic: pH = 6.42 ± 0.026. Along the course of the cervical canal, the pH value gradually increases. In the area of the internal pharynx, the pH value approaches neutral: 6.96 ± 0.013. As the sensor moves into the uterine cavity, a gradual increase in pH is observed from 7.14 ± 0.11 to 7.19 ± 0.11. On the mucous membrane of the uterine fundus, the environment is slightly alkaline: pH = 7.22 ± 0.007.
Publications for healthcare professionals addressing the issue of acidity in the female genital organs
- Murtazina Z.A., Yashchuk G.A., Galimov R.R., Dautova L.A., Tsvetkova A.V. Office diagnostics of bacterial vaginosis using hardware topographic pH-metry. Russian Bulletin of Obstetrician-Gynecologist. 2017;17(4): 54-58.
- Yashchuk A.G., Galimov R.R., Murtazina Z.A. A method for express diagnostics of disorders of vaginal biocenosis using hardware topographic pH-metry. Patent RU 2651037 C1.
- Gasanova M.K. Modern approaches to the diagnosis and treatment of serozometra in postmenopause. Abstract of dissertation. PhD, 14.00.01 - obstetrics and gynecology. RMAPO, Moscow, 2008.
Sperm acidity
The normal acidity level of sperm is between 7.2 and 8.0 pH. Deviations from these values are not in themselves considered pathology. At the same time, in combination with other deviations, it may indicate the presence of a disease. An increase in the pH level of sperm occurs during an infectious process. A sharply alkaline reaction of sperm (acidity approximately 9.0–10.0 pH) indicates prostate pathology. When the excretory ducts of both seminal vesicles are blocked, an acidic reaction of the sperm is observed (acidity 6.0–6.8 pH). The fertilizing ability of such sperm is reduced. In an acidic environment, sperm lose motility and die. If the acidity of the seminal fluid becomes less than 6.0 pH, the sperm completely lose their motility and die.
Skin acidity
The surface of the skin is covered with a water-lipid acid mantle
or
Marchionini mantle
, consisting of a mixture of sebum and sweat, to which organic acids are added - lactic, citric and others, formed as a result of biochemical processes occurring in the epidermis.
The acidic water-lipid mantle of the skin is the first barrier of protection against microorganisms. For most people, the normal acidity of the mantle is 3.5–6.7 pH. The bactericidal property of the skin, which gives it the ability to resist microbial invasion, is due to the acidic reaction of keratin, the peculiar chemical composition of sebum and sweat, and the presence on its surface of a protective water-lipid mantle with a high concentration of hydrogen ions. The low molecular weight fatty acids it contains, primarily glycophospholipids and free fatty acids, have a bacteriostatic effect that is selective for pathogenic microorganisms. The surface of the skin is populated by normal symbiotic microflora, capable of existing in an acidic environment: Staphylococcus epidermidis, Staphylococcus aureus, Propionibacterium acnes
and others. Some of these bacteria themselves produce lactic and other acids, contributing to the formation of the skin's acid mantle.
The upper layer of the epidermis (keratin scales) is acidic with a pH value of 5.0 to 6.0. In some skin diseases, the acidity level changes. For example, with fungal diseases the pH increases to 6, with eczema to 6.5, with acne to 7.
Acidity of other human biological fluids
The acidity of fluids inside the human body normally coincides with the acidity of the blood and ranges from 7.35 to 7.45 pH. The normal acidity of some other human biological fluids is shown in the table:
| Biological fluid | Acidity is normal, units. pH |
| Cytoplasm of cells | about 7.45 |
| A tear | from 7.3 to 7.5 |
| CSF (cerebrospinal fluid) | from 7.35 to 7.8 |
| Bile | from 8.0 to 8.5 |
| Human milk | from 6.9 to 7.5 |
| Pancreatic juice | from 7.5 to 9.0 |
| Synovial fluid (knee joint) | from 7.3 to 7.6 |
| Prostate juice | from 6.6 to 6.8 |
Publications for healthcare professionals addressing the issue of acidity in the digestive system
- Rapoport S.I., Lakshin A.A., Rakitin B.V., Trifonov M.M. pH-metry of the esophagus and stomach in diseases of the upper digestive tract / Ed. Academician of the Russian Academy of Medical Sciences F.I. Komarova. – M.: ID MEDPRACTIKA-M. — 2005. – p. 208.
- Churkin I.A. The use of targeted endoscopic pH-metry to assess the functional state of the mucous membrane of the rectum and sigmoid colon. Abstract of dissertation. PhD, 03.00.13 – physiology. ASMU, Tomsk, 2002.
- Khrustaleva E.V., Pedder V.V., Shishkina N.M., Lubyanskaya T.G. Relationship between the pH level of the mucous membrane of the oropharynx and the presence of fungal flora in patients with GERD // Medical Sciences. — 2013 — No. 6.
On the website, in the Literature section, there is a subsection “Acid-dependent gastrointestinal diseases”, containing articles for healthcare professionals on this topic.
Measures and standards of acidity
For verification and calibration of acidity measuring instruments in medicine and technology, special “acidity measures”, “buffer solutions”, and “standard titers” are produced.
They are capable of maintaining a strictly established value of the acidity of the solution, which does not change during measurements and for a certain time. For more information, see Standard Titers and Calibration Buffer Solutions. In the photo on the right: buffer solutions with pH=1.2 and pH=9.18 for calibrating pH probes.
Back to section
How to measure blood PH at home with a device, test strips?
How to measure blood PH at home with a device, test strips?
Of course, if you have any health problems, you should go to the clinic to see a doctor. But it often happens that we don’t have time to go to the hospital - don’t be upset. You can measure blood PH at home with a device or test strips.
A special device is sold at a pharmacy or any medical equipment store. It is inexpensive, but very useful for measuring blood PH at home. If you can’t find such a device, use test strips. They are sold in every pharmacy and cost pennies. If you don’t find any strips or a tester at the pharmacy, you can order everything you need online.
How to measure blood PH at home with test strips - tips:
- Prick the finger of your right hand with a scarifier, which is also sold in pharmacies.
- Squeeze some blood into a small container. It's good if you have a laboratory test tube.
- Dip the test strip into this blood, hold it for a few seconds, remove it from the tube, and evaluate the result.
- The scale for determining the alkaline reaction in the body is on the package with strips. Compare the color and find out the result.
When measuring PH values using the device, it is faster, easier and more convenient. You do not need to pierce your finger, the device will do everything on its own: puncture, sampling and give the result.
Where can I get a blood PH test?
Where can I get a blood PH test?
Laboratory tests are much more accurate than those obtained at home. If you decide to take a blood PH test in a special laboratory, you can go to the clinic at your place of registration or to any private clinic. The analysis will be ready on the day of blood sampling. The doctor himself may suggest that you take a test during a routine examination or preventive procedures if there are deviations in your health.
What does blood PH depend on?
What does blood PH depend on?
If the pH level becomes too low - less than 7.35 (acidic) or too high - more than 7.45-8 (alkaline), then the cells of our body begin to poison themselves with toxic emissions and die. Slags and toxins appear in large quantities. In this case, many people begin to remove these harmful substances from the body. But you just need to bring the PH levels of blood, urine and saliva back to normal. What does blood PH depend on?
This indicator depends on the following factors:
- Nutrition - you need to learn the basics of proper nutrition. Our body must maintain a balance of proteins, fats and carbohydrates.
- Stress resistance - constant nervous tension leads to acidification of the body. Learn to be calm and not get nervous over trifles.
- Obesity - When the body is acidic, it begins to accumulate fat. If you alkalize, you will immediately begin to lose weight, which means your well-being, skin and hair condition will improve.
The acid-base balance in the body depends on maintaining the correct proportions between intercellular and intracellular waters in the tissues. If the acid-base balance of liquids is not maintained constantly, it will be impossible to preserve life and the normal functioning of all organs and systems.
What does PH depend on?
Reasons for changes in blood pH
Metabolic changes in blood pH can occur as a result of kidney disease or kidney problems. Respiratory changes are related to how the lungs work.
When a change occurs in one direction, mechanisms exist to move the acid-base balance in the other direction. For example, if a person has respiratory acidosis, there must be a metabolic response from the kidneys to restore balance.
If the body does not reset its pH balance, it can lead to more serious illnesses. For example, this can happen if the level of acidosis is too severe, or if the kidneys are not working well.
Depending on the cause, changes in blood pH can be long-term or short-term.
The following sections will look at the specific causes of each type of blood pH change.
Metabolic acidosis
Metabolic acidosis can be caused by:
- kidney damage leading to a buildup of urea and other waste products in the blood
- heavy exercise, which produces lactic acid
- consumption of certain substances such as aspirin, methanol or paraldehyde
- loss of bicarbonate from the body, such as with chronic diarrhea
- infection
- excess acids called ketones in the blood
Ketoacidosis usually occurs in people with diabetes or due to alcohol abuse.
Respiratory acidosis
Respiratory acidosis occurs due to conditions that make breathing difficult. These include:
- lung diseases such as pneumonia or chronic obstructive pulmonary disease
- chronic heart failure
- severe obesity
- myasthenia gravis
- Guillain-Barre syndrome
- brain injury
The use of drugs such as opiates can also lead to respiratory acidosis.
Metabolic alkalosis
Some causes of metabolic alkalosis include:
- excess intake of bicarbonate, antacids or citrate
- Cushing's disease, in which there is too much of the hormone cortisol in the blood
- prolonged vomiting or severe dehydration
- excess fluid in the body
- using large amounts of laxatives
- some diuretics, which are medications that help the body get rid of excess water or salt
Respiratory alkalosis
Respiratory alkalosis often occurs due to situations or conditions that cause you to breathe faster or deeper than normal. These include:
- shock, fear or panic
- heat
- serious infection
- some lung diseases such as pneumonia
- pulmonary embolism
- liver failure
- overdosing on aspirin because the body overcompensates for high acid levels
How to reduce acidity and raise blood pH?
How to reduce acidity and raise blood pH?
Acid-base balance is our indicator of health. The more “sour” a person is, the faster he begins to age and get sick more. For the normal functioning of all organs and systems, the PH level in the body must be alkaline at least 7.35 units. How to reduce acidity and raise blood pH? Some tips:
- Eliminate meat products from your diet. You can fish, but in small quantities.
- It is important to make your nutrition correct. Get the right ratio of proteins, fats and carbohydrates. Use boiled and steamed dishes, exclude everything fried. Eat more fresh fruits and vegetables.
- Stop being nervous. Reconsider your attitude towards life - eliminate stressful situations.
- Practice separate meals. This will help the body quickly reduce acidity and normalize digestion. Products consumed separately will be better digested.
You can use special drops that are sold in pharmacies to alkalize the water. Alkaline water will help reduce acidity levels, and the kidneys, stomach and intestines will begin to work well. If your body is highly acidic, switch to a raw food diet.
But remember! Conducting experiments on your own is dangerous! Consult your doctor before following an alkaline diet or drinking alkaline water.
Blood acid imbalance
Acid-base balance is an important parameter that is maintained in human blood within certain limits. This is necessary for the normal functioning of various body systems, the occurrence of biochemical reactions, and the optimal functioning of enzymes.
Acids are substances that can give off hydrogen ions, and bases (alkalis) are substances that attach these ions. The acidity and alkalinity of solutions is assessed on a pH scale from 0 (solutions of strong acids) to 14 (solutions of strong alkalis). On the pH scale, neutral acidity is 7.
Normal blood acidity is 7.35 – 7.45 on the pH scale. A shift of this indicator below 7.35 indicates acidosis (a shift in the acid-base balance of the blood towards increasing acidity). When the pH deviates above 7.45, alkalosis occurs (excess of substances with alkali properties in the blood).
During the process of metabolism in the body, products are formed in large quantities that can cause a change in this parameter. The main role in the regulation of acid-base balance belongs to the lungs, kidneys and blood buffer systems.
During breathing, carbon dioxide is released through the lungs, which is formed during the metabolic process in the body. Carbon dioxide, when combined with water, forms carbon dioxide, therefore, if there is an excess of it in the blood, acidosis develops, and if the concentration of carbon dioxide is insufficient, alkalosis occurs.
The kidneys remove excess acids and alkalis from the body with urine. At the same time, these organs, within certain limits, can regulate the amount of acids and bases released and absorbed back, due to which the pH level in the blood is regulated.
Blood buffer systems are solutions of weak acids and alkalis, which combine with excess amounts of acids or bases (depending on the presence of acidosis or alkalosis) to neutralize them, thereby equalizing the pH level.
The cause of acidosis and alkalosis in most cases is the severe course of the underlying disease, in which the resulting changes in blood pH exceed the capabilities of the mechanisms regulating this parameter.
Synonyms Russian
Disorders of the acid-base balance of the blood, disturbances of acid-base homeostasis.
English synonyms
Acid-Base Disorders, Acid-Base Homeostasis.
Symptoms
Manifestations of acidosis and alkalosis are often masked by manifestations of the underlying disease, which caused a change in the acid-base balance of the blood.
Acidosis may have the following symptoms:
- nausea, vomiting
- increased breathing rate
- headache
- disturbance of consciousness (up to coma)
- drop in blood pressure (in severe forms of acidosis)
- heart rhythm disturbances.
Manifestations of alkalosis may include:
- headache
- dizziness
- depression of consciousness (up to coma)
- cramps in various muscle groups
- heart rhythm disturbances
General information about the disease
The acid-base balance in the blood is a vital parameter, the normal values of which are 7.35 - 7.45 on the pH scale.
A pH deviation below 7.35 indicates acidosis. When the pH shifts above 7.45, alkalosis occurs.
Depending on the causes of development, acidosis and alkalosis are divided into metabolic (metabolic) and respiratory (breathing).
Respiratory acidosis develops as a result of the accumulation of large amounts of carbon dioxide in the blood, which combines with water to form carbon dioxide. This causes an increase in blood acidity. This condition can develop with breathing disorders that cause decreased pulmonary ventilation.
This may be the result of lung diseases (for example, bronchial asthma), damage to the nervous system (for example, with brain injuries), diseases, muscles and nerves that lead to loss of the ability to make effective breathing movements (for example, with amyotrophic lateral sclerosis).
The opposite condition is respiratory alkalosis, which occurs when the lungs remove excess carbon dioxide from the body. The mechanism for the development of this type of alkalosis is based on an increase in the rhythm and depth of breathing.
Such breathing disorders can occur in the presence of pathology from various organs and systems (for example, injuries, brain tumors, lung diseases, cardiovascular failure).
Metabolic acidosis can develop for the following reasons:
- increased production of acids in the body. An increase in acid production in the body can be observed in conditions accompanied by metabolic disorders. For example, in diabetes mellitus, the use of glucose by cells is impaired due to a lack of the hormone insulin.
At the same time, the body begins to produce energy not from glucose, but from fats - an alternative way to obtain energy. The breakdown of fats in the liver is accompanied by the formation of large quantities of ketone acids, which leads to acidosis.
- impaired renal function. The kidneys play an important role in regulating the acid-base balance in the blood. In case of kidney diseases that lead to disruption of their functions, the processes of acid secretion and absorption of substances with an alkaline reaction may be disrupted, which can cause acidosis.
- loss of large quantities of alkalis with digestive juices. This condition can be observed with severe diarrhea, surgical interventions on the intestines.
- poisoning with poisons and toxic substances. The breakdown of these substances in the body can occur with the formation of large amounts of acids, which can cause acidosis.
The main causes of metabolic alkalosis are the following:
- loss of large amounts of acidic gastric contents. It can be observed with profuse vomiting, aspiration of stomach contents using a special probe.
- use of diuretics
- increased excretion of hydrogen ions by the kidneys. Such processes can be observed with an excess of the adrenal hormone aldosterone. Aldosterone is involved in the regulation of water and electrolyte balance in the body. An increase in its level can occur both in diseases of the adrenal glands and in pathologies of other organs (for example, heart failure).
Thus, the development of acidosis or alkalosis is often associated with the occurrence of pathological processes in which the resulting changes in acid-base balance exceed the compensatory capabilities of the body. In this case, normalization of the patient’s condition according to basic principles plays an important role in treatment.
How to increase acidity and reduce blood pH?
How to increase acidity and reduce blood pH?
It is bad for the body when the alkaline balance of the blood is greatly increased and has high levels. How to increase acidity and reduce blood pH? Adviсe:
- Eat acid-containing foods - grains, legumes, protein foods (meat, eggs).
- Eat foods rich in dietary fiber.
- Three times a day you can take 1 tablespoon of apple cider vinegar with honey.
- Vitamin C lowers pH levels.
- Do breathing exercises with deep breaths.
- If there are no medical contraindications, you can use dietary supplements - food enzymes and others.
- Correct the vitamin status in the body by taking multivitamin complexes.
Also, to increase acidity, it is necessary to carry out prevention and adequate treatment of the genitourinary system.
What are electrolytes and why are they so important?
More than half of our body weight consists of water. It is a universal solvent: only in an aqueous environment can all complex biochemical processes occur. Water performs a transport function, carrying various substances throughout the body, as well as taking part in the removal of metabolic end products. In addition, water is the main plastic material and takes an active part in thermoregulation. Some minerals are important electrolytes—substances that carry electrical charge when dissolved in a fluid (blood). Electrolytes are divided into cations (positive charge carriers) and anions (negative charge carriers). Cations include potassium and sodium, and anions include chlorine and its compounds. Most of them are found in bone and muscle tissue, as well as in the blood.
Electrolytes help the body maintain normal fluid levels. That is why, among the various pathogenetic links underlying many diseases, electrolyte imbalances occupy an important place.
Sodium (Na+). It is the main cation of extracellular fluid - 96% of the total amount of sodium in the body is found outside the cells. It takes part in the conduction of excitation in nerve and muscle cells, in the formation of an alkaline blood reserve and the transport of hydrogen ions. Sodium affects the state of the cardiovascular system. Since excess Na from food is excreted by the kidneys, its balance is constantly maintained in the body. Adequate production of aldosterone by the adrenal cortex and normal functioning of the gastrointestinal tract play an important role in the regulation of sodium balance.
Sodium metabolism is closely related to water metabolism. Natremia does not reflect the total amount of sodium in the body, but is closely correlated with the amount of water in the extracellular space. When there is an excess of Na, water is retained, and when there is a deficiency, it is excreted.
Hyponatremia is a decrease in sodium concentration in the blood. Most often this is due to:
- severe debilitating diseases accompanied by decreased diuresis;
- post-traumatic and postoperative conditions;
- extrarenal sodium losses;
- excessive intake of water in the antidiuretic phase of post-traumatic or postoperative conditions;
- uncontrolled use of diuretics.
Hypernatremia is an increase in sodium concentration in the blood. It can be observed with water depletion, salt overload, diabetes insipidus, and aldosteronism.
An excess of sodium, including as a result of its excessive intake (abuse of salt and salty foods), causes increased blood pressure, dehydration, increased load on the kidneys, and dry eyes and skin. With its lack, water is retained in the body due to the action of the hormone vasopressin, which leads to swelling, especially in the legs, congestion and heart failure.
Potassium (K+) . It plays an important role in the physiological processes of muscle contraction, heart function and nerve impulses, as well as in fermentation processes. Maintaining its normal concentration in the blood depends on intake with food, the functioning of the gastrointestinal tract, kidneys, and acid-base balance. To assess electrolyte balance, only very low and very high concentrations are key. Potassium is present in all body fluids, but most of it is concentrated in cells. Only a small amount is found in the blood.
Potassium enters the body with food and is excreted by the kidneys. Its level is regulated by the hormone aldosterone, since even slight fluctuations can result in negative health consequences - shock, respiratory and heart failure, arrhythmia and cardiac arrest. Electrolyte imbalance disrupts the body's pH to the acidic (acidosis) or alkaline (alkalosis) side, which can lead to life-threatening consequences and require immediate treatment.
Hypokalemia is a decrease in potassium concentration in the blood. It can be caused by:
- displacement of potassium into cells;
- alkalosis (respiratory, metabolic);
- aldosteronism;
- periodic hypokalemic paralysis;
- taking corticosteroids.
With a lack of potassium, muscle weakness appears, which can lead to hypoventilation, chronic renal failure, alkalosis, decreased tolerance to carbohydrates, encephalopathy, dynamic intestinal obstruction, cardiac arrhythmia (fibrillation is possible).
Hyperkalemia is an increase in potassium concentration. It can be caused by the release of potassium from the cell due to its damage, as well as by the retention of potassium in the body, most often due to excess intake of the cation into the body. Hyperkalemia is manifested by characteristic signs of neuromuscular damage (weakness, paresthesia, ascending paralysis, nausea, vomiting) and intestinal obstruction. The danger of this condition lies in the disruption of myocardial function.
Chlorine (Cl-) . This is a negatively charged ion that, together with potassium and sodium, helps regulate the amount of fluid in the body and maintain acid-base balance. Chlorine is present in all body fluids, but most of it is found in the blood and extracellular space. The Cl- concentration reflects the sodium level and changes in proportion to its fluctuations. However, if the acid-base balance is disturbed, the level of chloride in the blood may fluctuate independently of sodium, since chlorine acts as a buffer. It helps maintain optimal pH levels in cells, moving in or out as needed. Cl enters the body with food and is excreted by the kidneys.
Joint measurement of the levels of chlorides, potassium and sodium allows us to determine the so-called anion “window” - the difference in the concentration of anions and cations in the blood. Its abnormal value is not a specific marker, but suggests the presence of toxins in the body or the likelihood of metabolic disorders caused by fasting or diabetes.
Also, Program 116 “Electrolyte Balance in the Body” from DILA includes determining the levels of phosphorus, ionized calcium and determining blood pH.
Ionized (free) calcium Ca2+. Calcium is an essential macronutrient that takes part in the formation of bone tissue and teeth, conduction of impulses along nerve fibers, as well as in the contraction of muscles, including the myocardium. In addition, calcium takes part in blood clotting processes and is responsible for normal intestinal motility, preventing constipation and allergic reactions, and regulates the synthesis of calcitonin, one of the important parathyroid hormones.
Ionized calcium accounts for about half of the total calcium in the body. Its concentration changes throughout the day and is regulated by vitamin D, parathyroid hormone, and calcitonin.
Low serum macronutrient levels (hypocalcemia) are associated with symptoms of neuromuscular dysfunction:
- numbness;
- weakness and pain in the legs;
- violation of cardiac contractility.
With increased calcium concentrations (hypercalcemia), decreased appetite, nausea, abdominal pain, and arrhythmia are observed.
Phosphorus . This macronutrient is necessary for the formation of bone tissue and takes part in cellular energy metabolism. The processes of metabolism of phosphorus and calcium are closely related, therefore, for the normal absorption of minerals, their certain ratio with each other is necessary. In addition, the concentration of phosphates is influenced by the amount of parathyroid hormone and vitamin D. The bulk of all phosphorus is concentrated in skeletal tissues.
Phosphorus deficiency (hypophosphatemia) can be caused by:
- acid-base balance disorders;
- malnutrition;
- malabsorption;
- hypercalcemia;
- disorders affecting the processes of excretion in the kidneys.
The cause of excess phosphorus (hyperphosphatemia) may be excessive intake of the mineral from food, hypocalcemia and kidney damage.
Blood pH . The acid-base balance is the most important parameter that is maintained in the human blood within a certain range necessary for the normal functioning of all systems and organs, the occurrence of biochemical reactions, and the optimal functioning of enzymes. A pH shift below normal indicates acidosis (a shift in the acid-base balance of the blood towards increased acidity). When the pH deviates above normal, alkalosis develops (excess of substances with alkali properties in the blood).
Acidosis is manifested by the following symptoms:
- nausea and vomiting;
- increased breathing rate;
- headaches;
- disturbance of consciousness (up to coma)
- drop in blood pressure (in severe forms)
- arrhythmia.
Manifestations of alkalosis may include:
- headache;
- dizziness;
- depression of consciousness (up to coma);
- cramps in different muscle groups;
- heart rhythm disturbances.
The cause of acidosis and alkalosis in most cases is the severe course of the underlying disease, in which the resulting changes in blood pH exceed the capabilities of the mechanisms regulating this parameter.
How does calcium affect blood pH?
How does calcium affect blood pH?
Calcium is an alkaline substance. How does calcium affect blood pH? Our body is a smart “system”. In order to prevent critical indicators of the acid-base balance, with strong acidification, it begins to extract calcium and magnesium from our bones and teeth.
When the body is acidified, it will be useful to take a course of calcium. But this substance is well absorbed if taken along with magnesium and vitamin D3. The pharmacy sells special vitamin complexes with calcium. There is a lot of magnesium in fresh herbs and green vegetables.
Detailed description of the study
Electrolyte metabolism is an important part of overall metabolism. With its help, a constant environment in the body is maintained for normal cell functioning. Potassium, sodium, and calcium are important cations (positively charged ions). They are involved in maintaining osmolarity (ion concentration) and acid-base balance in the blood, regulate metabolic processes, influencing the functioning of enzymes, and have a number of specific properties. All three macroelements regulate impulse transmission in nerve and muscle tissue, ensuring muscle movement and, in particular, affect the contractility and excitability of the heart.
Sodium and calcium are found primarily outside cells, including the plasma, while potassium is primarily found inside them. Such a balance is necessary to maintain a certain charge of the cell membrane, with the help of which impulse transmission is possible, as well as the transport of substances into and out of the cell (glucose, amino acids).
Sodium (Na+) is the main extracellular cation. Table salt (NaCl) is the main source of sodium. The macroelement maintains normal osmotic pressure in body fluids, which ensures their proper functioning. Thus, with a lack of Na, symptoms of dehydration occur, and with an excess, edema occurs.
Sodium is excreted through the kidneys and part of it reenters the blood (reabsorbed). Thanks to the influence of hormones on this process, its level in plasma is regulated. Thus, the kidney hormone angiotensin II increases reabsorption, and vasopressin (a pituitary hormone) decreases it.
An increase in the level of sodium in the serum (hypernatremia) is caused by its excess intake from food, disruption of the kidneys and adrenal glands, increased water loss through the gastrointestinal tract (vomiting, diarrhea), and skin (increased sweating). Symptoms: swelling, shortness of breath, high blood pressure.
The reasons for a decrease in macronutrients (hyponatremia) are low dietary intake, vomiting, diarrhea (with improper replenishment of electrolytes), kidney disease, and heart failure. Hyponatremia manifests itself as weakness, loss of consciousness, low blood pressure, decreased urine output, and decreased skin elasticity.
Potassium (K+) comes from food (fruits, vegetables) and is absorbed in the small intestine. Part of the K+ is reabsorbed when filtered by the kidneys, this process enhances the hormone aldosterone. The amount of a macroelement in the plasma is affected by the pH of the blood - with acidosis (increased acidity), potassium leaves the cells, and with alkalosis (decreased acidity) - vice versa.
Even minor fluctuations in its level can lead to disruptions in the body's functioning. Signs of decreased concentration (hypokalemia) are: decreased muscle tone, weakened reflexes, general weakness. With an increase in potassium (hyperkalemia), a person may experience changes in skin sensitivity, paresis and paralysis, cardiac arrhythmia, even cardiac arrest.
Calcium (Ca2+) in plasma is in three forms: ionized, bound to proteins and anions. Total plasma calcium is a combination of these forms. It is the main macronutrient for the mineralization of bones and teeth. Ca2+ is also involved in impulse transmission in nerve and muscle tissues and is necessary in the regulation of blood clotting.
The macroelement enters the body mainly through food, found in dairy products, fish, eggs, green vegetables, and nuts. Calcitriol (the active form of vitamin D) and parathyroid hormone take part in the regulation of Ca2+ metabolism. They increase the calcium content in the blood by enhancing its absorption in the intestines, resorption (destruction) of bone tissue and increasing reabsorption by the kidneys. The hormone calcitonin reduces the level of the macroelement in plasma, mainly due to bone mineralization. This way, the optimal level of calcium in the body is maintained.
Low serum macronutrient levels (hypocalcemia) are associated with symptoms of neuromuscular dysfunction - numbness, weakness and pain in the legs, and impaired cardiac contractility. An increased concentration of calcium (hypercalcemia) is manifested by decreased appetite, nausea, abdominal pain, and arrhythmia.
A comprehensive blood test for potassium, sodium, and calcium allows you to most fully assess the state of electrolyte metabolism. This, in turn, helps to identify metabolic disorders of the body and take timely measures to eliminate them.
How to maintain a constantly normal blood pH level?
How to maintain a constantly normal blood pH level?
If acidity levels are normal, it is recommended to regularly take tests and check the PH level. How to maintain a constantly normal blood pH level? Adviсe:
- Make it a habit to eat right. Eat at least 5 servings (1 serving - 100 grams) of fresh vegetables and fruits. There are food products that are especially enriched with vitamins, minerals and contribute to the balance of nutrients.
- Lead a healthy lifestyle and exercise. Quit smoking and drinking alcohol - all this strongly and quickly acidifies the body.
- Drink mineral water without gases, freshly squeezed juices, herbal teas.
- Eliminate fatty, high-calorie, smoked foods, coffee, tea from your diet.
Harmful compounds that accumulate during acidification of the body do not leave the body, but are deposited on the walls of blood vessels. To get rid of the effects of acidification, it is necessary to carry out long-term cleaning measures. Therefore, it is better to always keep your blood pH, as well as urine and saliva, normal.
How to quickly and economically measure pH
Litmus indicator paper provides a quick and economical way to measure the pH (hydrogen value) of any necessary liquid and mixtures of liquids (urine, saliva, feces, semen, vaginal acidity, breast milk, solutions, water, drinks, etc.).
Litmus paper is necessary both in the family and for the specialist conducting the patient’s examination, applicable in chemical laboratories, and used for research activities.
In chemistry, there are substances that have the ability to change their color in the presence of acids and alkalis. These substances are called indicators and are used to determine the reaction medium. The environment can be acidic, alkaline and neutral. Filter paper is impregnated with these substances.
Litmus is a coloring substance extracted from certain types of lichen. Its composition is complex. Litmus is a weak acid that is used to soak paper.
How to use indicator paper:
It is necessary to dip a narrow strip of paper into the required solution for two to three seconds. Compare with the supplied color chart and calculate the values.
In a neutral solution at 25°C, pH = 7. In acidic solutions, pH 7, the greater the alkalinity of the solution, the greater its value. Conclusion: the lower the pH, the greater the concentration of H+ ions, i.e., the higher the acidity of the environment, and vice versa, the higher the pH, the lower the concentration of H+ ions, i.e., the higher the alkalinity of the environment.
Indicator paper parameters: pH measurement from 1 to 14. Indicator paper can be in the form of strips, rolls, boxes, tubes, pencil cases, tear-off. Universal indicator paper is used only for approximate determination of pH values over a wide range with an accuracy of about one pH unit or tenth.
PSH - METER - IS IT NEEDED?
To accurately identify the value of this indicator, a pH meter is used in routine methods. The use of indicators to determine the exact value is not practiced due to the subjective determination of color or the low accuracy of the indicator itself. However, the advantage of indicators is their low cost, clarity of analysis, and speed.
Psh meters have various characteristics, on the basis of which its cost is formed, namely, PH measurement range: 0.00 - 14.00, operating temperature, division value: 0.1 pH, accuracy: 0.1 pH. The cost ranges from 15 to 100 dollars.
For soap and cream making, in principle, the cheapest one will do. It has the following characteristics: PH measurement range: 0.00 - 14.00, operating temperature: 0 - 50°C, division value: 0.1 pH, accuracy: 0.1 pH. The PN meter must be calibrated.
If the PN meter is uncalibrated and shows incorrect values, then it needs to be calibrated. An example of calibrating a ph meter 009. However, other models are calibrated using the same principle!
What is needed for calibration? Actually, a psh meter, a slotted screwdriver (included in the kit), a calibration solution and, of course, straight hands, where would we be without them - not a single task can be done without them. I believe that it is enough to calibrate the budget ph meter 009 at one point, having a solution of ph 4.00 or ph 6.8. The solution must be at room temperature! If it is stored in the refrigerator, take it out in advance and shake it well just before calibration.
Place the switched on PN meter into the calibration solution. Wait a minute. There is a screw on the back of the meter; it must be turned clockwise or counterclockwise, depending on the indicator on the display. You must match the values on the display with the pH of your solution! That's actually the whole technological process!
HOW TO USE THE PSH METER
Typically, instructions are included with the device.
Below is an example of instructions for the Miniature pH-meter-tester pH-PAL
- remove the protective cap at the bottom of the housing;
- turn on the device by sliding the top switch to the right;
- immerse the device in the test solution up to the grooved mark;
- stir vigorously for 5-6 seconds. Read the readings after they have stabilized;
- if the electrode was dry, wait a little longer, which will allow the device to carry out temperature compensation;
- After each measurement, thoroughly rinse the electrode with distilled water;
- After completing the measurements, turn off the device and put on the protective cap.
PH STANDARDS
Solutions and liquids with respect to their acidity are considered:
- neutral at pH = 7
- acidic at pH 7
Acidity of urine
If the urine pH level fluctuates between 6.0 - 6.4 in the mornings and 6.4 - 7.0 in the evenings, then the body is functioning normally. The most optimal level is slightly sour, in the range of 6.4 - 6.5. A urine pH value below 5.0 indicates that it is strongly acidified, and above 7.5 indicates that it is strongly alkaline.
The reaction of urine determines the possibility of stone formation: urate - in an acidic environment, oxalate - in a neutral-acidic environment, phosphate - in a more alkaline environment. For example, uric acid stones virtually never occur when the urine pH is greater than 5.5, and phosphate stones never form unless the urine is alkaline. The best time to determine your pH level is 1 hour before or 2 hours after a meal.
Check the pH level twice a week 2-3 times a day.
Using indicator litmus paper pH test, you can easily, quickly and accurately monitor the response of urine to changes in the type of diet, the use of medications or dietary supplements. Positive pH dynamics can serve as a criterion for the correctness of the chosen diet or treatment.
The acidity of urine varies greatly depending on the food taken, for example, eating plant foods increases the alkaline reaction of urine. The acidity of urine increases if a person’s diet is dominated by meat foods rich in proteins.
Heavy physical work increases the acidity of urine.
Increased acidity of urine is observed with increased acidity of the stomach. Reduced acidity of gastric juice does not affect the acidity of urine.
The acidity of urine changes in many diseases or conditions of the body, so determining its acidity is an important diagnostic factor.
Saliva acidity:
The acidity of saliva depends on the rate of salivation. Typically, the acidity of mixed human saliva is 6.8–7.4 pH, but with high salivation rates it reaches 7.8 pH. The acidity of the saliva of the parotid glands is 5.81 pH, of the submandibular glands - 6.39 pH. In children, the average acidity of mixed saliva is 7.32 pH.
Optimal measurement from 10 to 12 hours. It is better to measure it on an empty stomach, two hours before or two hours after a meal. Salivation decreases in the evening and at night.
To increase salivation, in order to increase the pH of saliva, it is good if there is a piece of lemon on the plate; it even with visual perception increases salivation. Food should look appetizing, served on beautiful dishes, appetizingly decorated with herbs and/or vegetables, it should, as they say, please the eye! Not only the saliva flows, but also the juices in the body, preparing for the process of digesting food. This is the mental phase of digestive secretion.
Acid gastroesophageal and pharyngolaryngeal refluxes reaching the oral cavity play a leading role in the occurrence of oral pathology. As a result of the ingress of hydrochloric acid, the acidity of mixed saliva decreases below 7.0 pH. Saliva, which normally has alkaline properties, at low pH, especially at values of 6.2–6.0, leads to focal demineralization of tooth enamel with the appearance of erosions of hard dental tissues and the formation of cavities in them - caries. The amount of mucus on the mucous membrane increases, the gums become swollen and inflamed.
When the acidity in the oral cavity decreases, the acidity of dental plaque decreases, which causes the development of caries.
Bacteria in the mouth thrive in the absence of air. Saliva, rich in oxygen, actively prevents their reproduction. Bad breath occurs when the flow of saliva slows down, for example during sleep. Excitement, hunger, pronouncing a long monologue, breathing through the mouth (for example, with a runny nose), stress - dry out the oral cavity, leading to a decrease in the pH of saliva. A decrease in saliva flow inevitably occurs with age.
You can use a slightly alkaline mouth rinse with water with the addition of soda and also take it orally between meals, proposed by Professor A.T. Ogulov. – slightly alkaline pH 7.4-8. Rinsing the mouth with soda water occurs for various inflammatory diseases of the gums and teeth and for general acidification of the body.
You can set the desired pH of water for rinsing or ingestion using litmus indicator paper. There cannot be recipes with the required proportions, because... Each region has its own water, with its own pH. Therefore, it is necessary to have indicator paper on hand.
Vaginal acidity
The normal acidity of a woman's vagina ranges from 3.8 to 4.4 pH and averages 4.0 to 4.2 pH.
Vaginal acidity in various diseases:
- cytolytic vaginosis: acidity less than 4.0 pH
- normal microflora: acidity from 4.0 to 4.5 pH
- candidal vaginitis: acidity from 4.0 to 4.5 pH
- Trichomonas colpitis: acidity from 5.0 to 6.0 pH
- bacterial vaginosis: acidity greater than 4.5 pH
- atrophic vaginitis: acidity greater than 6.0 pH
- aerobic vaginitis: acidity greater than 6.5 pH
Lactobacilli (lactobacillus) and, to a lesser extent, other representatives of normal microflora are responsible for maintaining an acidic environment and suppressing the growth of opportunistic microorganisms in the vagina. In the treatment of many gynecological diseases, restoration of the lactobacilli population and normal acidity comes to the fore.
Sperm acidity
The normal acidity level of sperm is between 7.2 and 8.0 pH. Deviations from these values, in and of themselves, are not considered pathology. At the same time, in combination with other deviations, it may indicate the presence of a disease.
An increase in the pH level of sperm occurs during an infectious process. A sharply alkaline reaction of sperm (acidity approximately 9.0–10.0 pH) indicates prostate pathology.
When the excretory ducts of both seminal vesicles are blocked, an acidic reaction of the sperm is observed (acidity 6.0–6.8 pH). The fertilizing ability of such sperm is reduced. In an acidic environment, sperm lose motility and die. If the acidity of the seminal fluid becomes less than 6.0 pH, the sperm completely lose their motility and die.
The acidity of tears is normal - from 7.3 to 7.5 pH.
Acidity in the stomach.
- The minimum theoretically possible acidity in the stomach is 0.86 pH.
- The maximum theoretically possible acidity in the stomach is 8.3 pH.
- Normal acidity in the lumen of the body of the stomach on an empty stomach is 1.5–2.0 pH.
- The acidity on the surface of the epithelial layer facing the lumen of the stomach is 1.5–2.0 pH.
- The acidity in the depths of the epithelial layer of the stomach is about 7.0 pH. Normal acidity in the antrum of the stomach is 1.3–7.4 pH.
The cause of many diseases of the digestive tract is an imbalance in the processes of acid production and acid neutralization. Long-term hypersecretion of hydrochloric acid or lack of acid neutralization, and, as a consequence, increased acidity in the stomach and/or duodenum, causes so-called acid-dependent diseases. Currently, these include: peptic ulcer of the stomach and duodenum, gastroesophageal reflux disease (GERD), erosive and ulcerative lesions of the stomach and duodenum while taking aspirin or non-steroidal anti-inflammatory drugs (NSAIDs), Zollinger-Ellison syndrome, gastritis and gastroduodenitis with high acidity and others.
Low acidity is observed with anacid or hypoacid gastritis or gastroduodenitis, as well as with stomach cancer. Gastritis (gastroduodenitis) is called anacid or gastritis (gastroduodenitis) with low acidity if the acidity in the body of the stomach is approximately 5 or more pH units. The cause of low acidity is often atrophy of parietal cells in the mucous membrane or disturbances in their functions.
Acidity in the intestines:
- Normal acidity in the duodenal bulb is 5.6–7.9 pH.
- The acidity in the jejunum and ileum is neutral or slightly alkaline and ranges from 7 to 8 pH.
- The acidity of small intestine juice is 7.2–7.5 pH. With increased secretion it reaches 8.6 pH.
- The acidity of the secretion of the duodenal glands is from pH 7 to 8 pH.
- The acidity of pancreatic juice is from 7.5 to 9 pH.
- The acidity of colon juice is 8.5–9.0 pH.
In the lower parts of the colon, pH values of acidity gradually increase, reaching a maximum pH value in the region of the rectosigmoid junction.
- The acidity of feces is normally from 6.0 to 8.0 pH.
- The acidity of meconium (original feces of newborns) is about 6 pH.
- The acidity of human breast milk is 6.9-7.5 pH
Blood acidity:
The acidity of human arterial blood plasma ranges from 7.37 to 7.43 pH, averaging 7.4 pH. The acid-base balance in human blood is one of the most stable parameters, maintaining acidic and alkaline components in a certain balance within very narrow limits. Even a small shift from these limits can lead to severe pathology. When shifting to the acidic side, a condition called acidosis occurs, and to the alkaline side, alkolosis occurs. A change in blood acidity above 7.8 pH or below 6.8 pH is incompatible with life.
The acidity of red blood cells is 7.28–7.29 pH.
Normal blood revitalizes lymph cells that can destroy tumor cells. There are many lymphatic cells (eg NK cells, LAK cells) in the human body. Their uniqueness lies in the fact that they are able to distinguish normal cells from diseased and damaged ones, and destroy the latter. This is the function of the human body's immunity. greatest activity of lymphatic cells in destroying diseased cells occurs at pH 7.4. However, usually around the affected cells, there is a more acidic environment, which interferes with the activity of lymphocytes, which work better at a slightly alkaline pH.
By consuming foods that have an alkalizing effect, you can adjust the pH balance within 0.5 units, creating a favorable environment for the action of lymphocytes and the destruction of affected or abnormally constructed cells.
Cancerous tissue has increased acidity, unlike normal tissue, and the body protects it with a fibrous membrane whose pH is alkaline. If you continue to follow the acidic diet, the membrane dissolves and the cancer cells are released.
HOW TO CHECK YOUR BODY’S ENVIRONMENT INDEPENDENTLY
1. Check the pH value - the reaction of saliva and urine on a scale of colored indicator (litmus) paper. If saliva and urine are within the pH range of 5.0-5.7, there is a predisposition to cancer, but this does not mean that the person will get sick. If saliva and urine are within the pH range of 7.0-7.4, you are protected from cancer. 2. You can do a bioimpedance analysis (diagnosis of body composition). By the amount of water in a bound state, you will determine whether you have a shift in the acid-base balance. If an excess of such water is definitely an acidic environment, a deficiency is alkaline.
IF THE BODY IS ACIDIFIC:
Nowadays, this is observed very often in connection with poor nutrition and attitude towards one’s body. Acidification of the body is primarily caused by:
- the predominance in the diet of such products as sugar, meat, chicken, fish, sweets, pasteurized dairy products, flour products and cereals;
- the second factor is the consumption of incompatible foods, for example, proteins with carbohydrates;
- Many preservatives and food additives that modern products are so rich in, especially those with a long shelf life, are also oxidizing agents;
- alcoholic drinks;
- coffee, tea, chocolate, tobacco.
To alkalize the blood, the body uses minerals - calcium, sodium, potassium, iron, magnesium. And this leads to physical weakness, fatigue, decreased mental activity and insomnia, irritability and depression. The leaching of calcium from bone tissue causes a serious disease - osteoporosis.
What to do if the body is acidified:
The daily diet of a healthy person should include at least 75-85% of alkalizing foods, and in the diet of a person suffering from any disease, their share should be increased to 90%. Alkalinizing foods include vegetables and fruits. And it is in this sequence, and not vice versa, since there is an unspoken rule: the closer a vegetable or fruit is to the soil surface, the higher its content of alkalizing macroelements (for example, potassium). Potassium, found in peeled potatoes, basil, dried apricots, and many other vegetables and fruits, helps fight acidification (latent acidosis) and create favorable conditions for the absorption of nutrients and medications. The most useful in this sense are fresh tomatoes, beets, dried apricots, and melons. Freshly prepared vegetable or fruit juices alkalize the blood more effectively. The healthiest ones are carrot, celery and watermelon. Alkaline valences dominate in vegetables and fruits, so their consumption eliminates acidosis.
Your menu should definitely include grated raw beets and carrots, finely chopped cabbage, dill, celery, onions and garlic. It is very useful to eat young green shoots of plants, honey, herbal teas, soy sauce, seaweed, and wheat sprouts.
Once a week, it is advisable to arrange fasting days for yourself, eating only raw vegetables and fruits , or even drinking only juices and purees on one of these days.
Most legumes and cereals, with the exception of buckwheat and millet, increase blood acidity during normal preparation. However, after soaking or sprouting, they acquire an alkalizing effect. Raw nuts and seeds should be soaked half an hour before meals, cereals - 0.5-2 hours before cooking, legumes - overnight.
When the acid-base balance shifts to the alkaline side (as a rule, this can be observed in vegetarians), a lack of water is created in the tissues, the skin becomes dry and dehydrated. There must be a measure for everything.
Physical work and sports slightly shift the body's reaction to the alkaline side.
A person’s mood is of no small importance. A good cheerful mood normalizes the acid-base balance.
Once a week, when the body is acidified, it is advisable to arrange healing days for yourself, eating only vegetables (1.5 kg of vegetables, divided throughout the day), boiled and sometimes raw in the summer, only heat-treated in the autumn-winter) and Be sure to have clean hot water.
If you are sick, you must give up any meat food and broths.
If you are going to have surgery, you need to do it in an alkaline mode of the body, and follow a plant-based diet after the operation.
Please note - alkalizing foods (for example, fruits) consumed with sugar (a strong acidifier) acidify the body (blood).
OXIDIZING AND ALKALIZING PRODUCTS:
The degree of their action will be noted by the number of advantages:
Some foods that acidify the body: sugar! (+++), game (++++), oysters (++++), crayfish (++++), veal (+++), eggs (+++), chickens (+++) , fish (++), mussels (+++), coffee (+++), jam (+++), baked beans (+++), beef liver (+++), lean pork (++) , skinny bacon (++), ham (++), pickled plums (++), green bananas (++), dried peas (++), white flour (++), barley (++), hominy and corn flakes (++), starch (++), peanuts (++), hard cheese (++), white bread (++), boiled lamb (++), stewed lamb (+), black bread ( +), dried beans (+), soft cheese (+), cream (+), beef (+), full-fat bacon (+).
Some foods that alkalize the body: figs (++++), fresh beets (++++), celery (++++), berries (++++), grapefruit (++++), lettuce ( ++++), champignons (++++), fresh tomatoes (++++), dried apricots (++++), fresh apricots (+++), pears (+++), sea buckthorn (+ ++), lemon (if consumed without sugar) (+++), orange (+++), watermelon (+++), melon (+++), prunes (+++), pepper (++ +), fresh beans (+++), currants (+++), cabbage (all types) (+++), pistachios (+++), cucumbers (+++), dandelion (leaves ) (+++), parsnips (+++), plums (+++), peaches (+++), whole milk (+++), kumiss (+++), whey (+++) , ripe bananas (++), apples (++), grapes (.++), cherries (++), raisins (++), dates (++), onions (++), green peas (++ ), radishes (++), almonds (++), carrots (++), potatoes with skin (++), cranberries (+), asparagus (+), lard (+). published
What foods acidify the blood: table
Plan your diet and lifestyle in such a way that you do not have problems with blood alkaline levels. A healthy diet will help you maintain health and youth throughout your life. So, what foods acidify the blood? Table:
What foods acidify the blood: table
If your blood is highly acidified, change your eating habits. Proper nutrition has been in fashion for several years now, but there are still many people in the world who do not know what this term means.
What foods alkalinize the blood: table
If you have health problems, take a blood test to check your PH level. Acidified blood must be alkalized, otherwise failures in the functioning of organs and systems will lead to the most unpleasant consequences. What foods alkalinize the blood? Table:
What foods alkalinize the blood: table
You will see more detailed information about foods that alkalize and acidify the blood in this article on our website. It is important to remember that in a neutral biological environment our body has an amazing ability to heal itself. Therefore, strive to maintain your acid-base balance normal so as not to get sick and stay young for a long time.
Acid-base balance: what is it?
It would seem that the entry into the digestive tract, and further into the blood, of products with an acidic or alkaline reaction should change the composition of the blood. In fact, the body's buffer systems ensure the stability of the acid-base balance, preventing fluctuations outside the safe range.
List of buffer systems:
- Bicarbonate (hydrocarbonate) system – provides at least 50% of the adaptive abilities of the hemostatic system;
- Hemoglobin system – 35% safety;
- Blood protein system – 10% buffer capacity;
- Phosphate system – 5-6% buffer safety.
These systems, supporting the vital functions of the body, prevent a shift in the acid-base balance in any direction, despite the fact that the body consumes foods of various compositions. Buffer systems have an inexhaustible supply of safety, since they are constantly supported by the excretory system, which is activated at the level of reflexes when it is necessary to remove metabolic products.