Symptoms
The average platelet volume, or more precisely, the deviation from normal parameters, has its own clinical manifestations.
However, the problem is that any disorder can be completely asymptomatic, or the clinical manifestations are so mild that they go unnoticed. If the average platelet volume is elevated, the following symptoms may occur:
- bleeding gums;
- easy formation of bruises that do not go away for a long time;
- nasal hemorrhages;
- excessively heavy periods in females;
- skin itching;
- weakness and fatigue;
- constant drowsiness;
- vision problems.
When platelet counts are below normal, symptoms will include:
- hemorrhages in the retina of the eye;
- formation of hematomas;
- frequent nosebleeds;
- the appearance of subcutaneous hemorrhages.
Retinal hemorrhage
In addition to the above symptoms, it is necessary to seek medical help as soon as possible in the following situations:
- a sharp decrease in body weight;
- extreme fatigue (to such an extent that a person cannot get out of bed);
- constant nosebleeds;
- hypertension;
- increased heart rate;
- cyanosis of the skin and excessive pallor of the mucous membranes.
Such symptoms are common for adults and children.
Explanation of the platelet aggregation test
In the platelet aggregation test, indicators of 25–75% indicate good hematopoiesis. This means that tissues and organs are normally supplied with oxygen, and there are no blood clots.
Platelet rate
| Age | Indicator, x 10^9/l |
| Newborn | 100–420 |
| Child under one year old | 160–320 |
| 1–4 years | 150–300 |
| 15–18 years old | 180–340 |
| Men over 18 years old | 180–400 |
| Women over 18 years old | 150–380 |
What does a low level of thrombotic cells indicate in an adult?
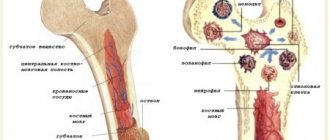
Unlike red blood cells, whose life cycle is approximately 120 days, thrombotic plates are destroyed on days 9-12 of their existence. Their quantity is continuously renewed by the bone marrow to maintain the physiologically necessary amount in the blood.
As with other blood cells, platelets have values called normal; they are defined in the range 180-320*10 9 units per liter, or 180-320 thousand units per microliter. If, according to the results of the analysis, it turns out that the number of thrombotic cells does not reach the normal level, this indicates the development of thrombocytopenia in an adult or child.
Thrombocytopenia (platelets significantly lower than normal) occurs due to the accelerated destruction of thrombotic cells or disruption of their production, which occurs in a number of diseases.
Activation
To perform their main function - repairing damage in the vessel wall - platelets must enter an active state. Like most cells in our body, this process proceeds according to the following scheme: signal - receptor - intracellular signal - amplifier - regulator - response (Fig. 3). The signal for activation is the appearance in the bloodstream of an agonist - a special signaling molecule, which should appear only when necessary and bind to a specific molecule that penetrates the platelet membrane (receptor). The agonist interacts with one “tail” of the receptor, protruding from the outside, and this leads to a change in the other, on the cytosol side, where the next signaling molecule, the secondary messenger, appears. It triggers the synthesis of several more messengers, which, in turn, triggers several more, and so the signal propagates in the cytosol and is amplified through a cascade of intracellular reactions, which ultimately leads to a complex platelet response. It is important that in the platelet there are special regulatory systems that modulate the concentrations of intracellular messengers at different stages of activation, so that, for example, there is no reaction to trace amounts of the agonist.
Rice. 3.
Platelet activation scheme
How is this scheme implemented in our body? In blood vessels, platelets are pushed out of the main flow by red blood cells and move along the walls, conducting a kind of monitoring of their condition. One of the first signals for platelet activation is collagen, the main protein of connective tissue, exposed when a vessel is damaged. Having detected collagen, they bind to it through special receptors, simultaneously becoming activated and firmly attaching to the site of damage. The interaction of a platelet with collagen leads to the launch of the mentioned intracellular signaling cascade and the appearance of a secondary messenger in the cytosol - inositol triphosphate (IP3). This small water-soluble molecule is able to move quickly in the cytosol and serves as a signal for the release of calcium ions from intracellular stores. And an increase in its intracellular concentration can lead to various responses of the platelet: splashing out the contents of granules (secretion), changing shape, attaching to the vessel wall (adhesion), bonding with other platelets (aggregation), and the appearance of procoagulant activity (Fig. 4). After the circulatory system has already recognized the damage to the vessel, three more natural platelet activators appear in the blood - thrombin, ADP and thromboxane A2. The thrombin protein is formed from its precursor, prothrombin, in the blood plasma, but in large quantities - already on the membranes of activated platelets. When their dense granules are secreted, large amounts of ADP (a small molecule that primarily performs energy functions in cells) are released, and much less ADP is released from damaged endothelial cells lining the inner surface of blood vessels. Thromboxane A2 is synthesized from arachidonic acid, located in the membranes of activated platelets. The binding of these three activators to their receptors on the platelet membrane leads, as in the case of collagen, to the appearance of IP3 in the cytosol and an increase in the calcium concentration in it (Fig. 4). Thus, all three soluble activators and collagen act through the same pathway, but induce different platelet responses. For example, thromboxane A2 provokes the release of dense granules, but ADP does not. Activation separately by collagen or thrombin causes all of the above responses simultaneously, and together leads to the appearance of a group of procoagulant platelets and the synthesis of thrombin on their membranes. Apparently, there are still insufficiently studied differences in the signaling triggered by different agonists. To prevent accidental activation from turning a platelet into a real “bomb”, carried in the bloodstream and triggering the entire coagulation system, intact endothelial cells in the body constantly secrete prostacyclin and nitric oxide, which block cell activation, preventing an increase in calcium concentration in them.
Rice. 4.
Scheme of the main pathways of platelet activation and its responses:
ADP
- adenosine diphosphate,
IP3
- inositol triphosphate,
ER
- endoplasmic reticulum
Signaling is one of the most complex and poorly understood areas in platelet research. There are many questions about the structure of each receptor and signaling pathway, and the simplest of them is: why are there so many activators at all?
How to increase?
When looking for ways to increase platelets in the blood, one should not forget the main thing - self-medication can be life-threatening. Medicines used to urgently increase thrombotic mass have a lot of side effects and should not be used without a doctor's prescription.
- Platelets are elevated in an adult - what does this mean, and how to reduce them?
If platelets are low due to medications (aspirin, etc.), you should stop using them.
There are no products that can effectively affect platelets if they are low, nor any folk remedies. These drugs can only be used as auxiliary, but not the main methods of increasing platelets.
The main way to eliminate pathological thrombocytopenia is to treat the disease that led to it.
Consequences of a low platelet count
The main and most terrible violation of this condition is the possibility of developing massive bleeding even due to a small injury, which is difficult to stop. Such problems are especially dangerous during pregnancy, childbirth and menstruation. Uterine bleeding is very difficult to treat even with a normal platelet level, and if it is reduced, in many cases it can be fatal.
Normal and changes in platelet levels in the blood
In addition, this condition can cause hemorrhage in the brain and in the eyeball and retina. Such disorders can be triggered by even a slight increase in blood and eye pressure.
Attention! The likelihood of such conditions can be reduced by following a correct lifestyle and following recommendations to prevent bleeding due to a low platelet count.
Structure
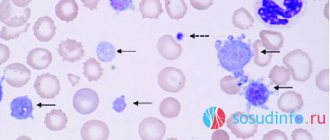
Rice. 1.
Micrograph of non-activated platelets []
Platelets (from the Greek θρομβοζ - 'clot' and κυτοζ - 'cell') are specialized non-nuclear blood cells, having the shape of a disk with a diameter of about 3 microns and a thickness of about 0.5 microns (Fig. 1). They are formed during the fragmentation of large bone marrow cells—megakaryocytes—and circulate in the bloodstream at a concentration of 200–400 thousand cells per 1 μl of blood. Platelets live in the bloodstream for an average of 5–9 days, and then are destroyed in the spleen and liver.
The structure of a platelet is quite complex. Externally, it is limited by a bilipid layer of the membrane, numerous invaginations of which (an open tubular system) provide a reserve surface for changing shape (Fig. 2). It supports it and at the same time allows for significant changes in the cytoskeleton (framework) of the cell. Inside there are the endoplasmic reticulum (a repository of calcium ions necessary for signaling and the platelet performing its functions) and mitochondria (organelles that ensure respiration). The cytosol contains granules containing substances that spill out into the extracellular space upon cell activation (transition to a new state). Dense granules contain nucleotides (ATP, ADP, GTP, GDP), serotonin, calcium ions in high concentrations, α-granules contain various proteins (including blood clotting factors), and lysosomes contain some enzymes (collagenase, elastase and etc.).
Rice.
2. Scheme of the platelet structure []
After platelet activation, a negatively charged lipid, phosphatidylserine, appears on the outer surface of its membrane. Some coagulation factors bind to it with the help of calcium ions, forming special complexes. They greatly accelerate the reactions leading to gelation of blood plasma at the site of injury (this process is called plasma hemostasis). In other words, phosphatidylserine provides a procoagulant function of platelets that promotes plasma hemostasis.
Why is the lifespan of these blood cells so short-lived (red blood cells, for example, live three to four months), because normally, in the absence of serious damage to blood vessels, they practically do not work? Why do they look like disks? Why does a platelet need mitochondria if its energy expenditure is extremely modest? Why did nature need to accelerate plasma coagulation reactions on cell membranes? Why do α-granules contain coagulation proteins, which are also found in blood plasma? These are just some of the questions that do not yet have clear answers.
What does this mean during pregnancy?
Among the physiological causes of low platelets in women, pregnancy is named, and for the early stages this is indeed a normal situation, if we are talking about acceptable low values. In the early stages, pregnant women experience an increase in the volume of circulating blood due to plasma, while the mass of red blood cells and platelets is produced at a less rapid pace. As a result, the effect of hemodilution is observed - blood thinning, accompanied by a reduced content of red blood cells and platelets.
Typically, this transient phenomenon does not have any significant impact on hemostasis. If platelets are reduced to a level of 50 units per microliter or lower, emergency measures may be required to stabilize the indicators. Especially if there are few platelets a couple of weeks before giving birth, this means that the body is not preparing for the upcoming blood loss and can suffer greatly during childbirth.
- Thrombocytopenia - symptoms and treatment
This is when the value of a general blood test becomes obvious - thanks to it, it is possible to promptly detect a low platelet count, suggest the reasons for the deviation and take action.
The range of conservative methods for increasing low platelets during pregnancy is limited, but the expectant mother will not be left without help, since the arsenal of medicine includes drugs with minimal effects on the fetus.
What we found and how we did it
Biophysical approach
Like any complex system, the formation of a blood clot in an artery needs to be managed. Identifying the mechanisms that regulate biological processes is one of the traditional tasks of biophysics, so the problem of regulating arterial thrombus formation has long attracted the attention of not only doctors and physiologists, but also biophysicists. For more than two decades, the Department of Biophysics of the Faculty of Physics of Moscow State University has been developing a direction related to the analysis of the principles of the structure and regulation of the hemostasis system: for example, in the classic works of Professor F.I. Ataullakhanov and his students demonstrated the autowave nature of the propagation of the blood plasma coagulation process in the absence of flow [5], [6].
Establishing the mechanisms that regulate thrombus formation in arterial blood flow is one of the main tasks of our research team, whose members are Professor of the Department of Medical Physics M.A. Panteleev, Professor of the Department of Biophysics F.I. Ataullakhanov, senior researcher Department of Biophysics D.Yu. Nechipurenko, as well as students and graduate students of the Faculty of Physics.
In vitro and in silico study
Recent studies carried out by us in collaboration with colleagues from France and the USA made it possible to link the surface distribution of dying platelets with the process of thrombus compression [7]. Using confocal microscopy in in vitro thrombus experiments, we showed that procoagulant platelets form in various parts of the growing thrombus and then move to its surface (Fig. 1).
Figure 1. Dynamics of movement of procoagulant platelets in a thrombus. a — Confocal micrographs of thrombi at different time points. Green color corresponds to the fluorescence of dying cells (a fluorescent marker of cell death is used). b — Blood clots at different points in time. Green color corresponds to the fluorescence of dying cells, purple color corresponds to the fluorescence of platelets attached to the thrombus (a fluorescently labeled antibody to the surface proteins of the platelet is used). c — The main quantities used to analyze the movement of platelets are the movement vector d , the translocation angle α between the direction of movement and the initial radius vector of the center of the dying cell, drawn from the center of the thrombus. d — Results of analysis of the modules of average movement speeds and translocation angles of dying cells (green) and “fresh” platelets attached to the surface of the thrombus (purple). Scale: 10 micrometers.
[7]
This redistribution is accompanied by the formation of fibrin on the surface of the thrombus. Since dying (procoagulant) platelets interact rather weakly with other cells and do not participate in the contraction process, it has been suggested that their redistribution is the result of mechanical displacement during the process of active compression of the thrombus. To test this hypothesis, a computer model of platelet aggregate compression was created, which demonstrated the functionality of the formulated hypothesis (Fig. 2).
Figure 2. Simulation of cell aggregate contraction. a — Platelet aggregate before and after compression. Spheres imitating procoagulant cells, which do not participate in the contraction process and interact relatively weakly with other spheres, are marked in green. Contraction is described as a decrease in the equilibrium length of the pair potential (Morse) interaction between the centers of the spheres. b — Aggregate before and after contraction, in which the “procoagulant” spheres, initially located inside the aggregate, had different radii. The spheres that remained inside the aggregate after contraction are marked in purple, and those outside the aggregate in green. c — The value of the absolute values of the movements of procoagulant platelets in experiments (ex vivo) and “procoagulant” spheres in the model (in silico). d — The fraction of spheres displaced as a result of compression of the aggregate onto its surface. Calculation results for spheres of different radii are shown.
[7]
An important evidence base for the work was experiments with the blood of unique genetically modified mice, whose platelets are deprived of the ability to exhibit mechanical activity and, therefore, ensure thrombus contraction. In accordance with the predictions of the model and the formulated hypothesis, dying cells did not move to the surface of the thrombus in the absence of contraction (Fig. 3). The lack of surface distribution of dying platelets was also accompanied by the lack of surface localization of fibrin.
Figure 3. Comparison of the distribution of procoagulant cells and fibrin for normal and genetically modified mice. a — Distribution of procoagulant platelets (green) in normal mice (top panel) and modified mice (bottom panel). The outline of the thrombus, constructed from the image in the differential interference contrast mode, is marked in yellow. b — Analysis of the ratio of the total fluorescence of the surface of dying cells located outside the dense part of the thrombus to the fluorescence of the same surfaces inside the thrombus for normal (WT) and genetically modified mice (MYH9). c — Distribution of procoagulant surfaces (green) and fibrin (purple) in thrombi from wild-type mice (top panel) and thrombi from genetically modified mice (bottom panel). Scale: 10 micrometers.
[7]