What is “platelet aggregation”
Platelets are colorless blood cells whose function is to protect the body from blood loss. When body tissues are damaged, platelets immediately rush to the site of injury and block it, preventing excessive blood loss. This blocking occurs due to the fact that platelets instantly stick together and form a block that closes the wound. This blocking process is called platelet aggregation. The process occurs in two stages:
- Blood cells stick together
- Later they adhere to the walls of the vessel, forming a blood clot or thrombus.
When the body is healthy, this function is protective. But if pathological changes occur in the body, the formation of blood clots can disrupt nutrition in vital organs and tissues.
Induced aggregation with ADP
Description Platelets play a significant role in human life: they prevent blood loss by connecting with each other on the wall of a damaged vessel. This process of platelets sticking together is called aggregation. It is important that this parameter does not deviate from the normal values, otherwise this is fraught with blood loss or the formation of a blood clot that can block the entire diameter of the vessel and lead to tissue death.
It is important that this parameter does not deviate from the normal values, otherwise this is fraught with blood loss or the formation of a blood clot that can block the entire diameter of the vessel and lead to tissue death.
Platelet aggregation: what is it?
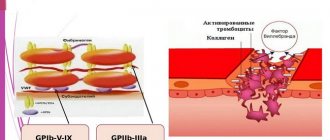
The process of platelet aggregation into conglomerates is as follows: when a vessel is injured, platelets combine with von Willebrand factor and collagen of the subendothelial layer.
Aggregation of platelets with collagen causes activation of red blood cells, and they become sources of components that stimulate aggregation: thromboxane and serotonin. Platelet disaggregation is the reverse of aggregation.
Platelet aggregation is a protective function of the body, but it is also subject to the influence of pathological reactions.
On the one hand, platelet aggregation saves us from excess blood loss, which is important during surgical operations, major injuries and childbirth; on the other hand, hyperaggregation leads to the formation of a blood clot.
Blood clots are very dangerous: their movement through the circulatory system into the heart, brain or pulmonary artery can lead to rapid death. The study of platelet aggregation takes into account their ability to connect: with deviations above the norm, a heart attack or stroke may occur, below the norm - blood loss, anemia, exhaustion.
What is platelet aggregation in the blood? To determine the value of platelet aggregation, it is necessary to take a blood test from a vein into a test tube, and in this case, when there is no wound, it is necessary to create conditions for the combination of platelets into a conglomerate.
For this purpose, induced platelet aggregation is introduced in the laboratory.
Conditions become as close as possible to the body’s environment; substances identical in chemical composition to human substances that can cause thrombus formation are taken as an inducer of platelet aggregation.
Inductors can be:
- Serotonin.
- Collagen.
- Adrenalin.
- Arachidonic acid.
- Adenosine diphosphate (ADP).
The way to measure platelet aggregation is to pass light through the blood plasma.
The difference between the light density of plasma before blood clotting and after clot formation is the aggregation activity of platelets. Additionally, the rate of platelet aggregation in 1 minute, the shape and type of waves are determined.
Spontaneous platelet aggregation is calculated without the use of an inducer.
Degree of platelet aggregation:
- norm with ADF – 30.7 – 77.7%
- with collagen – 46.4 – 93.1%
- with adrenaline – 35 – 92.5%
Preparing for the test
A reliable assessment of the result can be obtained if the following conditions are met: Do not eat 12 hours before the test. The day before blood sampling, do not perform physical activity or experience emotional turmoil.
7 days before the test, stop taking a number of medications (if this is not possible, then inform the nurse).
The day before the test, avoid alcohol, smoking, garlic and coffee. Come to donate blood healthy, without inflammation in the body.
Deviation from the norm
A decrease in platelet aggregation can occur for the following reasons:
- The presence of thrombocytopathy.
- Treatment with antiplatelet drugs.
Thrombocytopathy is a disease in which platelet functions (clotting, thrombus formation) are impaired. The disease manifests itself by the presence of bruises and bleeding, which subsequently leads to anemia.
If platelet aggregation is reduced, then drug treatment is prescribed, which should be carried out by a qualified specialist.
Increased platelet aggregation can occur with:
- Hypertension.
- Atherosclerosis of joints.
- Stroke and heart attack.
- Diabetes mellitus.
- Thrombosis.
If platelet aggregation is increased, then conservative therapy with the use of antiplatelet agents cannot be avoided. Be sure to visit a doctor to get tested and receive treatment recommendations.
During pregnancy, deviations from normal platelet aggregation values are possible.
A reduced level indicates the danger of bleeding, an increased level indicates toxicosis as a result of loss of fluid from the body and is fraught with miscarriage.
During pregnancy, the degree of platelet aggregation: the norm is 30-60% for any inducer. A platelet aggregation test is prescribed in cases of complicated pregnancy, poor medical history, and before planned conception.
What does platelet aggregation disorder lead to?
If the aggregation process is disrupted, this can lead to negative consequences. So, if the activity of colorless blood cells is increased, this can lead to a stroke or heart attack. And if platelet production is reduced, this can lead to large blood loss: a person suffers from frequent bleeding that does not stop for a long time, leading to exhaustion and anemia.
It is in order to prevent these consequences that platelet aggregation analysis is performed. Indications for testing are:
- Frequent bleeding (nasal, uterine)
- Poorly healing wounds.
- Bruises that appear from the slightest injury.
- Constant swelling.
To assess the platelet component of hemostasis, the number of platelets in the blood is counted. Platelet pathology is the cause of bleeding in almost 80% of cases. Thrombocytopenia is its most common manifestation. Thrombocytopenia should be diagnosed in cases where the platelet count is less than 150 × 109/L. The duration of bleeding is an important screening test, the main advantage of which is that it allows you to simultaneously qualitatively assess the number of platelets in the blood, their adhesive and aggregation functions, the functional properties of the blood vessel wall, as well as a pronounced deficiency of plasma coagulation factors (for example, factors VIII and IX, the deficiency of which is the cause of hemophilia A and B). Prolongation of bleeding time reflects a violation of hemostasis due to thrombocytopenia, thrombocytopathies (impaired platelet functions - adhesion and aggregation), disorders of the vascular wall, or a combination of these factors. Prolongation of bleeding time with a normal number of platelets in the blood suggests a violation of their functions. This is the main value of the test, because... To assess the adhesive and aggregation properties of platelets in laboratory conditions, complex and expensive equipment (aggregometer) is required. When platelet function is not impaired, bleeding time remains normal even when the platelet count drops to 100.0 x 109/L. Below this level, the bleeding time gradually increases in a linear relationship with the platelet count. Spontaneous bleeding occurs if the platelet count falls below 50.0 x 109/L; fatal bleeding is almost inevitable if the platelet count drops to 5.0 x 109/L. The duration of bleeding increases under the following conditions:
- Severe thrombocytopenia;
- Impaired platelet function - thrombocytopathies, which can be congenital (Bernard-Soulier syndrome) and acquired (pernicious anemia, acute and chronic leukemia, multiple myeloma, long-term use of aspirin);
- Marked decrease in plasma coagulation factors;
- If the resistance of the capillary wall is impaired (lack of vitamin C, defects in capillary contraction - microangiopathies).
A shortening of bleeding time has no diagnostic significance and is most often the result of a technical error during the test or indicates an increased spasticity of the capillaries. It is impossible to assess the susceptibility to thrombus formation using the bleeding duration test. An increased number of platelets in the blood - thrombocytosis, carries with it the risk of increased coagulation and is manifested by thrombosis. In clinical situations, the risk of thrombosis becomes real if the platelet count reaches 1000.0 x 109/L.
Study of platelet aggregation functions Aggregation processes are studied using an aggregometer, which reflects the progress of aggregation graphically in the form of a curve; ADP, adrenaline and collagen are often used as aggregation stimulators.
Disorders of platelet aggregation in various diseases (according to the results of studies using an aggregometer)
| Type of thrombocytopathies | Aggregation stimulator and aggregation disorder | ||||
| ADF | collagen | adrenalin | ristocetin | ||
| primary wave | secondary wave | ||||
| Thrombasthenia | Pathology | Pathology | Pathology | Pathology | Norm |
| Essential atrombia | Pathology | Pathology | Pathology | Pathology | Norm |
| Aspirin-like defect | Norm | Pathology | Pathology | Pathology | Norm |
| Bernard-Soulier syndrome | Norm | Norm | (+,-) | (+,-) | Norm |
| Wiskott-Aldrich syndrome | Pathology | Pathology | Pathology | Pathology | Norm |
| von Willebrand disease | Norm | Norm | Norm | Norm | Reduced pathological |
Note: (+,-) – has no diagnostic value.
Platelet pathology is the cause of bleeding in almost 80% of cases. Thrombocytopenia is its most common manifestation. Thrombocytopenia should be diagnosed in cases where the platelet count is less than 150 × 109/L. There are acute and chronic thrombocytopenia. The latter is diagnosed when its duration exceeds 6 months. Individuals with platelet levels above 50×109/L rarely experience bleeding. However, a decrease in platelets below 100×109/L can accompany serious pathology. In this regard, the cause of each case of thrombocytopenia must be clarified. With thrombocytopenia, severe thrombocytopathies and von Willebrand factor (VWF) deficiency, the bleeding time is significantly prolonged. Bleeding is associated with insufficiency of the adhesive-aggregation function of platelets - a violation of the formation of a platelet plug in damaged vessels. This may be due to either a significant decrease in the number of platelets in the blood, or their dysfunction, which is most often based on the absence or blockade of receptors on the platelet membrane that interact with stimulators (agonists) of aggregation of these cells (VWF, adrenaline, ADP, fibrinogen, arachidonic acid and prostaglandins), or the absence in platelets or a violation of the release from them of granule components containing these aggregation stimulants. Modern hematology analyzers display thrombocytometric curves (histograms of platelet distribution by volume). There is a connection between the size of platelets and their functional activity, the content of biologically active substances in platelet granules, the tendency of cells to adhere, and changes in platelet volume before aggregation. The presence of predominantly young forms of platelets in the blood leads to a shift of the histogram to the right; old cells are located in the histogram on the left, since as platelets age, their volume decreases. An increase in the content of young forms of platelets is observed during blood loss and indicates enhanced regeneration. An increase in the content of old forms, vacuolated platelets, forms of irritation and a decrease in the content of mature platelets are characteristic of various inflammatory processes, intoxications, and malignant neoplasms.
Thrombomodulin is an integral membrane protein, a thrombin receptor located on endothelial cells of blood vessels and involved in the anticoagulation system. It determines the speed and direction of the hemostasis process, actively limits and regulates blood clotting. The endothelium, forming thrombomodulin, blocks active coagulants secreted by the liver and found in the blood plasma, primarily the most active coagulation factor, thrombin. Bound thrombin is excluded from the blood coagulation system. Thrombin, having joined thrombomodulin, acquires new qualities: together with the anticoagulant proteins C and S (protein S cofactor), it forms an antiplatelet and antithrombotic complex, which prevents coagulation and inhibits fibrinolysis. Thus, the thrombomodulin-protein C system performs an anticoagulant function. Moreover, thrombin, modified by interaction with thrombomodulin, loses the ability to convert fibrinogen into fibrin and cause platelet aggregation. When the vascular wall is damaged, thrombomodulin is “separated” from the endothelium and enters the blood. Its increase in the blood is observed in patients with prethrombotic conditions, vasculitis, etc. The degree of increase in thrombomodulin in the blood has diagnostic and prognostic significance. Thrombomodulin is significantly reduced in some diseases, such as atherosclerosis, which can increase blood clotting and increase the risk of thrombosis.
Factor B illebrand is a complex multimeric adhesive glycoprotein, a carrier-stabilizer of the procoagulant protein F VIII:C, which is an adhesion protein in the processes of hemostasis. Von Willebrand factor can bind collagen and possibly other endothelial structures and mediates platelet adhesion to the subendothelium through binding of the platelet surface receptor glycoprotein Ib. Von Willebrand Factor-mediated platelet adhesion occurs most intensely at high shear rates, i.e. in the arteries. VIII-vWF is also a carrier of F VIII - antihemophilic globulin A. Von Willebrand factor in healthy people prevents the growth of blood clots in blood vessels by activating the formation of plasmin. Elevated levels of von Willebrand factor activity are an indicator of endothelial damage in vascular disease, which may be significant for hypertensive vascular complications. Von Willebrand disease is a congenital hemorrhagic diathesis. In its mild form, this disease is the most common hemorrhagic disorder in men, with an incidence of 1:100. This is a disease of a heterogeneous nature, caused either by defects in protein structure or by a decrease in the concentration of von Willebrand Factor. Classification of the disease is based on the clinical picture, history and laboratory results, including clotting time and determination of antigen and von Willebrand factor activity. Determining the level of Von Willebrand Factor helps in the differential diagnosis of two main types of disease: type 1 and type 2. Determining the type of disease is important, since the choice of patient management tactics depends on it.
Fibronectin is a receptor for fibrin-stabilizing factor. Promotes platelet adhesion by participating in the formation of a white blood clot; binds heparin. By joining fibrin, fibronectin thickens the blood clot. Under the influence of fibronectin, smooth muscle cells, epithelial cells, and fibroblasts increase their sensitivity to growth factors, which can cause thickening of the muscular wall of blood vessels (narrowing of diameter).
Explanation of the platelet aggregation test
In the platelet aggregation test, indicators of 25–75% indicate good hematopoiesis. This means that tissues and organs are normally supplied with oxygen, and there are no blood clots.
Platelet rate
| Age | Indicator, x 10^9/l |
| Newborn | 100–420 |
| Child under one year old | 160–320 |
| 1–4 years | 150–300 |
| 15–18 years old | 180–340 |
| Men over 18 years old | 180–400 |
| Women over 18 years old | 150–380 |
Disturbances in the hemostatic system and its correction in pregnant women with metabolic syndrome
The frequency of metabolic syndrome according to various authors is 5–20% (I. Despres, A. Marette, 1994). It should be noted that obesity is one of the main components of metabolic syndrome, and the number of obese women, including pregnant women, is progressively increasing.
There is a significant number of works devoted to the effect of obesity on the course of pregnancy and childbirth. However, a few studies have been devoted to the study of some metabolic parameters. Thus, G. A. Chernukha (1987) showed a violation of the hormonal profile in the system of hemostasis and lipid metabolism in obese pregnant women. V. N. Serov et al. (2000) noted changes in metabolic processes in pregnant women with obesity and underweight, in particular hypo- and dyslipidemia, and decreased glucose tolerance.
An important component in pregnant women with metabolic syndrome is impaired blood flow in the maternal system due to obesity, impaired placental insufficiency, altered hormonal profile: increased leptin levels, altered insulin ratio and altered water-electrolyte balance.
However, in the literature available to us, we did not find any studies that would link obesity in pregnant women with the presence of metabolic syndrome.
The purpose of our study was to study changes in the hemostatic system, the frequency of metabolic syndrome and its correction in pregnant women with metabolic syndrome and to determine the impact of these changes on the course of pregnancy and childbirth.
The task was set to find out the pathophysiological mechanism of the development of blood hypercoagulation and endothelial damage in pregnant women with metabolic syndrome. The study included 43 pregnant women with metabolic syndrome aged 27 to 37 years in the third trimester of pregnancy, including 8 pregnant women who developed moderate gestosis. As a control, 15 healthy pregnant women of the same gestational age were observed. All pregnant women underwent a clinical examination including an oral stress test for glucose tolerance. The inclusion criteria for pregnant women in the main group were: arterial hypertension, visceral obesity, hyperinsulinemia and hyperglycemia.
Laboratory research
Blood was taken from the cubital vein on an empty stomach with the addition of the anticoagulant 0.6% ethylenediaminetetraacetic acid (EDTA). The level of fibrinogen, antithrombin III (AT III), plasma tolerance to heparin, fibrinolytic activity of blood, thromboxane B2 content, cAMP in platelets and prostacyclins were determined. Quantitative indicators of platelet aggregation were assessed by spontaneous aggregation induced by adenosine diphosphate (ADP) and collagen, and blood insulin was determined. To determine platelet aggregation, blood was taken into tubes containing a 3.8% sodium citrate solution in a 9:1 dilution. The quantitative characteristics of the rate and intensity of aggregation were assessed on an automatic two-channel laser aggregation analyzer using software.
To study spontaneous platelet aggregation, an aggregation curve recorded for 10 minutes without induction was analyzed. An ADP solution at concentrations of 1×10-5 M, % and 1×10-7 M, %, collagen, platelet activating factor, adrenaline 10-4 M, aggristin was used as an aggregation inducer. The protocol for studying platelet aggregation in platelet-rich plasma included measuring the maximum aggregation rate as a percentage. NO was determined using the method for determining NO2 nitrites and nitrates as products of nitric oxide metabolism in human serum using high-performance liquid chromatography on a Shimatsu device. Blood serum was frozen and stored at -70 degrees. During the analysis, the serum was thawed, diluted with an equivalent amount, the buffer and proteins were removed by filtration through an UltraFree Units filter in a centrifuge at 5 thousand rpm for 15 minutes. Quantitative analysis was performed by comparison with an external standard at a wavelength of 214 nm, followed by dilution.
Research results
Physiological pregnancy is accompanied by significant changes in the hemostatic system. To address the issue of the development of blood hypercoagulation in conditions of complicated pregnancy, we conducted a study of the hemostatic system and functional activity of the blood in pregnant women of the main and control groups. From the table 1 shows that in the control group the level of fibrinogen is 2.36, and in the main group 3.74, plasma tolerance to heparin in the control group is 948.0, and in the main group 724.0. A very important indicator is the AT III level. In the control group it is 37.6, and in the main group - 27.3. An increase in the level of fibrinogen in the main group is one of the important links in the microcirculation disorder, which is accompanied by a blockade of the transport of substances, in particular a disruption in the delivery of oxygen, glucose, lipids, and proteins to the fetus. Violation of membrane permeability is accompanied by a change in the nature of oxidative processes, in particular an increase in the level of anaerobic oxidation, the development of ischemic and dystrophic changes in fetal tissues.
Clinically, this is manifested by a decrease in fetal weight and asphyxia. A great danger of developing thrombosis during pregnancy is the combination of high levels of fibrinogen and a reduced AT III level. It is known that the anticoagulant activity of blood is ensured by a number of biochemical factors. One of the most active anticoagulants is the plasma cofactor of heparin AT III. It is a universal inhibitor of almost all enzyme coagulation factors (especially thrombin and factor Xa). A critical decrease in the level of AT III in pregnant women in the main group causes a preeclamptic state. Comparing the quantitative indicators of platelet aggregation in healthy women and in pregnant women with metabolic syndrome, we noted significant differences. What are the reasons for these changes? An important role is played by changes in the physicochemical properties of platelets during pregnancy and insulin resistance.
The resulting ischemia in organs and tissues disrupts the energy potential of platelets with the development of membrane depolymerization, corresponding to the loss by the cell of one of the forms of “free energy,” i.e., the energy of the transmembrane potential. Platelet aggregation in patients with metabolic syndrome, induced by ADP, increases significantly - up to 79.1% (in healthy individuals 61.0%). Platelet aggregation stimulated by collagen significantly increases - up to 69.0% (57.0% in healthy individuals). Collagen-induced aggregation is very important, since in the case of a slight morphological change in the vessel wall, conditions arise for the formation of an intravascular platelet thrombus.
Endothelial damage with collagen exposure is an important trigger for platelet aggregation. With metabolic syndrome in pregnant women, platelet aggregation changes significantly under the influence of adrenaline. If in healthy women with physiological pregnancy it is 13.4%, then with metabolic syndrome it increases to 30.2%. Adrenaline-induced platelet aggregation in pregnant women with metabolic syndrome indicates a sharp increase in the sensitivity of the platelet receptor apparatus to adrenaline.
It follows from this that even minor emotional stress and moderate physical activity can provoke activation of platelet aggregation with possible progression to the development of a preeclamptic state. The pathophysiological basis for changes in platelet aggregation during gestosis is a violation of the cell's cAMP activity. It is the high level of platelet cAMP that allows it to maintain functional viability: metabolism, energy and information exchange.
In pregnant women in the control group, the platelet cAMP level is 4.1 pmol (3×108 cells); in women with metabolic syndrome, the cAMP level decreases to 3.0 pmol (3×108 cells). Preeclampsia is characterized by increased levels of fibronectin, which constantly interacts with platelets. Normally, its content is 4 mg × 109 cells. In platelets, it associates with alpha granules and is released from them after stimulation with thrombin or collagen, promoting cell adhesion by collagen. This plays an important role in the activation of the hemostatic process. Also, with gestosis, a low content of AT III is detected, which accounts for about 80% of the total primary anticoagulant activity of the blood.
Pregnant women with preeclampsia had high titers of beta-thromboglobulin and platelet factor IV. Clinicians attach an important role to the condition of the endothelium in preventing the development of excessive blood coagulation during pregnancy. The antithrombogenic properties of the vascular wall are ensured by the influence of a number of components: 1) prostacyclin, which is an inhibitor of platelet aggregation and provides a pronounced vasodilation effect; 2) anticoagulants (AT III and alpha-2 macroglobulin); 3) plasminogen proactivator produced in blood vessels; 4) thrombomodulin, formed on the surface of endothelial cells. In healthy individuals, platelets do not adhere to intact endothelium. When the endothelium is damaged, platelets easily adhere to the subendothelium, which is due to a decrease in the content of glycosaminoglycans and prostacyclin synthesized by the endothelium. The level of prostacyclin in healthy individuals during pregnancy is 65.0 pg/ml; in pregnant women with metabolic syndrome it decreases to 51.0 pg/ml.
The discovery of NO production by endothelial cells in the 80s once again confirmed the enormous morphofunctional importance of the endothelium, which was previously considered as a passive dividing surface between the blood and the extravasal space. NO has many functions, the main ones being the maintenance of normal vascular function and the full adaptation of the cardiovascular system to pregnancy. NO has vasodilating antiplatelet activity and is also an inhibitor of mitogenesis.
To determine the significance of NO levels in pregnant women in the development of blood hypercoagulation, we compared its quantitative indicators in pregnant women of the main and control groups. In individuals in the control group, the NO level is 23.2, and in individuals in the main group its value decreases to 21.16. It is very important to note that the NO level increases only 6 months after birth to 25.57. This indicates that the disruption of endothelial function is caused specifically by pregnancy. In patients with gestosis, significant damage to the endothelium occurs, hypoxia and the circulation of free radicals, as well as aggressive forms of oxygen, increase. In this case, NO is spent on binding free radicals, as a result of which one of its important functions, vasodilating and antiplatelet, suffers.
The level of AT III is significantly reduced, and this makes it possible to increase the thrombotic activity of the blood (Table 1). Added to this is a decrease in the fibrinolytic activity of the blood: if in the control group it is 20.3%, then in metabolic syndrome it decreases to 11.2%. Of particular importance is the increased sensitivity of platelets to various aggregates (collagen, adrenaline and ADP), which in various types of pathology, such as gestosis, can lead to thrombotic complications. It is also important that cAMP in platelets is reduced in pregnant women with metabolic syndrome to 3 × 108 cells pmol, while in the control group this figure is 4.1 × 108 cells pmol.
The reduced level of prostocycline (6-keto-PGF1-alpha in plasma - pg/ml) is important in matters of platelet activity; if in the control group the production of prostocycline is 65 pg/ml, then in pregnant women with metabolic syndrome the level of prostocycline is reduced to 51 pg/ml. The von Willebrand factor increases in the blood; in pregnant women with metabolic syndrome it is 2.18 units/ml, and in the control group it is 1.04 units/ml, thus enhancing the aggregation ability of platelets. Thus, the combined effect of high levels of fibrinogen and an increase in platelet aggregation activity, combined with reduced indicators such as AT III and prostacyclin, contribute to the development of thrombohemorrhagic syndrome in pregnant women with metabolic syndrome and is one of the triggers for the addition of preeclampsia.
In order to reduce perinatal risk and the incidence of other pregnancy complications, a number of patients with metabolic syndrome underwent complex therapy, which included:
- hypochloride protein diet with the inclusion of foods rich in “natural” antioxidants (prunes, raisins, blueberries, black currants);
- one of the drugs from the group of “synthetic” antioxidants: vitamin E (300 mg/day), ascorbic acid (100 mg/day), glutamic acid (3 g/day);
- preparations of the membrane stabilizer group containing polyunsaturated fatty acids: Essentiale Forte H 2 capsules - 3 times, Lipostabil 2 capsules - 3 times and the food supplement Eikonol 1 capsule 1-2 times a day;
- anticoagulant therapy (Fraxiparin 0.3 or 0.6 ml/day s.c. depending on the hemostasiogram parameters);
- antiplatelet therapy - Cardiomagnyl 75 mg/day.
The patients were treated with Cardiomagnyl 75 mg once a day and Fraxiparine 0.3–1.2 times a day. The mechanism of action of acetylsalicylic acid is based on irreversible inhibition of the enzyme cyclooxygenase (COX-1), as a result of which the synthesis of thromboxane A2 is blocked and platelet aggregation is suppressed. Fraxiparine exhibits a high ability to bind to the blood plasma protein AT III. This binding leads to accelerated inhibition of factor Xa, which accounts for the high antithrombotic potential of nadroparin. The antithrombotic effect of nadroparin includes activation of tissue factor conversion inhibitor (TFPI), activation of fibrinolysis through direct release of tissue plasminogen activator from endothelial cells and modification of the rheological properties of blood (decreasing blood viscosity and increasing the permeability of platelet and granulocyte membranes). Nadroparin calcium is characterized by higher anti-factor Xa activity compared to anti-factor IIa or antithrombotic activity and has both immediate and prolonged antithrombotic activity.
Against the background of the treatment, there was an improvement in the condition of pregnant women and a decrease in the indicators presented on the hemostasiogram, as well as an improvement in biochemical parameters in pregnant women with metabolic syndrome during the therapy (Table 2).
Thus, the functional activity of the endothelium during pregnancy (especially during gestosis) is sharply disrupted. Oxidative stress during pregnancy, especially in women with metabolic syndrome, stimulates endothelin production with the corresponding development of hypertension. Consequently, dysfunction of endothelial cells stimulates the release of endothelin, thromboxane, angiotensin II, inhibits the production of nitric oxide and leads to placental ischemia and the addition of preeclampsia (Table 1).
The outcome of pregnancy and childbirth was monitored in 58 women admitted to the department of pathology of pregnant women of the maternity hospital of City Clinical Hospital No. 70 in Moscow. The control group consisted of data from the course of pregnancy and childbirth in 15 women with metabolic syndrome.
Analysis of the course of pregnancy in women with metabolic syndrome indicates that pregnancy often occurs with various complications presented in Table. 3.
Of significant interest is the analysis of the course of labor in women with metabolic syndrome. The data is presented in table. 4.
A characteristic feature of the course of labor in women with metabolic syndrome is untimely rupture of amniotic fluid, labor anomalies, a large fetus and bleeding in the third stage of labor.
Thus, in women in labor with metabolic syndrome, the average amount of bleeding was 600–900 ml (one woman in labor in this group had bleeding of 1100 ml). In all cases, the use of conservative treatment methods was ensured.
The high percentage of bleeding in parturient women with metabolic syndrome can be explained by impaired contractility of the uterus, as evidenced by the significant frequency of labor anomalies.
Of particular interest is the frequency of operative delivery in women with metabolic syndrome. If in maternity hospital No. 70 the rate of cesarean section is 22–23% over the years, then for women in our study it was 33.6%.
Indications for cesarean section were: clinically narrow pelvis, severe form of gestosis, persistent weakness of labor, not amenable to drug correction.
Thus, the incidence of metabolic syndrome in obese women was 89.2%. Metabolic syndrome adversely affects the course of pregnancy and childbirth, which is the reason for a significant increase in the frequency of surgical interventions, especially delivery by cesarean section. The analysis gives grounds to classify pregnant women with metabolic syndrome as a high-risk group for the development of gestosis and complications of labor.
conclusions
- Metabolic changes in women during pregnancy (increased blood pressure, hyperglycemia, insulin resistance) pose a risk of developing preeclampsia.
- In the pathogenesis of DIC (disseminated intravascular coagulation) in pregnant women with metabolic syndrome, damage to the vascular endothelium, increased platelet aggregation and impaired rheological properties of the blood play a role.
- To treat DIC syndrome in pregnant women with metabolic syndrome, it is necessary to include antiplatelet agents and anticoagulants in complex therapy. Carrying out complex therapy including antiplatelet agents and anticoagulants is a pathogenetically based prevention of the addition of gestosis and allows you to prevent or delay the addition of gestosis and significantly improve the outcomes of pregnancy and childbirth.
Literature
- Arkhipov V.V., Kayupova G.F., Samatova Z.A. Course of pregnancy and childbirth, condition of the fetus and newborn in obese women // Health. Bashkortostan. 1999, no. 3, p. 33–34.
- Belovitskaya N. State of the platelet-vascular link of the hemostasis system in patients with NIDDM and coronary artery disease. M., 1990, p. 144.
- Butrova S. A. Metabolic syndrome: pathogenesis, clinical picture, diagnosis, approaches to treatment // Rus. honey. magazine 2001, 2, 56–60.
- Vasilenko A. Changes in platelet and procoagulant components of hemostasis in patients with arterial hypertension // Medical Affairs. 1992, no. 8, p. 65–66.
- Ginzburg M. M., Kozupitsa Ch. S., Kryukov N. N. Obesity and metabolic syndrome. M., 2000, p. 14–15.
- Mamedov M. N. Clinical and biochemical features and ways of drug correction of metabolic syndrome. Diss. Doctor of Medical Sciences M., 2001.
- Chazova I. E., Mychka V. B. Metabolic syndrome. 2004, p. 9.
- Chernukha G. E. Clinical significance of disturbances in homeostasis indicators in obese pregnant women. Diss. Ph.D. M., 1987.
- Bernardi F., Marcheti G., Pinotti M., Arcieri P. Factor VII gene Polymorphisms Contribute About One Third of the Factor VII Level Variation in Plasma // Atherosclerosis, Thrombosis and vascular Biology. 1996, v. 16, no. 1, 72–78.
- Bray GA Obesity. Part I. Pathogenesis//West J. Med. 1988, v. 194, no. 4, p. 429–441.
- Despres JP The impact of overstate on the multifactorial risk profile of abdominally obese patients // Diabetes, 1998, 48, 1, 307.
- Edwards LE, Hellerstedt WL, Alton IR et al. Pregnancy compactions and birth outcomes in obese and normal-weight woman: effects of gestational weight change // Obstet. Gynec. 1996, no. 87 (3), p. 389–394.
- Galtier-Derenze F., Bringer Y. Maternal overweight and pregnancy // Diabetes Metab. 1997, v. 23, no. 6, p. 192–195.
- Goldenberg RL, Tamura T. Pregnancy weight and pregnancy outcome // JAMA. 1996, no. 275 (14), p. 1127–1128.
- Stico-Rahm A., Niman V., Hamsten A., Nilsson Y. Secretion of plasminogen activator inhibitor 1 from cultudef human umbilical vein endothelian cells is included by very low density lipoprotein // Atherosclerosis. 1990, 10:1067–1073.
E. I. Sokolov, Doctor of Medical Sciences, Professor, Academician of the Russian Academy of Medical Sciences I. B. Manukhin , Doctor of Medical Sciences, Professor A. A. Mochalov O. B. Nevzorov , Doctor of Medical Sciences, Associate Professor
MGMSU , Moscow
Contact information for authors for correspondence
References
- Clinical laboratory diagnostics, National guidelines, Volume 1, Dolgov V.V., Menshikov V.V., 2012, pp. 761-765
- Kozlovsky V. I., Kovtun O. M., Seroukhova O. P., Detkovskaya I. N., Kozlovsky I. V. Research methods and clinical significance of platelet aggregation. Focus on spontaneous aggregation // Vestnik VSMU. 2013. No. 4.
- Puri RN, Colman RW.ADP-induced platelet activation.Crit Rev BiochemMol Biol. 1997;32(6):437-502. doi: 10.3109/10409239709082000. PMID: 9444477.
- Murugappa S, Kunapuli SP. The role of ADP receptors in platelet function.FrontBiosci. 2006 May 1;11:1977-86. doi:10.2741/1939. PMID: 16368572.
- Barbara Lunghi, Anna Lecchi, Rosa Santacroce, Mariangela Scavone, Rita Paniccia, Andrea Artoni, Christian Gachet, Giancarlo Castaman, Maurizio Margaglione, Francesco Bernardi, Marco Cattaneo. Severe bleeding and absent ADP-induced platelet aggregation associated with inherited combined CalDAG-GEFI and P2Y12 deficiencies.Haematologica 2020;105(7):e361-e364
Platelet hemostasis
Study of platelet aggregation ability
Evaluation of patients with bleeding requires a thorough history, including family history, medication history, physical examination, and only then laboratory testing.
Quantitative and functional platelet abnormalities are common in patients with bleeding disorders.
Types of platelet dysfunction:
| Platelet dysfunction | Probable diagnosis |
| Impaired platelet adhesive function | Bernard-Soulier syndrome Velocardiofascial syndrome Glanzmann's thrombasthenia Congenital afibrinogenemia Von Willebrand disease (possibly a platelet type of von Willebrand disease) Collagen receptor defects |
| Platelet receptor abnormalities | TxA2 receptor defects α-adrenergic receptor defect P2Y12 receptor defect Antiplatelet drugs (eg, clopidogrel) |
| Platelet granule abnormalities | Deficiency of dense granules - deficiency of α-granules - gray platelet syndrome |
| Abnormalities in secretion of the contents of platelet granules, or disruption of signal transduction in platelets | Primary secretion defects Aggregation defects that are similar to those seen in platelet pool storage disorders, but with normal granule and TxA2 content Defects in platelet surface receptor agonists - epinephrine - thromboxane - ADP - collagen Defects in protein G activation (defects in intracellular signal transduction) |
| Arachidonic acid metabolism disorder | Cyclooxygenase defects Thromboxane synthase deficiency Taking drugs |
| Disturbance of the procoagulant mechanism of platelets | Scott syndrome |
| Platelet cytoskeleton defects | MYH9-associated disorders Wiskott-Aldrich syndrome |
Before performing specific tests examining the functional properties of platelets, it is recommended to perform the following laboratory tests:
- Complete blood count with determination of platelet count and average platelet volume. This is important for hereditary macrothrombocytopenia (Bernard-Soulier syndrome), microthrombocytopenia (Wiskott-Aldrich syndrome), and for some bone marrow diseases.
- Study of blood smear and platelet morphology. Necessary for establishing diagnoses such as pseudothrombocytopenia, gray platelet syndrome. In some cases, for example, with Hegglin's thrombocytopenia, a blood smear may show the presence of Dele bodies (light gray-blue oval basophilic leukocyte inclusions located in the peripheral cytoplasm of neutrophils).
Specific laboratory tests to determine the functional properties of platelets include examinations such as:
- screening tests reflecting the functional activity of platelets, for example, activated clotting time (ACT), bleeding time (BT) and PFA-100, - aggregometry, for example, classical Born aggregometry , - flow cytometry to quantify the presence or absence of platelet membrane glycoproteins, — specialized studies used in research laboratories.
The principle of the Born aggregometry method:
The method for measuring platelet aggregation using the Born turbidimetric method is based on recording changes in light transmission in a platelet suspension. These changes are due to a decrease in light scattering and an increase in light transmission (transparency) of the suspension during the formation of platelet aggregates.
Classical Born aggregometry uses platelet-rich plasma (PRP). In the aggregometer, BTP is mixed in a cuvette at a temperature of 37°C. When an agonist is added, the resulting platelet aggregates absorb less light, which is detected by a photocell.
PRP – platelet-poor plasma, PRP – platelet-rich plasma.
The principles of Born aggregometry can be clearly seen in the animated English-language presentation, kindly provided by the authors of the scientific website https://www.platelet-research.org - https://www.platelet-research.org/3/aggregometry.htm.
Pre-analytical variability
Drugs that block platelet function include NSAIDs (non-steroidal anti-inflammatory drugs), specific antiplatelet drugs such as clopidogrel, as well as some antibiotics, antidepressants, beta blockers, etc. Foods such as garlic, turmeric can also affect platelet function and caffeine, high-fat foods (these can cause chylomicrons in the plasma, which interferes with normal light transmission during platelet aggregation testing). A number of factors can influence the accuracy of platelet aggregation results:
- very high or low platelet count;
- temperature - blood samples for platelet aggregation testing should be stored at room temperature;
— pH — platelet aggregation should be carried out at a physiological pH value;
- fibrinogen concentration.
Platelet agonists:
The addition of a platelet agonist to PRP leads to activation of platelets, a change in their shape from discoid to spherical, which is associated with an increase in optical density during platelet activation. The only exceptions to this are adrenaline (epinephrine), which does not change the shape of platelets, and ristocetin, which causes platelet agglutination rather than aggregation.
There are two types of agonists:
- strong agonists - collagen, thrombin, ThA2, arachidonic acid - directly cause platelet aggregation, synthesis and secretion of ThA2 platelet granules.
- weak agonists - ADP and adrenaline - cause platelet aggregation without causing secretion.
Strong agonists at low concentrations may act as weak agonists, but weak agonists, even at high concentrations, will not act as strong agonists.
With some weak agonists (ADP and epinephrine), the platelet aggregation curve may have a biphasic appearance - an initial wave of aggregation (primary wave), followed by a secondary wave of aggregation, which is usually irreversible. At higher ADP concentrations, the two waves of aggregation merge and there is no biphasic signal.
The aggregation response to the agonist is enhanced by the release of TxA2 from membrane phospholipids and the secretion of ADP from dense granules. ADP and TxA2 are agonists that, interacting with their specific receptors, enhance the platelet aggregation reaction.
Working concentrations of the agonists used and their mechanism of action:
| Agonist | Working concentration (C) | Mechanism of action |
| ADF | Low –1.0 μM, 2.5 μM, 5.0 μM High – 10.0 μM | ADP binds to its receptor on the surface of platelets. Initial binding results in the release of intracellular calcium and a change in platelet shape, leading to the formation of a primary wave of aggregation. The secondary wave reflects the release of ADP from platelet storage granules. A low dose of ADP causes only primary aggregation and its effect is reversible. ADP binds to two G-protein receptors P2Y1 and P2Y12. Binding of ADP to the P2Y1 receptor causes a shape change and initiates platelet aggregation (primary wave) by mobilizing calcium. The P2Y12 receptor is considered the main ADP receptor and is responsible for complete platelet aggregation through inhibition of adenyl cyclase. The P2Y12 receptor is also a target of clopidogrel. The second wave of ADP-induced aggregation is suppressed by aspirin and NSAIDs. |
| Adrenalin | 5μM, 10μM | Epinephrine binds to the α2-adrenergic receptor on the surface of platelets, resulting in inhibition of adenyl cyclase and the release of calcium ions. Platelet aggregation with epinephrine is visually similar to aggregation with ADP, with an initial primary wave of aggregation, release of stored ADP from platelets, and a second wave of irreversible aggregation. As with ADP, this second wave of aggregation is inhibited by aspirin and NSAIDs. Adrenaline, like ADP, is considered a weak agonist. |
| Ristocetin | Low –0.5 mg/mL High –1.5 mg/mL,5 mg/mL | Ristocetin in high concentrations causes platelet agglutination through von Willebrand factor and the GPIb-IX-V complex. |
| Collagen | 1.4μg/mL | Collagen binds to the GPVI and GPIa/IIa receptors, inducing the release of granule contents, the TXA2 pool, and then leads to sustained activation of GPIIb-IIIa. The GPIa/IIa receptor is involved in platelet adhesion. The GPVI receptor is involved in platelet signaling and TxA2 generation. The lag phase is formed after the addition of collagen to PRP, usually in less than 1 min. |
| Arachidonic acid | 500μg/mL | Arachidonic acid is a precursor of TxA2 in platelets. Arachidonic acid is converted to TxA2 with the participation of cyclooxygenase and thromboxane synthetase. TxA2 is a potent inducer of platelet aggregation, causing the release of granules, the formation of even greater generation of TxA2 and then the sustained activation of GPIIb-IIIa receptors. |
| Thrombin | Low –50 nmol/L High – 100 nmol/L | Thrombin is the most potent physiological platelet activator, acting on the PAR1 and PAR4 receptors. Thrombin causes platelet aggregation even at low concentrations, this does not lead to complete aggregation, but it changes the shape of platelets. At higher concentrations, thrombin induces complete platelet aggregation. |
Abbreviations used: PRP - platelet-poor plasma, PRP - platelet-rich plasma, TxA2 - thromboxane A2, C - concentration, GP - glycoprotein.
View of the classic two-phase platelet aggregation curve:
With the introduction of adrenaline and low doses of ADP, a biphasic aggregation curve is usually formed, while with the introduction of other agonists, or at high concentrations of inducers, a merger of the first and second waves is possible. In biphasic aggregation, the second wave is associated with the release reaction of platelet granules.
Aggregation parameters.
The main parameters of the aggregogram can be interpreted only after a preliminary assessment of platelet activity according to the types of aggregator curves (reversible, biphasic, irreversible and no platelet aggregation).
The main parameters of the aggregogram are the intensity of primary ( Tpa ), secondary ( Twa ), maximum aggregation ( Tma ) and disaggregation ( Tda ). The dynamics of the aggregation process on the graph should be judged by the slope angles of the aggregogram curve at the stage of primary aggregation ( α1 ), secondary aggregation ( α2 ) and disaggregation (β ). A characteristic of the dynamics of the reaction of release of endogenous stimulators of platelet aggregation is the latent period for collagen-induced aggregation ( tlp ) and the time of onset of secondary aggregation ( twa ) is normal for biphasic aggregation curves (high doses of ADP and adrenaline).
For collagen-induced aggregation, it is also important to evaluate the shortening or lengthening of the latent period ( tlp ) compared to the value of this indicator in healthy people.
A qualitative assessment of the aggregogram allows not only to diagnose hyper- or hyporeactivity of platelets, but also to suggest the causes of their occurrence. To quantitatively register various types of aggregation diagrams, the following indicators should be calculated:
- with irreversible aggregation - Tma, Tpa, Tda, Tba, α1, α2, of which the most informative are Tba, α2 and Tba;
- with reversible aggregation - Tma and Tda.
Fluctuation range of the main indicators of the aggregation diagram:
| Aggregogram parameters | Range of changes | Aggregogram parameters | Range of changes |
| Tma | 30-50% | Tva | 30-45 s |
| Twa | 30-40% | α1 | 60-75º |
| Tda | 60-80% (of Tpa value) | α2 | 60-75º |
| Tpa | 10-20% | β | 30-45º |
| tlp | 25-30 s | ||
Reference values
Normal aggregation activity when using inductors is:
- Platelet aggregation with ADP (5.0 µmol/ml): 60-90%
- Platelet aggregation with ADP (0.5 µmol/ml): 1.4-4.3%
- Platelet aggregation with adrenaline: 40-70%
- Platelet aggregation with collagen: 50-80%
Data interpretation
| Tentative diagnosis | Changes in the aggregogram |
| Glanzmann's thrombasthenia or afibrinogenemia | Absence or significant decrease in aggregation upon stimulation with any agonists except ristocetin |
| Bernard-Soulier syndrome or von Willebrand disease | Absence or significant reduction of aggregation with ristocetin |
| Storage pool violation | Primary wave of aggregation with only ADP, adrenaline and collagen. |
| Action of aspirin or aspirin-like effect | No aggregation with arachidonic acid. Presence of a primary wave only with ADP. Decreased or absent aggregation with collagen. |
| Effect of clopidogrel | No aggregation with ADP |
| Type 2B von Willebrand disease (VWD) or platelet type VWD | Aggregation with low dose of ristocetin (0.5 mg/ml) |
Link to the manual “Clinical and laboratory diagnosis of platelet function disorders”. Vasiliev S.A., Berkovsky A.L., Melkumyan A.L., Suvorov A.V., Mazurov A.V., Kozlov A.A. Federal State Budgetary Institution Hematological Research Center, Ministry of Health of the Russian Federation, Moscow, 2013.
When preparing this section, data from the scientific website www.practical-hemostasis.com was used.
When should you take the Platelet Aggregation with ADP test (4 concentrations with adenosine diphosphate)?
- Before prescribing drugs that affect the coagulation system.
- Differential diagnosis of diseases accompanied by pathological bleeding.
- As part of a comprehensive assessment of the platelet component of hemostasis in patients before (major) surgery, as well as in pregnant women at high risk of developing obstetric complications.
- Determination of resistance to drugs from the group of antiplatelet agents.
- Identification of hereditary and acquired thrombocytopathies.