Structural features
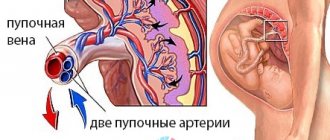
A normal umbilical cord connects the front of the abdominal wall to the placenta (an organ necessary for the bond between mother and baby). A properly formed umbilical cord has the following components:
- Two umbilical arteries. They carry blood from the fetus to the placenta.
- Umbilical vein. It works like arteries, only in the opposite direction.
- Urachus. This is the name given to the duct that connects the placenta to the baby’s bladder.
- Wharton's jelly. It is a connective matter that protects the vessels of the umbilical cord.
- Vitelline duct. Connects the intestines of the embryo with the yolk sac (responsible for hematopoiesis and the production of germ cells).
What is the pathology?
The only umbilical cord artery in the fetus is a pathology in which the structure of the connecting element changes (instead of two - one). This happens quite often: the probability is 1:20 for a multiple pregnancy and 1:200 for a singleton pregnancy. The risk of developing pathology increases if the expectant mother has diabetes.
One artery may be absent from the very beginning of pregnancy or may lose its function at any time (eg, atrophy). The structure of the umbilical cord is clearly visible on ultrasound from twenty weeks. Most often, a single umbilical cord artery is a single pathology and is not associated with any congenital anomalies (in 75% of cases). However, there is a chance that the child has developmental disabilities (25%). Most often, the pathology problem is accompanied by problems with the heart, kidneys, intestines and bones.
Yesil-Diagnostic, ultrasound in Petropavlovsk
A conventional ultrasound is a series of two-dimensional flat images of the fetus or its individual organs.
3D (3D) ultrasound is a method of obtaining an image, which is achieved by complex transformation of the obtained two-dimensional information into a three-dimensional three-dimensional model. Transformations are carried out using a special three-dimensional sensor and computer programs included in the ultrasound machine. The resulting volumes can be rotated and viewed from different angles.
4D (4D) ultrasound is the acquisition of a three-dimensional image of the fetus and its movements in real time. Thus, in addition to the three-dimensional image of the fetus, a fourth dimension is added - time, and it is possible to consider the motor activity and facial expressions of the fetus in motion.
Ultrasound in 3D and 4D modes can be done at different stages of pregnancy, but the result and quality of the image primarily depends on the position of the fetus in the uterus, the amount of amniotic fluid in front of its face, the presence of obstacles in the path of the ultrasound wave (uterine fibroids, uterine scar, subcutaneous tissue may obstruct examination and reduce image quality). So, if the fetus lies close to the wall of the uterus, then it is almost impossible to obtain a three-dimensional image of its face, just as it is impossible to see the face of a person who has rested his head against the wall. Sometimes you have to wait 15-20 minutes and continue the study after changing the position of the fetus.
The optimal period for obtaining an image of the fetal face is 20-26 weeks of pregnancy. At these stages, the fetus often changes its position, and there is enough water around it to see the face and body. After 30 weeks of pregnancy, the fetus is so large that it has to cluster well to fit in the uterus. However, obtaining a beautiful three-dimensional image of the face can be difficult and even impossible - it all depends on the position of the fetus.
It is important to understand that 3D/4D ultrasound is performed in addition to regular fetal ultrasound and cannot completely replace it. Measuring the size of the fetus and excluding malformations is carried out using conventional two-dimensional ultrasound. Only for some malformations does the use of three-dimensional ultrasound provide the doctor with additional information.
If an ultrasound examination reveals a single umbilical cord artery (two vessels in the umbilical cord instead of three)
What is a single umbilical cord artery?
A normal umbilical cord consists of three vessels - two arteries and one vein. Sometimes, instead of two arteries, only one artery and one vein are formed in the umbilical cord, thus, only two vessels are identified in the umbilical cord. This condition is considered a malformation of the umbilical cord, but this defect does not have any effect on the postpartum condition of the child and its further development.
Why can a single umbilical cord artery be identified in a fetus?
— Sometimes a single umbilical cord artery is detected in completely normal fetuses; After the birth of a child, this fact does not have any impact on its further development.
— Sometimes a single umbilical cord artery is combined with defects of the fetal cardiovascular system, therefore, when a single umbilical cord artery is identified, a detailed examination of the anatomy of the fetus and, in particular, the cardiovascular system is carried out. In the absence of other malformations, a single umbilical cord artery is able to provide adequate blood flow to the fetus.
— Somewhat more often, a single umbilical cord artery is detected in fetuses with Down syndrome and other chromosomal diseases. However, this marker is a “minor” marker of Down syndrome, so identifying only a single umbilical cord artery does not increase the risk of Down syndrome and is not an indication for other diagnostic procedures.
— A single umbilical cord artery sometimes leads to intrauterine growth retardation. In this regard, if a single umbilical cord artery is detected, an additional ultrasound is recommended at 28 weeks of pregnancy, and a planned one at 32-34 weeks. If a lag in the size of the fetus from the gestational age or disruption of blood flow in the vessels of the fetus and uterus is not detected, then the diagnosis of fetal growth retardation is excluded.
What to do if a single umbilical cord artery is identified in the fetus?
— identifying only a single umbilical cord artery does not increase the risk of Down syndrome and is not an indication for genetic consultation or other diagnostic procedures.
- control ultrasound at 28 and 32 weeks of pregnancy to assess the rate of fetal growth and its functional state.
If an ultrasound examination reveals choroid plexus cysts in the fetus
What are choroid plexuses?
The choroid plexus is one of the first structures to appear in the fetal brain. It is a complex structure, and the presence of both choroid plexuses confirms that both halves develop in the brain. The choroid plexus produces fluid that nourishes the brain and spinal cord.
Sometimes the fluid forms collections inside the choroid plexus, which appear as a “cyst” on ultrasound.
Choroid plexus cysts can sometimes be found on ultrasound between 18 and 22 weeks of pregnancy. The presence of cysts does not affect the development and function of the brain. Most cysts disappear spontaneously by 24-28 weeks of pregnancy.
Are choroid plexus cysts common?
in 1-2% of all normal pregnancies the fetuses have CSS,
in 50% of cases bilateral choroid plexus cysts are found,
in 90% of cases, cysts disappear spontaneously by the 26th week of pregnancy,
the number, size, and shape of cysts may vary,
cysts are also found in healthy children and adults.
Somewhat more often, choroid plexus cysts are detected in fetuses with chromosomal diseases, in particular with Edwards syndrome (trisomy 18, extra chromosome 18). However, with this disease, the fetus will always have multiple malformations, so identifying only choroid plexus cysts does not increase the risk of having trisomy 18 and is not an indication for other diagnostic procedures.
In Down syndrome, choroid plexus cysts are usually not detected. The risk of Edward's syndrome when CSS is detected does not depend on the size of the cysts and their unilateral or bilateral location. Most cysts resolve by 24-28 weeks, so a control ultrasound is performed at 28 weeks. However, if choroid plexus cysts do not disappear by 28-30 weeks, this does not affect the further development of the child.
If an ultrasound examination reveals markers of fetal chromosomal pathology
If an ultrasound examination found markers (signs) of a chromosomal pathology of the fetus, this does not mean that the fetus has a chromosomal pathology, and the pregnancy must be terminated. All women in whom ultrasound markers of chromosomal pathology of the fetus were found are offered invasive prenatal diagnostics - chorionic biopsy/placentobiopsy for the purpose of taking fetal cells and their fine microscopic analysis.
Chorionic biopsy is the removal of several villi from the fetal chorion under ultrasound guidance and counting the number of chromosomes in its cells. The chorion is an organ of the fetus from which the placenta is later formed. Its cells are identical to the cells of the fetus. Therefore, if the number and structure of chromosomes in chorion cells are normal, then chromosomal diseases in the fetus are excluded with a probability of more than 99%.
An injection is made with a thin needle through the anterior abdominal wall, the needle passes into the chorion and several chorionic villi enter it. The procedure is performed on an outpatient basis, disposable gloves and sterile needles are used, so there is virtually no risk of infection during the procedure.
Any procedure, even the simplest blood test, has a risk of complications. When performing chorionic villus biopsy, there is also a risk - the risk of miscarriage. However, if all the rules of the procedure are followed, this risk does not exceed 1%. Therefore, in cases where the individual risk of fetal chromosomal pathology is high enough, this procedure should be performed. To date, no other method has been developed in the world that allows one to obtain fetal cells for research without performing an intrauterine intervention. At the same time, the diagnosis of Down's disease or other chromosomal pathology can be excluded or confirmed only by examining fetal cells that were obtained during intrauterine intervention.
Chorionic biopsy is performed before 14 weeks of pregnancy, after this period a placenta forms in place of the chorion and then placentobiopsy is performed. The technique and risk of placentobiopsy are not fundamentally different from chorionic villus biopsy. We hope that this information will help you better understand why we conduct these studies and overcome the natural fear of the procedure that is caused by the unknown and the unprofessional information that is sometimes published in non-medical sources.
Diagnostics
To give birth to a healthy baby, any pregnant woman must be constantly monitored by a doctor and undergo all tests. If there is any suspicion, the unborn child must be carefully examined for various developmental defects and markers of genetic diseases. Don't be afraid of all these studies, because there is nothing more important than health.
The following methods will help identify the only umbilical cord artery during pregnancy:
- Ultrasound (ultrasound examination). The bladder must be full before passing.
- Doppler. Determines the presence and speed of blood flow.
- Examination by a gynecologist. Using special instruments, the doctor can listen to the baby’s heartbeat and, if there is any suspicion, prescribe additional examinations.
If a pathology is detected, doctors will suggest undergoing karyotyping of the fetus. This procedure involves the collection of amniotic fluid or chorionic villi, as well as detailed ultrasound and echocardiography.
The only umbilical cord artery during pregnancy can be seen on ultrasound during transverse scanning, in which the lumen of the vessels is best visible. Diagnosis of pathology is possible from the end of the first trimester. At earlier stages, the umbilical cord is not clearly visualized, so it will not be possible to make an accurate diagnosis. Difficulties may also arise if a woman has oligohydramnios, obesity, or multiple pregnancies.
Results of population-based studies of iEAP
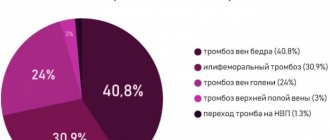
In a Canadian population-based study, there were 725 cases of iEAP among 196,752 singleton pregnancies at more than 20 weeks' gestation. over 23 years, an increase in the risk of adverse fetal outcome was found with a 95% confidence interval (CI) odds ratio (OR) from 1.09 to 5.94 [2]. The highest value of the latter was recorded for intrauterine/perinatal death (95% CI OR 1.97–5.82), which was statistically significantly different from that for pregnancy-induced arterial hypertension, cesarean section due to fetal distress, preterm birth after rupture of membranes (95 % CI OR 1.09–1.74). In addition, an increased risk (95% CI OR 1.21–5.94) of death in the first year of life, gestational age less than 37 weeks, was determined. or less than 34 weeks, birth weight less than the 3rd percentile or less than the 10th percentile, Apgar scores ≤3 points at the 1st minute or at the 5th minute of the child’s life, intensive neonatological care, weak labor , induction of labor, cesarean section, placental anomalies, amniocentesis, polyhydramnios, gestational diabetes mellitus.
In a Norwegian population-based study, there were 3,777 cases of iEAP among 918,933 singleton pregnancies at 16 weeks' gestation and older. up to 45 weeks over 16 years, the 95% CI OR for adverse fetal outcomes was 1.1–6.7 [3]. The greatest increase in risk among the latter was noted for intrauterine/perinatal death (95% CI OR 4.3–6.7), which was statistically significantly different from that of spontaneous preterm birth, preterm birth after rupture of membranes, intensive neonatological care, birth weight below 5th percentile, cesarean section (95% CI OR 1.1–1.9). Fetal weight greater than the 95th percentile, placental weight greater than the 95th percentile, umbilical cord length less than the 5th percentile, and preeclampsia were not associated with iEAP.
In the population studies presented, the main adverse outcomes for the fetus were intrauterine and perinatal death. It should be emphasized that the research power in Norway exceeds that in Canada by 5 times. Both studies examined similar or identical fetal outcomes: preterm birth and prematurity/immaturity, need for intensive neonatal care for infant survival, delivery by caesarean section, birth weight below the 5th and 3rd/10th percentiles. Moreover, while maintaining the comparability of the 95% CI OR values for these outcomes, they are in relation to non-overlapping sets with the 95% CI OR intrauterine/perinatal death. In other words, with increasing study power, the increase in the risk of intrauterine and perinatal fetal death is already statistically significantly different from all other fetal outcomes. In relation to the latter, the first acts as an increase in the risk of an unfavorable outcome of a higher level due to an increase in the level of significance of the connection with iEAP.
On the other hand, it is noteworthy that the increase in the probability of such a pregnancy outcome with iEAP, such as a significant increase in the risk of pregnancy-induced hypertension (95% CI OR 1.09–1.7), which was observed in Canada, becomes insignificant with the combination with clinically significant proteinuria in Norway (95% CI OR 0.8–1.6).
Thus, according to the results of the presented population studies, the greatest increase in risk with iEAP is characteristic of intrauterine and perinatal death, the smallest is for slow development and prematurity of the fetus, the possibility of being born by cesarean section, and the child’s need for intensive neonatological care.
For ease of perception and clarity, we converted the results of the study [3] into an ordinal scale. To do this, the 95% CI of the increase in the risk of an adverse pregnancy outcome for the fetus from 1.1 to 6.7 was divided into three equal parts (low at 1.1–2.96, moderate at 2.97–4.82, high at 4. 83–6.7). If the 95% CI for the increase in the risk of any adverse fetal outcome was found at two levels, it was assigned to one of them when the majority of the 95% CI was located at it.
To the low
(on average twofold) increase in the risk of an adverse pregnancy outcome for the child included spontaneous preterm birth, premature birth after rupture of membranes, intensive neonatological care, birth weight below the 5th percentile, cesarean section, Apgar score less than 7 points by 5 1st minute of the child’s life, placental weight less than the 5th percentile and umbilical cord length greater than the 95th percentile, marginal fixation of the umbilical cord on the placenta, placenta previa and abruption, curettage after childbirth, true umbilical cord knot and bleeding of more than 500 ml after childbirth.
The group with a moderate
(on average threefold) increase in the risk of adverse fetal outcome included cord fixation, manual separation, and placenta release.
In the group with a high
(on average fivefold) increase in the risk of an unfavorable fetal outcome were intrauterine and perinatal death of the child. During subsequent pregnancy, the statistical significance of the increase in the risk of recurrence of iEAP and marginal fixation of the umbilical cord on the placenta was determined.
The results of the two largest population-based studies of adverse fetal outcomes with iEAP allow us to conclude that the latter is an independent factor in the death of the fetus/newborn. In addition, using multiple logistic regression with control for the cofounding effect, it was shown that iEAP is an independent factor in increasing the risk of developmental delay and a fetus that is small for gestational age and very early preterm birth [6]. It follows that fetuses with iEAP require careful monitoring of their development and condition.
In the absence of clinical recommendations for the management of pregnancy and childbirth during iEF, the closest ones in terms of the purpose of monitoring the fetus are those with retarded fetal development (FGR), which occupies a leading place among the factors leading to stillbirth [7], in 5.4% of cases being the cause perinatal mortality, especially at 28–31 weeks. pregnancy [8]. Moreover, there is insufficient data from randomized controlled trials to provide information on the best monitoring schemes for fetuses with developmental delays [9]. In addition, the methods used for this, the monitoring regime and the timing of delivery differ in the communities of obstetricians and gynecologists in different countries [10]. In this regard, in the absence of Russian federal and regional clinical recommendations for monitoring a fetus with developmental delays, each doctor has the right to choose the position of any of them for his practical work.
Based on personal preferences, in our work we use recommendations for monitoring the fetus, which has an 8-fold increased risk of intrauterine death [11] (more than that for iEAP), adopted by the German Society of Gynecology and Obstetrics and based not only on evidence, but also at the maximum consensus of experts, when 95% of them agree with the choice of research results on which they are based [12].
Due to the fact that no single monitoring method provides a reliable unfavorable fetal prognosis, a combination of various diagnostic procedures is recommended: clinical examination, sonography (fetometry and assessment of amniotic fluid), Doppler (umbilical and middle cerebral arteries, cerebro-placental ratio - CPR) ), cardiotocography (CTG), including Dawes-Redman analysis, biophysical profile. The prognostic value of Doppler blood flow velocity (DSV) in the uterine arteries in the third trimester of pregnancy is unclear. DSC in the fetal aorta, umbilical vein and inferior vena cava is currently only recommended if performed as part of research studies, as evidence of its usefulness remains insufficient [12].
Clinical monitoring of the mother's condition should be carried out to identify early signs of preeclampsia in FGR caused by placental insufficiency [12]. At the same time, IEA, regardless of the presence or absence of FGR, acts as a factor increasing the risk of its perinatal death. In this regard, clinical monitoring of the fetal condition by measuring the height of the uterine fundus and auscultation of the fetal heartbeat should be combined with the identification of other nosological forms and pathological syndromes that contribute to the disruption of not only uteroplacental, but also fetal-placental blood flow, especially with pleural fixation of the umbilical cord and abruption of a normally located placenta, which act as both risk factors for FGR [9] and adverse fetal outcomes in iEAP [3].
A series of sonographic studies with fetometry and assessment of the volume of amniotic fluid in its deepest pocket with an interval of at least 2 weeks. should accompany the development of the fetus not only if FGR is suspected [12], but also with iEAP.
Taking into account that DSC results in the umbilical artery (UA) are associated with an unfavorable or favorable fetal outcome in FGR, respectively, when zero diastolic/negative (reverse) or normal blood flow is detected, if DSC results in the AP are normal, repeat control scans every 2 are sufficient weeks, and in case of pathological results, when there is a pulsatility index (PI) >95th percentile or reverse blood flow, monitoring should be carried out at least once a week. In the latter case, DSC of the middle cerebral artery (MCA) and ductus venosus (DV) is additionally required. PI SMA <5th percentile increases the risk of cesarean section and poor perinatal outcome. The latter is also associated with a low CPV, equal to the quotient of PI SMA divided by PI AP. Absence or reverse blood flow during DSC in the VP indicates severe acidemia and the risk of fetal death [12].
It should be emphasized that CPO is used as a tool for assessing the condition of the fetus in the third trimester - both small and corresponding to the gestational age, regardless of the results of individual measurements of PI AP and PI SMA. Moreover, a decrease in CPO is associated with a lower gestational age and low birth weight, a higher frequency of cesarean section with fetal distress during childbirth, an Apgar score at the 5th minute of the child’s life less than 7 points, neonatal acidosis, hospitalization in the intensive care unit neonatal care, adverse neonatal outcomes and perinatal death [13]. It is these outcomes that occur with iEAP [2, 3], so CPO can be considered decisive in assessing the condition of the fetus with iEAP in the third trimester of pregnancy.
The correlation between CPO and fetal cord blood pH was more pronounced when compared with the correlation between CPO and low fetal birth weight. In fetuses corresponding to the gestational age, with low and normal CPO, the pH values of the umbilical cord blood were found to be correspondingly lower in the former relative to the latter. Moreover, at full-term and premature births, there was a correspondingly weak and strong correlation between adverse outcomes and fetal weight [14]. In the latter case, PI SMA and PI AP were determined in fetuses, which are directly related to their perinatal mortality and morbidity [15]. At gestational age up to 32 weeks. The MCA DSC measurement had a sensitivity of 95.5% and a negative predictive value of 97.7% for major adverse perinatal outcomes, including fetal and neonatal death, neurological complications and necrotizing enterocolitis. However, among fetuses older than 36 weeks. with unfavorable outcomes, only 44% of cases had an SMA PI less than the 5th percentile. Moreover, regardless of gestational age, during the redistribution of blood flow to the brain, normal and abnormal (>95th percentile) PI APs were evenly represented [16]. A pathological decrease in CPR is ahead of that of SMA PI and can be standardized as 0.6765 MOM (multiple of median, the degree of deviation of the level of a numerical sign from the median) for a given period of pregnancy in order to help in identifying placental insufficiency, hypoxia and FGR [17].
A study was carried out on fetuses older than 34 weeks. 7 days before birth with an estimated gestational weight <10th percentile (confirmed after birth) with normal DSC AP results. Fetuses were divided into groups according to the presence and absence of pathological signs of malperfusion/inflammation of the placenta, which did not differ in the frequency of induction into labor, weight and gestational age of fetuses and placentas. It turned out that with comparable average values of MCA PI and CPO PI, a decrease in their value is less than the 5th percentile, an increase in the average PI of the uterine arteries is more than the 95th percentile, and a decrease in the normalized blood flow velocity in the umbilical vein is less than the 5th percentile turned out to be characteristic of placental malperfusion ( maternal in 64.5% and fetal in 15.5%) and its inflammation (in 20%) [18].
For comprehensive monitoring of the condition of a developmentally delayed fetus, CTG is used, but the study of a biophysical profile is impractical [12]. Pathological CTG patterns, together with such signs of placental hypoperfusion as an increase in mean PI and a decrease in fetal blood flow in the VP, become indications for delivery in fetuses with normal expected body weight [18].
As a result of the analysis of data accumulated in the literature on the use of ultrasound fetometry and DSC in the vessels of the fetus and mother, it became obvious that the decrease in CPR is more associated with fetal acidosis and is ahead of the decrease in PI of the MCA, regardless of the presence or absence of FGR. They, together with indicators of decreased blood flow in the VP and uterine arteries with an estimated fetal weight <10th percentile at a gestational age of >34 weeks. suggest maternal/fetal malperfusion or placental inflammation. This set of indicators is complemented by CTG patterns and the results of assessing the volume of amniotic fluid, which have independent significance as predictors of fetal outcome, as well as late clinical equivalents of the pathology of the fetoplacental complex. It is inappropriate to exclude DSC EP from fetal examination, as well as to neglect the use of a modified biophysical profile of the fetus, the success of which has been demonstrated in FGR from 28 weeks. pregnancy: in predicting the perinatal outcome (according to the Apgar scale at the 5th minute of the child’s life <7 points) it was better than the CPO with a sensitivity of 94.7%, specificity of 52%, negative predictive value (NPV) of 96.5%, also ahead according to sensitivity and PCOR, negative and reverse blood flow in the AP [19].
Thus, to assess the condition of a fetus with iUAP, a series of ultrasounds with fetometry and assessment of the amount of amniotic fluid and DSC of the uterine arteries, AP, and VP after 22 weeks will be useful. every 2 weeks, which is added in the third trimester by DSC of the MCA and assessment of the central nervous system, CTG (if there is heart rate variability, it can be used earlier). If there are pathological values of at least one parameter of the fetal condition, the entire complex of diagnostic studies can be repeated weekly or more often.
Recommendations on drug effects on the fetus, place and timing of delivery in anticipation of premature birth remain relevant not only for FGR, but also for IEA. Corticosteroids should be administered once after 24 weeks of pregnancy. up to 34 weeks If delivery is expected within 7 days, magnesium sulfate for fetal neuroprotection can be prescribed before birth up to 32 weeks. Delivery should take place in a perinatal center with a neonatal intensive care unit and an experienced team to provide immediate care if necessary. With early delivery, the risks of death of the child outside and inside the womb are compared, since his gestational age is a factor influencing survival [12].
In early and late FGR, deterioration of the fetal condition is reflected in pathological deviations in venous and cerebral Doppler parameters, respectively [12].
It is necessary to consider the possibility of delivery in the following obstetric situations: with pathological CTG (from the moment of registration), PI EP >95th percentile (depending on gestational age), absence or reverse A-wave in EP, negative or zero blood flow in AP (not later than 32 weeks), PI AP >95th percentile (after 37 weeks), PI SMA <5th percentile (no later than 37 weeks), decreased CPR (37 weeks), normal DSC results and isolated FGR without additional risks (38 weeks) [12]. Additionally, with iEAP and estimated fetal weight <10th percentile from 34 weeks. pregnancy, when Doppler signs of maternal/fetal malperfusion or inflammation of the placenta and pathological CTG are detected, it is advisable to decide on delivery by cesarean section. It is also advisable to do this with iEAP with FGR, PI MCA <5th percentile and pathological CTG up to 32 weeks, with iEAP regardless of the presence or absence of FGR, a decrease in CPR during repeated studies from 32 weeks, accompanied by the appearance of pathological CTG . In case of iEAP and placenta previa, a planned cesarean section is advisable, and if the placenta ingrowth is determined by ultrasound and Doppler examination or magnetic resonance imaging, excision of the site of placenta ingrowth within the intact myometrial tissue. In case of iEAP in combination with placental abruption and a live fetus, immediate abdominal delivery is required.
In FGR, cesarean section is not a mandatory method of delivery, and in case of normal DSC or PI AP results >95th percentile, induction of labor is possible, taking into account the higher risk of complications and the need for constant intrapartum monitoring of the fetal heart rate [12]. In addition, with iEAP, in terms of labor management, one should take into account the pronounced increase in the risk of manual separation and discharge of placenta, postpartum hemorrhage, and provide an algorithm of actions in accordance with current clinical recommendations [20]. With iEAP, especially in combination with pleural/marginal fixation of the umbilical cord on the placenta, registration of pathological CTG during labor serves as the basis for its surgical completion.
Nicotine cessation should be recommended for all pregnant women. Due to the lack or insufficient evidence of benefit in FGR, calcium supplements, progesterone, oxygen inhalation, plasma volume expansion, low-dose aspirin, sildenafil, pentaerythrityl tetranitrate, vasodilators, and low molecular weight heparin are not recommended for use. β-blockers are known to contribute to intrauterine growth retardation and should therefore be avoided [9].
Thus, the presented obstetric tactics completely cover all possible adverse fetal outcomes identified in population studies [2, 3].
Risk factors for iEAP are: low fetal weight during a previous pregnancy, multiple pregnancy, smoking, neurological diseases of the mother [2], iEAP during a previous pregnancy [3], high pregnancy parity (4+), diabetes mellitus, epilepsy, chronic arterial hypertension, previous cesarean section maternal section and pregnancy resulting from assisted reproductive technologies (ART) [21].
It follows that to prevent IEA, a pregnant woman should be advised to quit smoking, follow an optimal diet and exercise regimen, compensate for diabetes mellitus, and stabilize blood pressure. While taking antiepileptic drugs, with a uterine scar, multiple pregnancies, the birth of a small-for-gestational age child, and iEAP during a previous pregnancy, high parity of the current pregnancy, or the occurrence of pregnancy after ART, it is necessary to specifically look for iEAP during the second ultrasound screening.
At the final stage of the analysis, in order to construct a risk rating for fetal anomalies with a 95% CI from 1.2 to 39.1 observed with EAP, we divided the magnitude of the significant association between them by 3 and received a low (95% CI 1.2–13. 83), moderate (95% CI 13.84–26.46) and high (95% CI 26.47–39.1) increased risk of their development. In EAP, the increased risk of fetal anomalies is defined as high for esophageal and anorectal atresia, moderate for esophageal and anorectal stenosis, trisomies 13 and 18, low for renal agenesis, congenital heart disease, diaphragmatic hernia, short limbs, cleft lip and palate. , trisomy 21.
EAP is most closely associated with gastrointestinal atresia associated with trisomy 13 and 18, indicating common mechanisms of their development. If, after birth, foamy mucus appears from the baby’s mouth and nasal passages, esophageal atresia should be suspected and a gastric tube test and an elephant test should be performed in the delivery room [22]. If the anus is absent, then the question of transferring the child to a surgical hospital should be raised [23]. The pronounced association of EAP with trisomy 13 and 18 requires the obstetrician to be prepared to determine them after the birth of the fetus. Low-set ears, cleft lip and palate, polydactyly and microphthalmia in the newborn fetus suggest Patau syndrome, while a prominent occiput, overlapping toes, bowed lower limbs, micrognathia, low-set ears, short neck, and cardiac and renal malformations suggest Edwards syndrome.
In case of EAP, the obstetrician should be aimed at searching for the main fetal malformations associated with it in order to suspect them in a timely manner and recommend that the pregnant woman study the child’s karyotype (after the first or second screening for malformations during pregnancy or after birth). Risk factor management is available through quitting nicotine use, following a diet for diabetes mellitus, normalizing blood pressure for chronic arterial hypertension, and adopting and maintaining a healthy lifestyle.
Causes
Problems with the vessels of the umbilical cord are not fully understood, but most often they are associated with:
- infectious diseases that the pregnant woman suffered in the first trimester;
- intoxication;
- bad habits (alcohol, smoking, drugs or potent drugs);
- diabetes mellitus;
- radiation exposure, poor ecology, hazardous production;
- multiple pregnancy;
- chromosomal disorders.
The most dangerous period of pregnancy is 6-9 weeks. At this stage, the risk of pathologies of blood vessels, placenta and blood flow increases. Artery atrophy (one of them stops functioning) can be caused by: thrombosis, tumors, hematomas or nodes (on one of the arteries). It should be remembered that such conditions occur quite rarely, but require constant monitoring.
A woman's health condition can also lead to the formation of a single umbilical cord artery during pregnancy. Causes may include: diabetes mellitus, multiple pregnancy, kidney disease, heart disease.
The only artery of the umbilical cord. Fetal growth retardation. EAP and ZRP. My history
In general, this post was planned for the baby’s year. But there are a lot of girls who write in PM that I decided to write now. I hope the post will be useful (but in general I hope even more that it will not be useful to anyone).
I think in some communities the story of my pregnancy, childbirth and the first months of motherhood is already known by heart.
But after giving birth, frightened girls who also had this pathology began writing to me on various social networks. As it turns out, it's not that rare.
Therefore, I would like to describe and leave a detailed post about how I encountered the only artery of the umbilical cord, what I did, what happened to the child.
I’ll say right away that this is a story about a difficult pregnancy and the great difficulties that we went through. But I think it’s better to know that such scenarios are possible. If you are not ready to face reality, it is better not to read. For the timid, overall we escaped with little loss of life.
First paid screening at 11 weeks.
“You have one artery here in the umbilical cord... There should be two... This is not scary in itself, but EAP is a marker of chromosomal abnormalities (CA), but small... You have nothing else, so this is most likely the norm, but I will write this in conclusion »
I shrugged my shoulders - okay. I donated blood in the next room.
I read about this EAP on the Internet - many wrote that the artery was present at the second screening. So I calmed down for the time being.
Later the blood came, the risks were low, but the beta-hCG was high (3.53 according to the norm to 2), which in tandem with the EAP scared me.
To be on the safe side, I took NIPT for 5 syndromes - all negative.
The first screening in the gastrointestinal tract, ultrasound at 15-16 weeks, the second screening in the gastrointestinal tract - everywhere EAP. Everyone reassures: “this happens normally.” But further...
Ultrasound at 24 weeks - “shortening of the tubular bones.”
Ultrasound 26 weeks - “well, your due date is definitely not 26 weeks, two weeks less, probably the LCD made a mistake.”
Ultrasound at 32 weeks - “grade 1 fetal growth restriction, weight 1620 g.”
I know the reason for ZRP!
I study information about the EAP in detail, looking on social networks for girls who have encountered this. Let me compile some personal statistics:
- 2-3% of cases of genetic abnormalities (but a whole bunch of pathologies were visible on ultrasound)
- 10% of cases of FGR
- 15% of cases of developmental defects (mostly congenital heart disease, also problems with the kidneys, gastrointestinal tract) - it must be said that in general almost everything is operable without dire consequences
- 70% of cases - everything is fine, baby dolls are born 3500 grams / 50 cm