Dissecting aortic aneurysm
Aortic dissection is a fairly rare but potentially dangerous disease, occurring in 1 out of 10,000 hospitalized patients (however, a significant proportion of patients die in the hospital stage), in 1 case in 400 autopsies, in 1 out of 100 who die suddenly, 3-4% of all sudden deaths from cardiovascular diseases. Aortic dissection is the most common catastrophe among acute aortic diseases. If left untreated, the early mortality rate for dissection is 1% per hour (one person in a hundred dies every hour) on the first day, 75% within two weeks, and over 90% within the first year. However, the survival rate of patients can now be significantly increased thanks to timely diagnosis and early treatment of this terrible condition. Early clinical recognition and various diagnostic techniques for imaging the aorta are an integral part of the management of patients with aortic dissection.
Etiology of aortic dissections
1. Diseases and conditions accompanied by cystic degeneration of the media: a) long-term arterial hypertension (AH) b) congenital connective tissue defects (Marfan, Ellers-Danlos, Turner syndromes, polycystic kidney disease) c) old age (60-70 years) 2. Congenital heart defects: a) coarctation of the aorta b) bicuspid (in 7-14%) and unicuspid valve 3. Atherosclerosis of the aorta 4. Pregnancy 5. Chest trauma, severe physical and emotional stress 6. Systemic vasculitis (especially often granulomatous, giant cell arteritis) 7. Chemical and toxic effects (drugs) 8. Iatrogenic causes. The etiology of aortic dissections is varied, but the main ones are 2 factors that contribute to media degeneration, manifested by disorganization of collagen, smooth muscle elements and elastic fibers: hypertension and age. Hypertension is detected in approximately 84% of patients with RA. The peak incidence of aortic dissection occurs in the 6th–7th decades of life, with men affected 2–3 times more often than women. Marfan syndrome occurs in a small number of cases (6-9% of aortic dissections), most often at a relatively young age with localization of the dissection in the proximal part. A hereditary connective tissue defect is characterized by pathology of the skeletal muscular system (asthenic physique, arachnodactyly, chest deformation, kyphoscoliosis, high arched palate, weakness of the eye ligaments (iridodenosis - lens trembling due to weakness of the ligament of cinnamon), subluxation of the lens, retinal detachment, high myopia), as well as cardiovascular complications (aortic root dilatation, aortic regurgitation, aortic dissection and aneurysm, mitral valve prolapse). Most patients die from complications before reaching 40 years of age, although with timely and adequate correction, life expectancy can be normal. About half of aortic dissections occur in pregnant women over 40 years of age, more often in the third trimester, rarely in the early postpartum period. The reasons for the development of aortic dissections in this category of patients are not completely clear; importance is attached to an increase in blood volume, cardiac output and an increase in blood pressure. Women with Marfan syndrome are at especially high risk of aortic dissection during pregnancy. Sometimes the diagnosis of Marfan syndrome is established after the diagnosis of RA in the postpartum period. Direct trauma to the aorta leads to local tear, hematoma or rupture and only in rare cases to aortic dissection. Various groups of patients after valve replacement suffer from aortic dissection, which usually develops several months and years after surgery. About 18% of acute aortic dissections occur in patients with previous cardiac surgery. Patients with aortic valve replacement are at highest risk. Iatrogenic dissections are associated with angiography and balloon dilatation, aortic cannulation during cardiopulmonary bypass. Delamination in these cases is associated only with technical errors. Aortic dissection also occurs at sites where the aorta has been resected or sutured.
Pathogenesis of aortic dissections
Almost always, aortic dissection begins with a tear in the inner lining in one or two places. In addition to this mechanism, the primary mechanism may be the spontaneous formation of an intramural hematoma due to a rupture of the vasa vasorum, which can lead to the formation of a hematoma without an intimal defect (aortic dissection without an intimal rupture in 3-13%) or with subsequent intimal rupture in the clinical picture of a classic dissection. Intramural hematoma is considered an atypical manifestation of aortic dissection and it is still unclear whether it is an early or subsequent stage of aortic dissection. Rarely, aortic dissection can be promoted by a penetrating atherosclerotic ulcer, most often leading to the formation of a pseudoaneurysm with aortic rupture. Any processes leading to weakening and degeneration of the components of the aortic media, elastic fibers, and smooth muscles predispose to aortic dissection.
RA classification
Aortic dissections are classified by anatomical location and timing. According to the most common classification according to M. DeBakey, aortic dissections are divided according to the place of origin and the degree of involvement of various sections: type I - the place of origin of the rupture is the ascending aorta, and the dissection extends to the arch and often distally to the thoracic and abdominal aorta; type II - the rupture is localized in the ascending part, the dissection is limited only to the ascending aorta; type III - intimal tear is localized in the descending thoracic aorta, spreads more often antegrade distally, along the descending aorta at various lengths, involving either only the entire thoracic region and/or both the thoracic and abdominal regions, and rarely retrogradely to the arch and ascending aorta. Since the treatment tactics for types I and II are similar, anatomical classification is currently common: type A is the proximal or ascending type (which includes DeBakey types I and II), type B is distal or descending (corresponds to DeBakey type III ). According to autopsy data, type A is 2-4 times more common. This type is associated with a relatively high incidence of early complications (rupture with tamponade, stroke, aortic regurgitation), is characterized by high pre-hospital mortality and requires emergency surgical treatment. In most cases, dissection occurs in 2 locations: the ascending aorta - within a few cm of the aortic valve (65%) and the descending aorta - slightly distal to the origin of the left subclavian artery (20%), as the most hemodynamically vulnerable areas. Isolated dissection of the arch (10%) and abdominal aorta (5%), as well as individual arteries arising from the aorta, especially the carotid and coronary, is also possible. For aortic dissections involving the ascending aorta, surgical treatment is indicated, but without its involvement, conservative treatment is recommended. Depending on the time of onset of the disease, acute (less than 2 weeks) and chronic (more than 2 weeks) aortic dissections are distinguished. More than 2/3 of cases of aortic dissection are acute.
Aortic dissection clinic
The clinical picture of the disease is determined by 3 pathoanatomical factors underlying the dissection: dissection of the aortic wall, the development of an extensive intramural hematoma and compression or separation of the aortic branches supplying vital organs (heart, brain and spinal cord, kidneys), followed by ischemia. Sudden aortic dissection itself causes pain. The formation of an intramural hematoma in the area of the ascending aorta leads to compression of the coronary arteries, narrowing of the LV outflow tract, acute circulatory failure, and proximal coarctation. An extensive intramural hematoma, containing a large amount of blood, creates a kind of “oligemic syndrome.” Symptoms of aortic dissection can vary, because... dissection is a dynamic process and the initial picture of the disease may differ from the final one. They can mimic almost all cardiovascular, neurological, surgical and urological diseases. The leading and most common (in 90-96% of cases) aortic dissection syndrome is pain (except for patients with impaired consciousness). The pain is unusually intense and occurs suddenly, with maximum severity at the beginning of the dissection, in contrast to myocardial infarction (MI), where it gradually increases. In some cases, the pain can become unbearable. The pain has a tearing, tearing, shooting nature, can be migrating from the site of origin in the direction of the dissection, and may initially be accompanied by vagal manifestations, nausea, vomiting, and increased blood pressure. The localization of pain in RA is determined by the location of the onset of dissection. Pain behind the sternum, in front of the chest, simulating MI, is characteristic of proximal dissection (more than 90% of cases), especially if it extends to the root and causes compression of the coronary arteries. With further dissection (type 1), the pain moves to the interscapular space, then moves along the spine. Migrating pain along the path of dissecting hematoma is observed in 17-70% of patients. Pain in the neck, pharynx, jaw, face, teeth indicates involvement of the ascending aorta and arch. Pain in the chest behind, back, and lower extremities is characteristic of distal dissection, and it is initially localized in the interscapular space. The absence of pain in the interscapular space is sufficient evidence against distal dissection. When aortic dissection types I and II spread to the abdominal aorta, the pain is localized in the epigastrium, hypogastrium, and lower back, simulating acute diseases of the gastrointestinal tract and urological diseases. An asymptomatic (painless) course (except for patients with impaired consciousness) may occur in patients with chronic dissection. Less common initial signs of aortic dissection (with or without pain) may be: - symptoms of cerebral or spinal cord ischemia, peripheral neuropathy, syncope without local neurological symptoms (in 4-5%), which are more often associated with rupture of aortic dissection in pericardium or pleural cavity; - aortic insufficiency and acute circulatory failure; - renal ischemia; — ischemia of the digestive organs; - cardiac arrest and sudden death. Physical examination findings in aortic dissection are variable and, to varying degrees, are related to the location of the aorta and the degree of cardiovascular involvement. In other cases, even in the presence of extensive dissection, objective data may be subtle or completely absent. 1) Hypertension at the onset of the disease (with a possible clinical picture of shock) is observed more often with distal dissection (in 80-90% of cases), less often with proximal dissection. Arterial hypotension is more common with proximal dissection. Its causes are most often cardiac tamponade, or intrapleural or intraperitoneal rupture of the aorta. 2) Asymmetry of the pulse (decrease in its filling or absence) and blood pressure in the upper or lower extremities is observed in half of the patients with proximal and in 15% with distal RA (with the involvement of the femoral or subclavian arteries). The narrowing is caused either by extension of the aortic dissection to one artery or another, with a decrease in the true lumen, or by proximal obstruction by an intimal flap overlying the ostium of the involved artery. Although the presence of pulse asymmetry in a patient with acute pain suggests RA, erroneous interpretations are possible. 3) Aortic regurgitation with a diastolic murmur of aortic insufficiency is an important sign of proximal dissection and occurs in 50-75% of patients. The murmur may have a musical tone and is better heard along the right edge of the sternum. It can be increasing, decreasing, of varying intensity, depending on the value of blood pressure. In severe aortic insufficiency, there may be peripheral signs: fast, galloping and high pulse and high pulse pressure. In some cases, with the development of congestive heart failure, due to acutely developed aortic insufficiency, the diastolic murmur may be subtle or absent. Four mechanisms can lead to the development of aortic insufficiency during proximal dissection: - expansion of the aortic root and aortic annulus, as a result of which the aortic valve leaflets cannot close in diastole; - asymmetrical dissection, when the pressure of the dissecting hematoma can reduce the location (or weaken the function) of one leaflet below the line of closure of the others; - rupture of the ring supporting the leaflet, or a tear in the leaflet of the aortic valve itself, resulting in a “dangling” leaflet; - prolapse through the aortic valve of a mobile intimal flap, which prevents complete closure of the valve leaflets during diastole, which can cause severe aortic insufficiency. In severe aortic regurgitation, sudden onset of heart failure may be an objective sign of RA. 4) Neurological disorders occur in 6-19% of all aortic dissections and include cerebrovascular disorders, peripheral neuropathy, disturbances of consciousness, and paraplegia. Cerebrovascular disorders occur in 3-6% of cases, due to involvement of the innominate or left common carotid artery. Less commonly, there may be disturbances of consciousness or even coma. When the spinal arteries are involved (usually with distal dissection), there may be paraplegia or paraparesis due to spinal cord ischemia. 5) More rare manifestations of aortic dissection may be: MI, renal infarction, etc. In 1-2% of cases of proximal dissection, the ostia of the coronary arteries may be involved and secondary MI may develop (more often - posterior/inferior, due to more frequent damage to the right coronary artery). Due to the presence of symptoms of aortic dissections, myocardial infarction may not be clinically evident. On the other hand, the ECG of acute MI may not recognize aortic dissection, and the use of thrombolysis can lead to fatal consequences. Therefore, in case of posterior/inferior myocardial infarction, one should not forget about the possibility of RA, and before thrombolysis, some authors consider it necessary to conduct an X-ray examination to exclude aortic dissection. The spread of dissection to the abdominal aorta can cause various vascular disorders: renal ischemia and infarction, leading to severe hypertension and acute renal failure; mesenteric ischemia and infarction of the corresponding area (in 3-5% of aortic dissections); acute ischemia of the lower extremities (with the spread of dissection to the iliac arteries). 6) The clinical manifestation of aortic dissection may be pleural effusions, usually on the left, due to either a secondary exudative reaction around the affected aorta or as a result of rupture or leakage of blood into the pleural cavity. 7) Very rare manifestations of aortic dissection can be: - pulsation of the sternoclavicular joint - hoarseness of voice - compression of the trachea and bronchi with symptoms of stridor or bronchospasm - hemoptysis with a rupture in the tracheobronchial tree - dysphagia - vomiting of blood with a rupture in the esophagus - Horner's syndrome - syndrome superior vena cava - pulsation of neck tissue - atrioventricular block (if the septum is involved) - fever of unknown origin caused by exposure to pyrogenic substances from the hematoma or associated effusion - murmurs caused by rupture of the dissected aorta in the cavity of the atria or right ventricle with the development of heart failure. Despite the variety of clinical manifestations of aortic dissection, it should be borne in mind that various diseases manifested by chest pain can mimic aortic dissection: MI, acute aortic regurgitation without aortic dissection, non-dissecting thoracic or abdominal aortic aneurysm, mediastinal tumors, pericarditis, bone muscle pain.
Methods for diagnosing aortic dissections
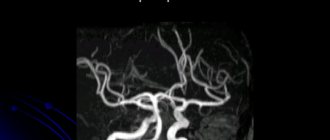
If aortic dissection is suspected, it is important to quickly and accurately verify the diagnosis. Chest X-ray, while not a method of verifying the diagnosis, can nevertheless be the first to reveal signs suspicious of aortic dissection. X-ray examination data are not specific, but may provide grounds for other research methods. The main radiological signs indicating the possibility of RA are: I. Expansion of the aortic shadow (in 81-90% of cases, according to our data), better identified in the left oblique projection (sometimes local protrusion in the area of dissection, less often - expansion of the upper mediastinum). Expansion of the aortic shadow was detected in 50% of patients with type I dissection (- and in 10% - type III. There was unevenness of the contours of the descending aorta, deformation of its shadow. 2. Separation (separation) of the calcified intima in the area of protrusion from the adventitia by more than 1 cm (normally up to 0.5 cm) - a presumptive, but also not diagnostic sign. 3. Change in the shadow of the contours of the aorta or mediastinum when compared with the data of a previous study. 4. Deviation of the trachea or pleural effusion (usually left-sided). 5. Sharply decreased or absent pulsations of an abnormally wide aorta. Although most patients with RA have one or more radiographic findings, 12% of patients have an unchanged chest x-ray. Absence of x-ray changes does not exclude the diagnosis of aortic dissection. Standard 12-lead electrocardiography reveals signs nonspecific for RA left ventricular hypertrophy and associated changes (ST segment depression, negative T wave).In 1/3 of patients, the ECG remains normal!!! However, taking an ECG is important for two reasons: - the absence of changes on the ECG in a patient with severe pain in the chest is the reference differential diagnostic criterion for RA with MI; — the presence of signs of AMI on the ECG (usually lower localization), when compared with X-ray data, allows not only to assume that the patient has aortic dissection, but also indicates the involvement of the coronary arteries. Laboratory signs are not very indicative in the diagnosis of aortic dissection: a. anemia - with significant sequestration of blood in the false canal or rupture in the cavity; b. small (moderate) neutrophilic leukocytosis (up to 12-14 thousand/mm3); V. increased LDH and bilirubin (due to hemolysis of blood in the false channel); d. normal levels of CPK and transaminases; D. Occasionally, the development of disseminated intravascular coagulation syndrome is possible. According to objective and routine examination methods, the diagnosis of aortic dissection can be made in only 62% of patients. The rest at the onset of the disease have signs of myocardial ischemia, congestive circulatory failure, non-dissecting aneurysm of the thoracic or abdominal aorta, symptoms of aortic stenosis, pulmonary embolism, etc. Among these patients with initially undiagnosed aortic dissection, 2/3 of aortic dissections were diagnosed by other research methods used to resolve other clinical issues. In 1/3 the diagnosis was made only at autopsy. The main methods for diagnosing aortic dissections are currently considered to be methods that allow visualization of the aorta: - aortography - contrast-enhanced computed tomography (CT) - nuclear magnetic resonance (NMR) - transthoracic and transesophageal echocardiography. Each technique has its own advantages and disadvantages. The choice of method depends on opportunity and experience. Aortography has long been considered the standard and the only accurate, highly sensitive method for diagnosing aortic dissections. Direct signs of aortic dissection during aortography are: visualization of two lumens (true and false), intimal flap, and indirect signs are deformation of the aortic lumen, expansion and deformation of its wall, abnormal origin of vascular branches, the presence of aortic regurgitation. Aortography allows: 1. to determine the extent of dissection 2. to identify the involvement of aortic branches 3. to determine the location of the initial rupture and the exact location of the proximal fenestration 4. the presence or absence of distal fenestration 5. to assess the degree of consistency of the aortic valve and coronary arteries. However, the false lumen, most often detected in the descending aorta, thromboses in 10-15% of cases; the true lumen is narrowed. With transfemoral access, the catheter may not enter the true lumen of the aorta. It is possible to detect the presence of an intimal flap (i.e., a detached inner membrane between the true and false lumen) in 1/3 of patients. The disadvantage of aortography is the possibility of obtaining false-negative results, which happens with weak contrast of the false lumen (due to its possible thrombosis), equally uniform contrast of both channels, small and local dissection. The difficulties of using this method include the risk of an invasive procedure and the introduction of a contrast agent (its intolerance), the impossibility of performing aortography in unstable (non-transportable) patients. In addition, the introduction of alternative diagnostic techniques has shown that the sensitivity and specificity of aortography are 77-88% and 95%, respectively. Thus, the false tract is visualized in 87% of patients, the intimal flap in 70%, and the site of the initial intimal tear in only 50% of patients with aortic dissections. Echocardiography is an accessible and non-invasive method for diagnosing RA. According to the literature, transthoracic echocardiography can detect 80% of aortic dissections. Currently, a special role in the diagnosis of aortic dissection is given to transesophageal echocardiography (the sensitivity of the method is 95%, and the specificity is 75%), which is the method of choice in case of an unstable patient’s condition, because can be quickly performed at the patient’s bedside, in the operating room, immediately before surgery, and does not require cessation of monitoring and ongoing therapeutic measures. Echocardiography allows visualization of dilation of the aortic bulb, increased aortic wall thickness, aortic valve function, identification of the mobile flap in the aortic lumen, and also provides additional information about cardiac structures and function. If transesophageal echocardiography is not possible, computed tomography with contrast injection is the method of choice. On contrast-enhanced CT, aortic dissection is identified by the presence of two distinct lumens, apparently separated by an intimal flap, or by a different rate (degree) of contrast opacification. The method has a sensitivity of 83-94% and a specificity of 87-100%. The advantages of CT are: non-invasiveness, although IV contrast is required; availability; the ability to establish a diagnosis of aortic dissection in the case of false lumen thrombosis; the ability to determine the presence of pericardial effusion. The main disadvantages of CT are: relatively low sensitivity for diagnosing aortic dissections; inability to identify an intimal flap in 1/3 of cases; the rarity of identifying the location of the initial rupture; inability to detect the presence of aortic regurgitation and involvement of vascular branches. NMR is a non-invasive technique that does not require IV contrast, while providing high-quality images in several planes. NMR facilitates the recognition of RA, allows the identification of branch involvement, and also diagnoses aortic dissection in patients with pre-existing aortic diseases. The sensitivity and specificity of the method are about 98%, while the sensitivity is 88% for identifying the site of intimal rupture and aortic regurgitation, 98% for diagnosing the presence of thrombosis and 100% for detecting pericardial effusion. The unusually high accuracy makes NMR the modern “gold standard” in the diagnosis of RA, especially in stable patients and with chronic dissection. However, the method still has a number of disadvantages: NMR is contraindicated in patients with a pacemaker, in the presence of a certain type of vascular staples, and some old types of prosthetics with metal artificial valves; is not a widely available method. Some authors consider the unstable condition of the patient, requiring intravenous administration of antihypertensive drugs and blood pressure monitoring, to be a relative contraindication to NMR.
Treatment of aortic dissections
Treatment for aortic dissection is aimed at stopping the progression of the dissecting hematoma. Death is usually caused not by the intimal rupture itself, but by subsequent vascular complications or aortic rupture. Without treatment, aortic dissection has a high mortality rate. The main cause of death in the proximal type of dissection is cardiac tamponade, less often - occlusion of the main branches of the aorta, and in the distal type - bleeding into the left pleural cavity and renal failure. Mortality in the proximal type among those treated conservatively is approximately 4 times higher than among those operated on. All patients with aortic dissection should be hospitalized in an intensive care unit to stabilize hemodynamics, monitor blood pressure, heart rate and diuresis, and, if necessary, monitor central venous pressure or pulmonary artery wedge pressure, regardless of further treatment tactics. Intensive therapy for acute dissection is aimed at relieving pain and reducing blood pressure (systolic - up to 100-120 mm Hg), using various schemes. The pain should be relieved with IV morphine. To reduce cardiac output and reduce the rate of LV ejection, b-blockers are used in increasing doses until the heart rate decreases to 60-80 per minute. Propranolol (Obsidan) is used intravenously at an initial dose of 1 mg every 3-5 minutes. The maximum initial dose should not exceed 0.15 mg/kg. Maintenance therapy - every 4-6 hours in doses from 2 to 6 mg, depending on heart rate. You can also prescribe metoprolol at a dose of 5 mg IV every 5 minutes. (up to three times). Labetalol, as an α-blocker, is 2-7 times less active than phentolamine, and as a β-blocker, it is 5-18 times less active than propranolol. Blocks α- and b-adrenergic receptors in a ratio of 1:3. Blood pressure decreases, mainly due to a decrease in peripheral resistance, with virtually unchanged cardiac output. Intravenous administration is carried out either as a bolus - 100-125 mg, or as a slow drip - 50-200 mg / day. in 200 ml of saline or glucose. The drug is contraindicated in cases of AV conduction disorders, a tendency to bronchospasm, and in the first months of pregnancy. If there are contraindications to the use of b-blockers (bradycardia, AV block, bronchospasm), calcium channel antagonists are now increasingly used. Nifedipine sublingual can be used immediately while other drugs are prepared for administration. The disadvantage of nifedipine is its weak negative inotropic and chronotropic effects, and therefore diltiazem and verapamil can be used. If beta blockers are ineffective, sodium nitroprusside can be used at a dose of 0.5-10 mg/kg*min IV. For refractory hypertension, as a result of involvement of the renal arteries, the most effective is the use of ACE inhibitors (enalapril - 0.625 mg intravenously every 4-6 hours with a gradual increase in dose). With normal blood pressure, only b-blockers are used (and if they cannot be used, diltiazem or verapamil) to reduce the rate of LV ejection. In case of hypotension, one should think about the possibility of cardiac tamponade, aortic rupture, which, if possible, requires rapid restoration of blood volume. For refractory hypotension, it is preferable to use norepinephrine and mesaton. Dopamine is used to improve kidney function and only in small doses. When the patient's condition is stabilized, diagnostic studies are immediately carried out to verify the diagnosis. If the patient's condition is unstable, it is preferable to perform TEE, against the background of continuous monitoring and therapeutic measures. Further tactics are determined by the type of separation. Sudden deterioration in patients with aortic dissection is associated with tamponade or aortic rupture. As has been proven in a number of retrospective studies, pericardiocentesis during hemotamponade worsened the prognosis and patients died suddenly after 5-40 minutes. after the procedure, therefore, carrying out this procedure in urgent conditions is possible only in the operating room with direct surgical intervention. In extreme cases, with severe hypotension, careful aspiration of only enough pericardial fluid to produce an acceptable rise in blood pressure is possible.
Surgical treatment of aortic dissection
In all cases, patients should be consulted by a vascular surgeon. Surgery is the treatment of choice for acute proximal aortic dissections, which may lead to complications such as rupture, tamponade, acute aortic regurgitation, or neurological impairment. Surgical treatment is also indicated for chronic ascending dissection with severe aortic regurgitation and localized aneurysm. In chronic distal dissection, surgery is indicated for aneurysms larger than 6 cm in diameter. For distal dissection, conservative therapy is justified due to the lower risk of early death, older age of patients, and the presence of severe atherosclerosis or pulmonary heart disease, which increases the risk of surgical intervention. With this type of dissection, surgical treatment is indicated when a saccular aneurysm forms, when vital organs are involved, limb ischemia continues despite conservative therapy, dissection, or retrograde spread to the ascending aorta. Surgical treatment is indicated for all patients with Marfan syndrome. Conservative (medical) treatment is the treatment of choice for uncomplicated distal dissection, stable isolated aortic arch dissection, and stable uncomplicated chronic dissection (more than two weeks old). The risk of surgical intervention increases in the presence of concomitant diseases, especially pulmonary emphysema, violation of the integrity of the aneurysm, cardiac tamponade, shock, and involvement of vital organs. Postoperative mortality with rapid surgical treatment was significantly lower (3%) than with long-term preoperative preparation (20%). The goals of surgical treatment are: - resection of the most damaged segment of the aorta containing an intimal rupture; — removal of the intimal flap; — obliteration of the entrance to the false canal; — prosthetics of the excised area of the aorta; - restoration of the aortic valve (valvuloplasty). Possible complications of surgery for aortic dissection include: 1. Early bleeding, infections, pulmonary and renal failure, and sometimes spinal cord ischemia with paraplegia. 2. Progression of aortic regurgitation. 3. Formation of a local aneurysm. 4. Repeated delamination. With the introduction of new surgical methods, postoperative survival is 80% for proximal dissection and 90% for distal dissection. Such methods that have reduced mortality in operated patients with aortic dissection are: thromboexclusion in typical cases of dissection of the descending aorta, the use of endovascular methods (balloon dilatation of the renal arteries), the use of special glue to fill the false lumen. Further long-term treatment of all patients after discharge from the hospital includes maintaining systolic blood pressure at a level not higher than 130-140 mm Hg. Art., prescription of antihypertensive drugs with a negative inotropic effect (beta blockers, calcium antagonists), repeated thorough examination, control radiography, CT, MRI every three months during the first year, then every 6 months. Late complications include: aneurysm formation after surgical repair at the site of repair 1/3 of late deaths may be associated with rupture of the false lumen after successful surgery.
How this happens: pathophysiology of the process
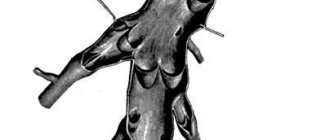
Typically, delamination occurs at the site of the damaged area of the inner wall. Most often, there is already an aneurysm there - an expansion of the lumen of the aorta. Naturally, the inner wall of the vessel is significantly thinned. Another important factor is high blood pressure (especially during a hypertensive crisis). In this case, under strong pressure, the damaged intima ruptures.
Blood then enters the muscle layer and pushes the fibers apart, spreading proximally and distally from the rupture site. This creates a so-called false flow. In some cases, it may stop in places where healthy tissue is located. If the aorta is affected over a significant area, the process spreads further, spreading to other arteries, which significantly worsens hemodynamics. In some cases, the protrusion formed due to false flow leads to the closure of the lumen of the outgoing arteries.
In cases where the outer wall also ruptures, massive internal bleeding occurs, which in 90% of cases leads to death.
Symptoms and clinical manifestations
Symptoms of aortic dissection depend on the course - acute or chronic. In the first case, the condition develops very quickly, the clinical picture is clearly defined. In the second, the process is slow, symptoms appear gradually and depend on the localization of the process.
The acute form is characterized by the following symptoms:
- intense pain that begins suddenly, appears behind the sternum or between the shoulder blades and gradually moves to the abdominal area and lower back;
- collapse, manifested by severe sudden weakness, pallor, sweating, lethargy and even loss of consciousness;
- dyspnea;
- fear of death;
- neurological disorders - loss of sensitivity or paralysis of a certain area of the body, muscle weakness;
- involuntary urination and defecation.
The abdominal variant of dissection has its own characteristics. When the abdominal aorta is damaged, the following symptoms occur:
- acute abdominal pain;
- intestinal dysfunction, bloating;
- symptoms of intoxication;
- numbness and pain in the legs.
In the chronic version, the following manifestations are characteristic:
pain syndrome, which manifests itself depending on the location of the pathology. The pain is dull, pressing, intermittent, and increases with increasing pressure;- shortness of breath on exertion;
- superior vena cava syndrome (swelling of the upper half of the body, cyanosis, tachycardia, swelling of the jugular veins);
- difficulty swallowing, hoarseness, bradycardia, pulmonary edema - due to compression of the mediastinal organs;
- congestion in the intestines, abdominal pain - with damage to the abdominal region.
Diagnostics
Correct diagnosis will make it possible to accurately determine the type and location of dissecting aortic aneurysm, which is of decisive importance for the choice of further tactics.
The acute form, due to its pronounced clinical manifestations, is determined quite easily, while the chronic form is often disguised as other diseases. This requires a careful differential diagnosis.
A physical examination reveals the following signs:
- cyanosis or pale skin;
- pulse difference in peripheral arteries;
- high blood pressure, a significant difference in the arms and legs, in some cases it is not determined;
- percussion – expansion of the boundaries of cardiac dullness.
To confirm, the following diagnostic methods must be used:
electrocardiography (ECG);- chest x-ray;
- echocardiography – this technique is the gold standard;
- Ultrasound of the abdominal organs;
- CT or MRI;
- contrast angiography.
List of sources
- Guide to Cardiology / ed. ON THE. Manaka. - Minsk: Belarus, 2003;
- Pyrochkin V.M. Dissecting aortic aneurysm. Textbook for 4-6 year students and doctors / V.M. Pyrochkin, E.V. Mironchik. - Grodno, 2004;
- Camm A.D., Luscher T.F., Serruis P.V., eds. Diseases of the heart and blood vessels. Guidelines of the European Society of Cardiology. M.: GEO-TAR-Media; 2011;
- Sprigins D., Chambers D., Jeffrey E. Emergency therapy: A practical guide: Translated from English. - M.: Geotar Medicine, 2000;
- Chazov E.I. Diseases of the heart and blood vessels. In 4 vols. M.: Medicine, 1992. T 3.
Treatment methods
This pathology requires immediate emergency medical care already at the diagnostic stage, followed by transfer of the patient to the intensive care unit. The intensive care protocol contains the following algorithm:
- pain relief – narcotic analgesics (morphine);
- pressure correction (dopamine, mesaton for collapse, sodium nitroprusside for elevated);
- infusion therapy to maintain blood volume and kidney function;
- oxygen therapy.
Further treatment of aortic dissection is usually surgical; conservative therapy is indicated only in cases of mild chronic forms.
The operation consists of eliminating the false channel and suturing or prosthesizing the walls of the vessel. If necessary, aortic valve repair is performed. There are two options - open heart intervention and endovascular technique. Anticoagulants and glucocorticoids are used to prevent complications.
In the future, the patient requires long-term rehabilitation and dynamic observation.
Maintenance drug therapy is prescribed - cardiotonics, antihypertensive and antisclerotic drugs.
The following recommendations reduce the risk of relapse:
- moderate physical activity;
- rejection of bad habits;
- avoiding junk food;
- physiotherapeutic procedures.