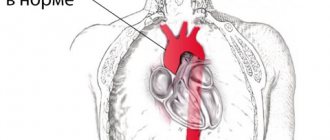
It branches like a tree, first into large branches (trunks), then into smaller branches and twigs, and is conventionally divided into several parts or sections:
- 1. The ascending aorta is the area from the aortic valve to the brachiocephalic trunk.
- 2. The aortic arch is a short section from which all the vessels supplying the arms and head (brachiocephalic arteries) depart. They anatomically form an arch connecting the ascending and descending aorta.
- 3. The descending (thoracic) aorta begins from the mouth of the left subclavian artery and continues to the diaphragm.
- 4. Below the diaphragm and before the bifurcation of the aorta (bifurcation) is the abdominal aorta.
Dividing the aorta into sections is very important for assessing risk and choosing optimal treatment tactics in patients with aortic aneurysms.
An aortic aneurysm is an area of local expansion.
What is an aneurysm
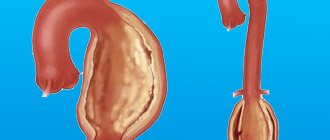
A thoracic aortic aneurysm is an abnormal bulge on the walls of the artery, formed due to intense blood pressure on a weak area of the vessel. An aneurysm can be:
- fusiform - has a convex shape, protrudes from both sides of the aorta;
- saccular - a rounded formation that protrudes from one side of the vessel.
A small aneurysm does not pose a health threat. However, if it is present, you must be observed by a doctor so as not to miss the moment of disease progression.
Classification of the disease
Pathology is divided into 3 types depending on the extent and location:
- In the ascending aorta with advancement along the arch. Occurs in 50% of the total number of recorded cases of the disease.
- In the ascending aorta. Diagnosed in 35% of cases.
- In the descending part of the aorta with progression down/up along the arch. Occurs in 15% of cases.
Stretching of the vessel occurs quickly - it does not require weeks. The process can be compared to inflating a balloon, which expands several times when filled with air.
Depending on the duration of the process, an aneurysm can be:
- acute – 1-2 days have occurred since the formation of aortic dissection;
- subacute – the endothelial defect appeared 2-4 weeks ago;
- chronic – the aneurysm formed 4 or more weeks ago.
Some patients ignore the symptoms of the disease and do not see a doctor for years. But the fact that the thoracic aortic aneurysm does not rupture is pure chance and luck. This can happen at any time, and in 90% of cases the person will die.
Symptoms of pathology
Symptoms of a thoracic aortic aneurysm differ depending on the location of the formation. In some cases, a person does not feel any discomfort at all, which greatly complicates the timely diagnosis of the disease.
Aneurysm of the ascending aorta is accompanied by:
- Acute pain in the heart area. This is due to the pressure of the damaged vessel on nearby organs and tissues;
- Shortness of breath, significantly increasing over time. It is problematic for a person with an aneurysm to walk long distances or climb stairs;
- Constant feeling of heartbeat. This is one of the most common complaints from patients. This phenomenon is associated with blood pressure on weakened vessel walls;
- Dizziness. May be accompanied by a severe headache that is not relieved by pills;
- Swelling of the face, upper half of the body. This phenomenon is due to the fact that the aneurysm compresses the superior vena cava.
Aneurysm of the aortic arch is characterized by the following symptoms:
- Swallowing problems. The swollen section of the vessel presses on the esophagus;
- Hoarse voice, periodic coughing. The main cause of the malaise is the pressure of the aneurysm on the recurrent nerve;
- Increased salivation. Occurs due to pressure from the damaged aorta on the vagus nerve;
- Breathing problems, shortness of breath. It manifests itself in the case of aneurysm pressure on the trachea and bronchi;
- Unilateral pneumonia. An aneurysm can put pressure on the root of the lung, preventing its normal ventilation. When an infection enters the body, in 94% of cases this condition develops into unilateral pneumonia.
An aneurysm of the descending aorta is accompanied by:
- Pain of unknown nature in the left arm, acute pain syndrome in the scapula area;
- Paresis, paralysis. It occurs due to the pressure of the aneurysm on the intercostal arteries, which disrupts the supply of oxygen to the spinal cord;
- Displacement of the vertebrae if the patient’s aneurysm is in a chronic condition;
- Painful sensations in the side, sternum, similar in nature to pain due to radiculitis/neuralgia.
Signs and symptoms of a ruptured aneurysm:
- Sudden, intense, and persistent pain in the abdomen, chest, or back
- Pain that radiates to the back or legs
- Sweating
- Sticky sweat
- Dizziness
- Low blood pressure
- Increased heart rate
- Loss of consciousness
- Dyspnea
- Weakness or paralysis on one side of the body, difficulty speaking, or other signs of a stroke.
Emergency cardiology deals with the treatment of such conditions.
Another complication of aortic aneurysms is the risk of blood clots. Small blood clots can develop in the area of the aortic aneurysm. If a blood clot breaks away from the inside wall of the aneurysm and blocks a blood vessel in another part of the body. This causes pain or blocks the flow of blood to organs and tissues, causing ischemia of the lower extremities, brain, and abdominal organs.
Reasons for development
A disease such as thoracic aortic aneurysm develops as a result of:
- Atherosclerosis. It is this pathology that in 90% of cases is the cause of aneurysm formation;
- Traumatic damage to a vessel. Occurs most often as a result of an accident, a fall from a great height, or a strong blow to the chest.
- Congenital pathologies. When a patient has a systemic connective tissue disease, the walls of blood vessels are in a chronically inflamed state.
- Complications after medical procedures. These include reconstructive operations on the aorta, cardiac catheterization and other interventions in the cardiovascular system.
- Infectious diseases. An aneurysm can develop in patients with tuberculosis, sepsis, syphilis, osteomyelitis, and pericarditis.
Previously, the pathology was diagnosed mainly in patients over 50 years of age. However, every year the disease becomes younger. Already today, young people aged 25-30 years often turn to doctors when an examination reveals a thoracic aneurysm.
Types of open surgical operations for aortic aneurysms:
- Bentalla-De Bono operation (replacement of the ascending aorta with a valve-containing conduit with a mechanical prosthetic aortic valve);
- David's operation (replacement of the ascending aorta while preserving the native aortic valve);
- Supracoronary aortic replacement;
- Prosthetics of the ascending aorta and its arch (Borst technique, the use of oblique aggressive anastomosis and other techniques);
- Thoracic aortic replacement;
- Abdominal aortic replacement.
Risk factors
The pathology has a number of risk factors, the presence of which significantly increases the likelihood of its development:
- Male gender. According to statistics, thoracic aneurysm is diagnosed 14 times more often in men than in women.
- Smoking. Specialists from the Moscow Regional Research Institute conducted mass screening diagnostics of patients with atherosclerosis and aneurysm. It turned out that 3/4 of the patients had a smoking history of more than 25 years.
- Age over 55 years. During this period, the walls of blood vessels stop producing collagen and elastin, as a result of which they become thinner.
- Hereditary predisposition. 14% of patients with thoracic aortic dissection have a similar pathology in close relatives.
- Insufficient physical activity. A sedentary lifestyle provokes blood stagnation in the extremities and puts a serious strain on the cardiovascular system.
- Obesity. Excess body weight negatively affects the functioning of the heart and vascular patency.
- Increased blood cholesterol levels. Scientists have long proven that the human body does not need cholesterol coming from outside to function properly - the liver produces it in sufficient quantities.
conclusions
The creation of an aortic team in a separate center made it possible to obtain good reproducible results in the treatment of patients with various pathologies of the ascending aorta. The extent of surgical correction for pathology of the ascending aorta in itself is not a significant risk factor. Further improvement of the treatment of this pathology is associated with scientific developments at the intersection of various clinical and biomedical specialties (surgery, radiology, molecular biology and genetics). An urgent task is to create a unified register of patients with aortic diseases. Identification of new informative biochemical markers may affect the timing and extent of surgery in patients of this profile.
There is no conflict of interest.
Potential Complications
The most common complication of the disease is rupture of a thoracic aortic aneurysm. In this case, blood flows freely into the chest, abdominal cavity, pericardium, and esophagus. This condition requires emergency hospitalization and surgery. A ruptured aneurysm in the thoracic aorta without medical assistance will invariably lead to the death of the patient.
Other complications of the pathology are:
- Compression, erosion of adjacent structures;
- Thromboembolism;
- Coronary artery occlusion.
These conditions are deadly. The aneurysm itself is a favorable environment for the formation of blood clots, which under pressure from the bloodstream can break off at any time. If a blood clot enters the lungs, heart or brain, the patient has a more than 90% chance of death. That is why it is so important to consult a doctor at the first symptoms of the disease, who will conduct a diagnosis and select the correct treatment tactics.
Diagnostics
The first thing a patient needs to do is make an appointment with a therapist. He will conduct an examination, collect anamnesis and issue a referral to a specialist.
In 50% of cases, pathology is discovered accidentally during an X-ray examination of the lungs. In 70% of cases, the aneurysm is audible with a phonendoscope and manifests itself in the form of characteristic noise. The problem can also be identified during an ultrasound examination of the heart.
Specific methods for diagnosing an aneurysm are:
- Magnetic resonance imaging;
- CT scan;
- angiography.
Based on the data obtained during the examination, the doctor decides on the most appropriate treatment method.
Surgery
It involves removing the damaged area of the aneurysm and replacing it with an artificial blood vessel - an aortic prosthesis. It is installed forever. The rehabilitation period after surgery ranges from 7 days to 1 month, depending on the patient’s age and the presence of concomitant diseases.
It is important to understand that aortic replacement does not guarantee the recurrence of an aneurysm in another part of the vessel. Therefore, it is necessary to strictly follow the doctor’s recommendations regarding nutrition and lifestyle.
The main indications for surgical treatment of an aneurysm are:
- Large size. The average is more than 5.5 cm in diameter. However, here everything depends on the initial diameter of the aorta. If the patient’s normal size is 3-3.5 cm, then surgery is indicated in case of aneurysm formation larger than 6.5-7 cm;
- Rapid growth - the damaged area of the vessel expands by more than 0.5 cm annually;
- An aneurysm provokes compression of the bronchi, the formation of an aortoesophageal/aortobronchial fistula;
- The vessel was stretched due to traumatic injury.
Rupture of abdominal aortic aneurysm
Emergency surgical interventions in patients with ruptured AAA are a complex problem. They are accompanied by high mortality and a large number of complications. At the same time, refusal to operate is tantamount to imposing a death sentence on the patient.
Based on the above, there is no alternative to surgical intervention for a ruptured aneurysm. However, surgical activity for this pathology cannot be expanded indefinitely, since in some cases the operation is obviously doomed to failure. In this regard, the surgeon must carefully assess the situation so that, on the one hand, not to perform a wasted operation, and on the other, not to deprive the patient of his last chance for salvation.
Five risk factors associated with mortality should be kept in mind: age over 76 years, signs of myocardial ischemia, hemoglobin < 90 g/l, creatinine > 190 mmol/l and lack of consciousness on admission to hospital.
Naturally, an abdominal aortic aneurysm is never an isolated manifestation of general atherosclerosis. Almost every patient has coronary heart disease, and many have atherosclerotic damage to the cerebral vessels. All this significantly aggravates the condition of patients, especially if there is a history of myocardial infarction or cerebrovascular accident. However, these circumstances do not make the forecast completely hopeless. However, the presence of a recent myocardial infarction or acute cerebrovascular accident in a patient completely excludes the possibility of surgical treatment of a ruptured AAA.
Impaired urinary function in this group of patients is a poor prognostic sign, since even with normal initial diuresis after aneurysmectomy, progressive renal failure often develops, which in many cases causes death. The fight for the life of a patient with a ruptured AAA should begin in the hospital emergency room. Ideally, catheters should be installed in the central vein, in the bladder, and the main indicators of hemostasis, blood type, Rh factor, etc. should be determined.
It is advisable to hospitalize the patient in the intensive care unit, where, along with ongoing preparation for the operation, ultrasound and, if possible, CT scans are performed. In addition, cardiac, pulmonary and renal function should be assessed. In cases where immediate surgery is not possible in some cases, there are bloodless methods to temporarily stop bleeding from a ruptured aortic aneurysm. Pneumatic body compression has been successfully used to stop bleeding from the aorta for a period of about 2-5 hours. However, the scope of its use is limited by the time of transportation of the patient.
Surgery for a ruptured abdominal aortic aneurysm belongs to the category of surgical interventions in which speed and a clear sequence of manipulations are of particular importance. The slightest mistake or deviation from the optimal technical version of the operation for a given situation can cost the patient his life. This is why it is necessary to elaborate on the technical details of the intervention.
The optimal surgical approach for ruptured abdominal aortic aneurysm is a median laparotomy from the xiphoid process of the sternum to the pubis. This surgical approach gives wide exposure of the infrarenal aorta and iliac arteries. Some surgeons use transverse laparotomy, making a horizontal incision in the anterior abdominal wall 3-4 cm above the navel. However, this access limits the surgical area, since under conditions of transverse laparotomy, exposure of the iliac arteries is achieved with great difficulty. In addition, the transverse approach is more traumatic and time-consuming compared to the vertical median one. As for thoracophrenolumbotomy, which allows extensive mobilization of the aorta along with the renal arteries and visceral branches, this surgical approach is simply not necessary for intervention for the rupture of an infrarenal aneurysm. In some cases, when the lesion is extensive and when revascularization of the lower extremities is necessary, a combined approach is required: midline laparotomy and femoral access (unilateral or bilateral).
After opening the abdominal cavity, the initial task of the surgeon is to achieve proximal control of the abdominal aorta, i.e. interruption of blood flow in the aorta above the aneurysm. There are three levels of proximal control of the abdominal aorta: infrarenal, suprarenal, subphrenic.
The upper pole of an abdominal aortic aneurysm is most often located 2 cm or more below the origin of the renal arteries. In this regard, one should strive to implement proximal control at the infrarenal level. This critical stage of the operation is performed as follows. After opening the abdominal cavity, the small intestine is moved to the right half of the wound or removed from the abdominal cavity altogether, wrapped in a damp towel. Next, the posterior parietal peritoneum at the root of the mesentery of the transverse colon, the posterior layer of the mesentery and the ligament of Treitz are dissected. The duodenum is shifted to the right, after which the infrarenal portion of the aorta is isolated with a finger. In most cases, this can be done relatively easily, because the tissues surrounding the aorta are displaced to the side by the para-aortic hematoma. Following this, a clamp is applied to the selected area.
In some cases, the left renal vein is a significant obstacle to applying an infrarenal clamp. When visual control is difficult in the setting of a para-aortic hematoma, it can be damaged, resulting in an additional source of bleeding. You should not immediately try to find a defect in the venous wall and sew it up, because this wastes valuable time. It is necessary to perform hemostasis with tamponade of the bleeding area and continue manipulations on the aorta. If the left renal vein proves to be an insurmountable obstacle to applying an infrarenal clamp to the aorta, it can be divided between the clamps near the orifice to preserve functioning collaterals at the renal hilum. After completion of the aortic surgery, the integrity of the transected (or accidentally damaged) renal vein must be restored. In principle, as a last resort, ligation of the renal vein is also acceptable, but this is an additional factor that can aggravate the already frequently developing renal failure in operated patients.
If difficulties arise with isolating the infrarenal aorta, you can use endovascular occlusion of the “neck” of the aneurysm using a Foley catheter or a Fogarty-type obturator balloon.
It should be said that in cases of profuse bleeding from a ruptured aneurysm, endovascular proximal control can be a life-saving measure. The method is not without its drawbacks. Complete obturation of the aorta is not always achieved, which causes leakage of blood in addition to the balloon. It is difficult to determine exactly where the balloon is located: below, at the level of, or above the renal arteries. If there is a sudden increase in blood pressure, the obturator may be displaced downward by the blood stream and cease to perform its function. Finally, balloon obturation, like external compression, is a temporary measure for emergency hemostasis, after which a clamp should be applied to the infrarenal aorta.
Significant difficulties for both proximal control and superior anastomosis arise if the upper pole of the aneurysm is located close to the renal arteries. In this case, it is permissible to apply a clamp at the suprarenal level. The duration of suprarenal aortic clamping should be reduced as much as possible, therefore, immediately after the proximal anastomosis of the aorta with the prosthesis, the clamp should be moved below the renal arteries.
The subphrenic level of proximal control of the abdominal aorta is indicated in cases where: the patient’s condition is so critical that there is no time left to isolate the “neck” of the aneurysm; There is an extensive retroperitoneal hematoma, making it difficult to find and isolate the infrarenal aorta.
The technique of subdiaphragmatic compression of the aorta is as follows. Immediately after opening the abdominal cavity, the left triangular ligament of the liver is dissected and its left lobe is retracted to the right. The cavity of the lesser omentum is opened, the aorta is bluntly isolated immediately below the hole in the diaphragm and compressed with fingers or a clamp (Fig. 17.4). Subphrenic clamping of the aorta is accompanied by ischemia of the kidneys and all abdominal organs, and therefore it should be short-term. It is necessary only for the period of infrarenal (or suprarenal) control, after which it must be immediately eliminated.
These are the methods and levels of proximal control of the abdominal aorta. Each of them has its own advantages and disadvantages, as well as indications for use. More than three decades have passed since Ch. Dubost (1951) performed the first operation for an aneurysm of the abdominal aorta. Over the past period of time, the surgical technique has undergone significant changes, and therefore the operation no longer fully corresponds to the term “aneurysmectomy.” It is now generally accepted that the entire aneurysmal sac should not be removed because this is fraught with injury to organs fused to the aneurysm, increases blood loss and duration of the operation, and results in severe postoperative intestinal paresis. In the modern version, the scope of the operation includes removal of the contents of the aneurysm (atheromatous and thrombotic masses), excision of the anterior and partially lateral walls of the aneurysmal sac, and therefore it would be more correct to call such an intervention “subtotal aneurysmectomy.” Total removal of the aneurysm is permissible if it is small, but this possibility is rare, since large aneurysms usually rupture.
When starting an aneurysmectomy, it is necessary to simultaneously decide which prosthesis to prefer: direct or bifurcation. Unlike a planned operation, the scope of which often includes reconstruction of the renal and other visceral vessels, as well as arteries of the lower extremities, with emergency aneurysmectomy the main task is to save the patient’s life. In this regard, the scope of intervention should, if possible, not be expanded. In particular, direct aortic replacement (tubular prosthesis) is preferable to bifurcation. Unfortunately, the possibility of direct prosthetics is not always possible.
As noted above, in order to reduce the time of the operation and reduce its morbidity, it is not necessary to completely cross the aorta in the area of the “neck” of the aneurysm. The anterior wall of the aneurysm is cut through a vertical incision, which has a T-shape at the proximal end. If direct replacement of the aorta is intended, the lower end of the incision is given the same shape. If the aneurysm involves both common iliac arteries, the incision is made in such a way that it becomes possible to sew a bifurcation graft into the slightly altered areas of the iliac vessels. After the aneurysm cavity is freed from thrombotic and atheromatous masses, the orifices of the lumbar arteries are sutured for final hemostasis. Next, they begin to apply a proximal anastomosis of the aorta with the prosthesis. This is the technically most difficult stage of prosthetics, especially if the stump of the infrarenal aorta is short.
The anastomosis is performed with a continuous enveloping suture. It is necessary to note the danger of cutting through the sutures of the posterior semicircle of the anastomosis. There is no free edge of the aorta and therefore the needle must be inserted to a depth twice the thickness of the aortic wall. In the event that the seam has cut through, it is advisable to apply several U-shaped sutures with synthetic “gaskets” that reliably fix the prosthesis to the aortic wall. If necessary, a blanket suture can be placed between the U-shaped seams. A distal anastomosis is performed in a similar manner, which is much easier to perform due to better exposure of the distal aorta. Before completion of the distal anastomosis, by temporarily loosening the infrarenal clamp, the prosthesis is “wetted” with blood, followed by washing out the clots using a syringe.
The moment of removing the clamps from the aorta is very important. In this case, rapid redistribution of blood occurs and metabolic disorders develop, i.e. revascularization syndrome (after a more or less long period of ischemia of the lower half of the body). In order to prevent possible hemodynamic disorders, the clamp from the aorta should be removed slowly under the control of systemic arterial and central venous pressure.
After removing the clamps, it is necessary to ensure the tightness of the anastomoses. If blood leaks from a needle or between sutures, a napkin moistened with alcohol should be pressed to this place, which is most often sufficient to achieve hemostasis. If this technique does not give the expected effect, it is necessary to apply additional U-shaped sutures. Then, in order to isolate the prosthesis from surrounding organs, the remaining sections of the wall of the aneurysmal sac are sutured over it after their partial excision.
The issue of drainage of the abdominal cavity and retroperitoneal space must be resolved depending on the specific situation. If the possibility of capillary bleeding from the aneurysm bed is foreseen, aspiration (non-suction) drainage should be installed; if the surgeon is confident that final hemostasis will be achieved, there is no need for drainage.
It is important to mention one more circumstance. During the period of isolating the aorta and applying clamps to it, embolism of the iliac arteries and arteries of the lower extremities (thrombi, atherosclerotic plaques) may occur. That is why, before completing the distal anastomosis, it is necessary to check the nature of the retrograde blood flow from both iliac arteries by sequentially briefly removing the clamps from them. However, even in this case, if the retrograde blood flow is good, it is advisable to inspect the arteries using a Fogarty catheter. After completing the vascular stage of the operation, it is necessary to examine the patient’s lower extremities to identify signs of ischemia.
Bifurcation aortoiliac replacement is indicated when the common iliac arteries are involved in the aneurysm, as well as when their mouths have pronounced atherosclerotic changes. Just as with direct aortic replacement, before completing distal anastomoses with the iliac arteries, it is necessary to inspect the peripheral arterial bed using a Fogarty catheter.
In case of widespread atherosclerosis of the iliac arteries, aortofemoral bypass surgery is indicated, which, to save time, is advisable to be performed by two teams of surgeons.
Particular attention should be paid to those cases where blood flow in the lower limb is poorly restored or not restored at all. This can be easily verified by the appearance of the limb, as well as by the results of an ultrasound examination. There is no point in expanding the scope of the operation through femoral-popliteal prosthetics and other revascularization methods, because In this category of patients, severe in many respects, such an intervention will be intolerable. Given this circumstance, in case of severe ischemia, including muscle contracture, it is logical to perform primary amputation of the limb.
In recent years, the installation of an endovascular stent has been used as an alternative to traditional surgical treatment for ruptured AAA. Unlike open surgery, this method is much less invasive. According to the limited literature data, stent placement reduces the mortality rate and the number of postoperative complications. However, for successful installation of an endovascular stent graft into an aortic aneurysm, it is necessary that the proximal “neck” of the aorta should not be shorter than 1.5 cm and there should be no pronounced calcification there. Kinking of the aorta and pelvic vessels makes stent placement more difficult.
A wide range of complications during implantation of endovascular stents has been described. According to the literature, distal migration of the stent prosthesis can occur in 10% of cases. Other complications may include stent occlusion of the renal arteries and graft occlusion (19%).
To summarize, we hope that the information presented in this chapter will serve to improve surgical care for patients with a ruptured abdominal aortic aneurysm.
Endovascular intervention
If the shape and location of the aneurysm are not critical, the endoprosthesis replacement technique is used. The operation proceeds as follows:
- a puncture is made on the patient’s thigh, through which a conductor (narrow silicone tube) is inserted into the aorta;
- A vascular prosthesis is inserted through a conductor into the damaged aorta, which is fixed to the normal parts of the vessel above and below the location of the aneurysm.
The intervention is classified as minimally invasive. It does not require abdominal access, making the operation much easier for the patient to tolerate. The rehabilitation period is 2-3 days. During the day after the operation, the patient is under the supervision of a doctor.
Forecast
If an aneurysm is detected, the patient needs constant monitoring by doctors. Even after surgery, it is necessary to undergo regular examinations to monitor the condition of the blood vessels. Today, no intervention provides a 100% guarantee that the aneurysm will not reappear in another part of the aorta. Therefore, you should take responsibility for your own health and not neglect regular visits to a specialist.
Statistics on aneurysm operations say that the mortality rate of patients six months after surgery and aortic replacement is 10-15%. Within 10 years from the date of surgery, this figure increases to 40%. This is due to the fact that most patients have concomitant chronic diseases.
In case of endovascular intervention, the prognosis is more favorable. The probability of complications and re-formation of an aneurysm is only 10%, while patient death occurs in only 2% of cases.
Medical observation
For small aneurysms, your doctor may recommend medical monitoring, which includes regular testing to make sure the aneurysm is not getting larger, as well as treatment for underlying conditions that may be contributing to the aneurysm's formation or enlargement.
Your doctor will give you regular tests to determine the size of the aneurysm. Once an aneurysm is diagnosed, you will have an echocardiogram in 6 months, as well as follow-up imaging tests.
If you have high blood pressure or have plaque in your arteries, it is likely that your doctor will prescribe medications to lower your blood pressure and reduce your risk of developing aneurysm complications. Such drugs include:
- Beta blockers. Beta blockers lower blood pressure by slowing your heart rate.
- Angiotensin II receptor blockers. Your doctor may also prescribe these drugs if beta blockers do not lower your blood pressure enough. These medications are recommended for people with Marfan syndrome, even if they do not have high blood pressure.
- Statins. These drugs for the treatment of atherosclerosis of the aorta can lower cholesterol levels, leading to a decrease in plaque deposits in the arteries and a reduced risk of developing aneurysm complications.
If you smoke or chew tobacco, it is important that you quit this bad habit. Tobacco use can worsen the aneurysm.
Prevention
There are no specific measures to prevent such pathology as thoracic aortic aneurysm. Doctors give general recommendations:
- Complete cessation of smoking, including hookah and electronic cigarettes.
- Complete abstinence from alcohol (the maximum allowed is 100g of medium-strength alcohol during the holidays).
- Regular exercise. However, physical activity should be moderate. It is recommended to contact a rehabilitation therapist and exercise therapy specialist, who will select appropriate exercises based on the patient’s diagnosis, the presence of concomitant diseases and the current state of health.
- Control of factors that can provoke a rise in blood pressure. These include stress, kidney pathologies, and prolonged exposure to the open air during the warm season.
- Control of atherosclerosis. This pathology requires treatment, because it can lead to other serious complications in addition to the aneurysm.
- Immediately consult a doctor in case of the slightest suspicion of malfunctions in the cardiovascular system, gastrointestinal tract or lungs. It is necessary to respond to the body's signals to prevent serious complications.
If an aortic aneurysm is already present, prevention consists of preventing the development of complications of the pathology:
- Taking anticoagulants prescribed by a specialist. Such drugs are not cheap, but they are vital. They prevent the formation of blood clots in the lumen of the aneurysm.
- Optimal physical activity. If the patient works in a position that involves heavy lifting or other physical labor, it is necessary to think about changing professions. Excessive force can cause rupture of the aortic wall.
- Control of hypertension. It is necessary to take all medications prescribed by the doctor to avoid increasing blood flow pressure on the thinned wall of the aneurysm. The patient must be aware that it can rupture at any moment.
- Control over psychological state. In medical practice, there have been cases where aortic rupture was caused by a minor stressful situation.
In order to monitor the condition of the cardiovascular system, it is necessary to visit a cardiologist annually. And do this not “for show,” but undergo a comprehensive examination. This way you can detect developing diseases in the early stages, which greatly increases the likelihood of successful treatment without incisions and pain. Take care of your health!
results
The Bentall-DeBono operation was most often performed - 177 (39.1%), the 2nd, 3rd and 4th places in terms of frequency of execution were taken by the combined operation of supracoronary VA replacement and AC replacement - 89 (19.6%), AC replacement in combination with VA repair - 67 (4.8%) and David I operation - 65 (14.3%). Supracoronary VA replacement operations were performed less frequently, either alone or in combination with AV repair. In 6 (1.3%) cases, the “elephant trunk” operation was performed, including 1 “frozen elephant trunk” operation using the E-Vita Open Plus hybrid device (Fig. 1).
Rice. 1. Surgical operations used in the treatment of aneurysms of the ascending aorta (n=453). 1. Bentall-DeBono - 177; 2. NP+PAK - 89; 3. PAK + PLAO - 67; 4. David - 65; 5. N.P. Ao - 33; 6. NP+PLAK - 9; 7. plao - 7; 8. “Elephant trunk” - 6.
David's operations had the longest duration of extracorporeal circulation (ECC) and myocardial anoxia (Fig. 2). In the immediate postoperative period, 13 (2.9%) patients underwent resternotomy due to bleeding. In 4 (0.9%) patients, fenestration and drainage of the pericardial cavity were required during their hospital stay. In 11 (2.4%) patients, severe manifestations of cardiovascular failure (CVF) were observed intraoperatively and in the early postoperative period, in 6 cases requiring the use of mechanical circulatory support (3 - intra-aortic balloon counterpulsator, 3 - left ventricular bypass). Complications of the immediate postoperative period are shown in Fig. 3. Hospital mortality was 2.6% (12 patients). The causes of unfavorable outcomes were acute heart failure (4 patients), cardiac arrhythmias (2), acute cerebrovascular accident (ACVA) (2), multiorgan failure (2) and uncontrolled intraoperative bleeding (2) with initial severe infectious damage to the structures of the VoA. There was no strict dependence of the complicated course of the immediate postoperative period on the volume of surgical correction.
Rice. 2. Parameters of extracorporeal circulation.
Rice. 3. Complications of the immediate postoperative period.
From January 1, 2006 to October 1, 2015, we performed 68 operations of valve-sparing aortic root replacement using the David I method for aneurysms and aortic root dissections. Of the 47 patients, 69% were men, 4 (5.9%) patients were operated on due to VA dissection. Hospital mortality was 2 (2.9%) patients, causes: acute CHF - 1 and fatal stroke - 1. In the immediate postoperative period, 7 patients underwent resternotomy for bleeding. When assessing the degree of aortic insufficiency (AI) in the immediate postoperative period, the vast majority of patients had grade 0-1 regurgitation; no severe AN was detected. In the long-term postoperative period, in the group of patients who underwent David's operations using the Valsalva aortic root prosthesis, no more than grade I AN was detected; in the group of patients with linear aortic prostheses, grade 0-I AN was observed in 76% of cases, grade II AN was observed in 16%, and severe AN was observed in 8% of cases. Of the last subgroup, 3 patients were reoperated (AC replacement was performed), 2 are under intensive observation. When studying predictors of relapse of AN in the long-term postoperative period, we identified factors such as the initial diameter of the fibrous ring (FC) of the AC (correlation coefficient with the degree of AN in the long-term postoperative period r
=0.55), position of the point of coaptation of the AC leaflets (
r
=–0.778), as well as the presence of asymmetrical prolapse of the AC leaflets (
r
=0.818).