Hyperthyroidism is a condition of hyperfunction of the thyroid gland, which is accompanied by excessive production of thyroxine and triiodothyronine. An increase in the level of hormones in the blood accelerates metabolic processes in the body and has a negative effect on almost all organ systems. Symptoms of hyperthyroidism reduce the patient's quality of life. Hyperthyroidism is a condition of hyperfunction of the thyroid gland, which is accompanied by excessive production of thyroxine and triiodothyronine. An increase in the level of hormones in the blood accelerates metabolic processes in the body and has a negative effect on almost all organ systems. Symptoms of hyperthyroidism reduce the patient's quality of life.
Adequate and timely treatment allows you to prevent complications and slow down pathological processes, up to the complete restoration of thyroid function.
Types of hyperthyroidism
There are three forms of the disease:
- Subclinical. There are no obvious symptoms, T4 levels are normal, triiodothyronine levels are low;
- Manifest. Characteristic signs of hyperthyroidism appear. T4 level is normal, triiodothyronine level is low;
- Complicated. Symptoms include heart failure, arrhythmia, psychosis and other severe conditions.
According to the level of occurrence of hyperthyroidism, there are:
- primary – pathology of the thyroid gland;
- secondary – the pituitary gland is affected;
- tertiary – processes develop in the hypothalamus.
Causes of hyperthyroidism
A number of endocrine diseases lead to thyroid dysfunction, such as:
- Graves' disease (Bazedow, Bazedow-Graves) . The syndrome is autoimmune in nature. The body produces antibodies that stimulate the thyroid gland to produce excess amounts of the hormone T4;
- Nodular goiter, toxic adenoma . The listed pathologies are accompanied by the formation of benign nodes in the tissues of the gland. The formations begin to produce hormones and cause hyperthyroidism. Doctors cannot yet say for sure why some adenomas synthesize T4 and others do not;
- Thyroiditis . The inflammatory process destroys thyroid cells. Hormones enter the bloodstream and cause hyperthyroidism. The autoimmune nature of thyroiditis is possible. The body produces antibodies against TSH receptors. The cells cause active growth and inflammation of the thyroid gland.
People with a hereditary predisposition to endocrine pathologies are most susceptible to the disease. Symptoms of hyperthyroidism are more common in women than in men. The development of the disease is also influenced by the environmental situation, chronic iodine deficiency, and stress.
Symptoms of hyperthyroidism
The first signs of dysfunction are uncharacteristic, so the disease is often confused with other somatic conditions. Symptoms of hyperthyroidism of the thyroid gland increase as metabolic processes in the body are disrupted.
Main signs of the disease:
- loss of body weight while maintaining appetite, volume and quality of food;
- arrhythmia, tachycardia;
- anxiety, depression;
- increased sweating;
- fine tremor of fingers and hands;
- indigestion;
- the formation of a visible goiter, changing the contours of the neck;
- increased fatigue.
A symptom of hyperthyroidism in men is a decrease in potency and libido. In women, increased levels of thyroid hormones cause menstrual irregularities. Pregnancy may end in spontaneous abortion. A symptom of thyroid hyperthyroidism in women can also be decreased fertility, including infertility. During menopause, the pathology in the initial stages is asymptomatic.
Tachycardia - symptoms
The main symptoms of tachycardia are:
- increased heart rate (90 or more beats per minute);
- increased heart rate (ventricular tachycardia);
IMPORTANT! The heart rate (HR) and pulse should normally match the number of beats per minute. Heart rate is the number of heart contractions per minute, and pulse is the number of dilations of the artery during the same time, at the moments of “ejection” of blood by the heart. With tachycardia and other heart diseases, heart rate and pulse indicators may differ.
- lowering blood pressure;
- a feeling of heaviness in the chest, even suffocation;
- shortness of breath, sometimes even with minor physical exertion or at rest;
- skin moisture;
- interruptions and “failures” in the work of the heart;
- dizziness, darkness in the eyes, presyncope (sometimes fainting associated with cerebrovascular accident);
- pain in the heart area - this symptom is characteristic of tachycardia against the background of vegetative-vascular dystonia (VSD) and some other pathological conditions.
If you find yourself with one or more of the listed symptoms and suspect that you have tachycardia, what should you do first? Of course, contact a cardiologist who will refer you to certain examinations and tests, and then prescribe treatment. You can take the necessary tests in advance so that you can come to the doctor with ready-made results.
Complications of hyperthyroidism
As the disease progresses, the symptoms of thyroid hyperthyroidism intensify, and complications may arise:
- Disturbances in the functioning of the heart . Atrial fibrillation is added to the general symptoms of hyperthyroidism. The patient does not tolerate physical activity well, and there is a disturbance in heart rhythm. Congestive heart failure may develop. The consequences are reversible. After eliminating hyperthyroidism, the signs of arrhythmia are completely cured.
- Increased bone fragility . In its advanced form, hyperthyroidism causes disturbances in the structure of bone tissue. Osteoporosis develops. The reason bones become brittle is that excess hormones prevent calcium from being incorporated into the bone.
- Eye diseases . Graves' ophthalmopathy adds to the symptoms of hyperthyroidism. The cause of the pathology is the growth and swelling of the tissues located behind the eyeballs. The patient feels pain in the eyes, sensitivity to light, and complains of double vision. Visual acuity gradually decreases. In advanced cases, blindness develops.
- Skin problems . Ocular symptoms in hyperthyroidism often develop in parallel with Graves' dermopathy. The skin becomes swollen and red, especially around the feet and legs.
- Thyrotoxic crisis . An increase in the level of thyroid hormones in the blood can cause a sudden fever and an increase in all the main symptoms. Thyrotoxic crisis is accompanied by tachycardia. In some cases, delirium (mental disorder) occurs. The patient requires emergency medical care.
Types of tachycardia
Tachycardia as such is divided into two groups:
- physiological , which is not considered a pathology and is a consequence of physical activity, and also occurs in preschool children, and then in adolescents during the period of hormonal changes. In addition, this “safe” tachycardia can also be caused by some external factors in addition to physical activity - stress, emotional arousal, heat, coffee, alcohol, some medications, sudden changes in body position, etc. In all these cases, a certain amount of adrenaline is released into the blood, which and serves as a “trigger” for increased heart rate;
- pathological tachycardia is already a disease, and it may be associated not necessarily with the cardiovascular system, but also with other systems of the body. Such tachycardia, if not detected in a timely manner, leads to various organic pathologies, including those related to the cardiovascular system - heart attacks, strokes.
The pathological form of the disease is also divided into types depending on the parts of the heart that “accelerate” its work - the atria (atrial tachycardia) and the ventricles (ventricular tachycardia). A subtype of ventricular tachycardia is sinus tachycardia.
Diagnosis of hyperthyroidism
Consultation with an endocrinologist
Diagnosis of hyperthyroidism begins with an appointment with an endocrinologist. At the appointment, the doctor asks the patient questions that will help in diagnosis: how long ago did the clinical symptoms appear, what is the dynamics of the disease, are there any other patients with goiter or thyroiditis in the family. A physical examination is required: palpation of the thyroid gland. In case of obvious hyperthyroidism, the doctor will try to palpate the contours of the organ, determine its position, uniformity of structure, and pain. After the examination, the patient receives directions for further examinations to accurately diagnose the cause of the disease.
Laboratory research
Hyperthyroidism is confirmed by blood tests to measure the level of thyroid hormones. Elevated levels of thyroxine in the absence or minimal amount of TSH indicate hyperfunction of the gland. A blood test is especially important in the early diagnosis of the disease in older people. For example, menopausal women may not have symptoms of hyperthyroidism. Pathology is detected only by the results of laboratory tests.
A blood test helps confirm the condition of the endocrine organ, but additional tests will be required to determine the cause of hyperthyroidism.
Instrumental research methods
Ultrasonography. Ultrasound of the thyroid gland allows you to determine the shape and size of the lobes, assess the degree of its enlargement, and detect nodes, cysts, and other neoplasms.
Radioisotope scintigraphy . The patient is given a special solution intravenously. The drug contains radioactive isotopes of iodine, which are actively captured by the thyroid gland. After some time, the doctor assesses the degree of saturation of the tissues and makes a conclusion about their functionality. A large amount of radioiodine indicates excessive production of thyroxine, which develops, in particular, with Graves' disease.
If, with severe symptoms of thyroid hyperthyroidism in a woman or man, a minimal amount of isotopes is observed in the gland, then the probable cause of the pathology is thyroiditis. The radioactive iodine uptake test helps in differential diagnosis.
Fine needle biopsy . Under the control of an ultrasound probe, the doctor takes samples of thyroid tissue for examination. Biopsy materials make it possible to determine the nature of the neoplasm: benign or malignant. The study results also provide information about autoimmune processes that may cause hyperthyroidism.
Treatment of hyperthyroidism
The approach to therapy depends on the cause of hyperthyroidism, the age and condition of the patient.
Conservative treatment methods:
- Radioactive iodine preparations . Isotopes accumulate in the tissues of the thyroid gland, suppressing compensatory mechanisms. The organ decreases in size, the symptoms of hyperthyroidism gradually weaken. Treatment takes several months. It is important that radioactive iodine suppresses gland function, so over time the patient may develop hypothyroidism, which is corrected by hormone replacement therapy.
- Antithyroid drugs . Medicines suppress the production of thyroid hormones. Symptoms decrease within a few weeks after taking the drugs in the correct dosage. The course of treatment is up to 1 year or longer. In some cases, hyperthyroidism is cured completely, but sometimes relapses occur. It is important that antithyroid drugs can cause allergies, individual intolerance, and can reduce immunity. The effects of this group of drugs cause disturbances in liver function. You can take medications only under the supervision of a doctor.
- Symptomatic treatment . Depending on the severity of the manifestations of hyperthyroidism, the doctor may prescribe medications to lower blood pressure, maintain heart rhythm, etc. Symptomatic treatment is continued until the patient’s general well-being improves.
In cases of severe hyperthyroidism, accompanied by significant enlargement of the thyroid gland, multiple neoplasms in functional tissue, as well as medullary cancer, surgical intervention is indicated. The doctor partially or completely removes the endocrine organ. After the operation, the patient is given a dosage of hormones that are taken for life.
Thyroid cardiopathy
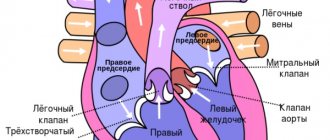
At the XI International Endocrinology Congress, held recently in Sydney (Australia), special attention was paid to cardiopathy caused by thyroid pathology. Of course, this problem is becoming more and more urgent as the prevalence of this pathology increases. Recently, more attention has been paid to thyroid disorders on the border between normal and pathological: subclinical thyrotoxicosis and subclinical hypothyroidism. Their high prevalence has been proven, especially in older people, mainly in women. A number of regions have even introduced screening for subclinical forms of thyroid disease in older age groups. A disturbance in the content of thyroid hormones in the body, even a slight increase or decrease, causes pathology of the cardiovascular system.Heart with subclinical hypothyroidism
Subclinical hypothyroidism is a pathological condition characterized by normal levels of total and free thyroxine (T4) and elevated levels of thyrotropin (TSH), or hypersecretion of TSH, in response to the administration of thyrotropin-releasing hormone (THH).
It should be remembered that in some cases, even with manifest hypothyroidism, especially in the elderly, there is no increase in TSH levels. This feature is due to environmental problems (environmental pollution with lead, cadmium, carbon monoxide, etc.), exposure to drugs (rauwolfia, clonidine, etc.), and protein deficiency in food. It has also long been noted that in the zone of iodine deficiency, the synthesis of TSH by the pituitary gland is reduced.
Cardiac “masks” of subclinical hypothyroidism are as follows:
- persistent hypercholesterolemia, atherogenic dyslipidemia;
- atherosclerosis;
- IHD;
- arrhythmias (sinus bradycardia or tachycardia, polytopic extrasystole, paroxysms of atrial fibrillation and flutter, sick sinus syndrome);
- cerebrovascular disease;
- arterial hypertension;
- MVP (and/or other valves), hydropericardium.
Subclinical hypothyroidism includes endemic goiter (EG). It has long been known that patients with EZ develop cardiac complaints, the sonority of heart sounds and heart rhythm may change. However, these changes in the activity of the cardiovascular system were previously considered to be mild and caused by autonomic dysregulation. The widespread use of ultrasound examination of the heart has revealed the frequent development of mitral valve prolapse (MVP) or other valves in EZ and other cases of subclinical hypothyroidism. MVP is a systolic protrusion of one or both mitral leaflets into the cavity of the left atrium above the level of the mitral annulus. In this case, a violation of the closure of the valves with the development of mitral regurgitation does not always develop. More than 50 diseases are known in which MVP can develop. However, EZ and hypothyroidism as etiological factors of MVP have not received due attention even in the literature of recent years. Meanwhile, even an insignificant deficiency of thyroid hormones in the body causes serious disturbances in metabolic processes, including dystrophic changes in the heart, accompanied by a decrease in the intensity of oxidative phosphorylation, a slowdown in protein synthesis, a decrease in oxygen uptake by the myocardium, and electrolyte shifts. Both the contractile myocardium and the stroma are affected. Creatine phosphate is deposited in cardiomyocytes and so-called myocardial pseudohypertrophy occurs. In the heart, as in other tissues, acidic glycosaminoglycans are deposited, leading to mucous edema of the myocardium and stroma. With EZ and hypothyroidism, the autonomic nervous system is involved in 100% of cases. Autonomic dystonia is characterized by vagal hypertonicity, that is, parasympathetic autonomic dysregulation of the heart occurs. As a rule, MVP in patients with EZ and subclinical hypothyroidism has a “silent” course: the number of heartbeats and cardiac configuration remain normal, but in most cases there is a decrease in one or both tones. Classic manifestations of MVP - mesosystolic, less often protosystolic, or late systolic click, precordial “click” (auscultatory phenomenon of resonance of the mitral leaflet) are not recorded. When analyzing electrocardiograms, deviations from the norm (sinus bradycardia, slowing of impulse conduction in different parts of the myocardium, decreased voltage of the waves, especially the T wave) are observed in 80% of cases, but are not of a regular nature. Ultrasonography has established that against the background of latent hypothyroidism, MVP often occurs, in some cases combined with prolapse of the tricuspid and/or aortic (extremely rare - pulmonary) valve. The displacement of the mitral valve leaflets into the atrium cavity reaches 3-7 mm; this is the I or II degree of MVP. Regurgitation is found only in isolated cases; the diastolic opening of the mitral valve is not impaired, the volume of the left atrium is normal and, therefore, serious hemodynamic disturbances do not develop. Nevertheless, MVP can be called an important diagnostic symptom of EZ and subclinical hypothyroidism. III degree prolapse, that is, more than 9 mm, is not typical for patients with subclinical hypothyroidism. In these cases, even in the presence of EZ, one should look for other causes of MVP. Despite the absence of regurgitation and hemodynamic disturbances in patients with EZ with MVP, the risk of prolapse complications remains. Typical complications of MVP are infective endocarditis, thromboembolism, and sudden death. Therefore, it is relevant to develop methods for the treatment of MVP in patients with EZ and subclinical hypothyroidism. It is known that for the treatment of MVP, (-adrenergic blockers are used to suppress the increased contractility of the left ventricular myocardium during MVP, as well as to increase its volume and prevent arrhythmias. It should be emphasized that (-adrenergic blockers are contraindicated in case of hypofunction of the thyroid gland, since they have an antithyroid effect and enhance hypothyroidism. In addition, parasympathicotonia also serves as a contraindication to the use of these drugs in such patients. Systematic replacement therapy with thyroid hormone drugs reduces or completely eliminates MVP in patients with EZ and subclinical hypothyroidism. On the contrary, without correction of thyroid insufficiency, prolapse of other valves may appear and regurgitation may increase.
Another echocardiographic finding in patients with subclinical hypothyroidism, including patients with EZ, may be hydropericardium, which is asymptomatic. Typically, the effusion is localized in the apex and along the right contour of the heart.
Heart with subclinical hyperthyroidism
Subclinical hyperthyroidism is a condition in which the serum TSH concentration (without pituitary insufficiency) is decreased, while serum thyroid hormone levels remain within normal limits.
Before making a diagnosis of subclinical hyperthyroidism, especially in the elderly, it is necessary to repeatedly determine the TSH level over time over several weeks, since a decrease in the basal TSH level can be observed with various non-thyroid diseases, depression, taking certain medications, etc. The true prevalence of subclinical hyperthyroidism in our country has not yet been studied. In England it is about 10% in women, in other countries it varies from 0.5% to 11.8%.
The causes of subclinical hyperthyroidism are different: it is a euthyroid variant of Graves' disease, a toxic adenoma of the thyroid gland, a consequence of the destruction of thyrocytes in subacute or chronic thyroiditis, as well as insufficient adequate treatment of overt hyperthyroidism. The most common cause of a decrease in TSH levels is the use of thyroxine (drug-induced subclinical hyperthyroidism). It often occurs during pregnancy. Gestational hyperthyroidism, caused by increased levels of human chorionic gonadotropin during developing pregnancy, can also often be subclinical. Iodine-Basedowism, high iodine consumption with imperfect mass prevention of EZ in many cases occurs as subclinical hyperthyroidism. For the clinician, it is important to answer the question whether subclinical hyperthyroidism affects health, and primarily the cardiovascular system, or is it just a laboratory finding.
The effect of thyroid hormones on the circulatory system is well known. They play an important role in regulating energy metabolism in the body. Their effect on mitochondrial processes in cells, including cardiomyocytes, has recently been clarified. Thyroid hormones regulate the lipid composition of mitochondrial membranes, the content of cytochromes and cardiolipins in cells, etc., ultimately stimulating cellular respiration. These effects are divided into short-term (several hours) and long-term (several days). In subclinical hyperthyroidism, these processes are disrupted. Thus, when examined as part of the Framingham Study, patients with a TSH level of less than 0.1 milliU/L were found to have a significantly higher incidence of atrial fibrillation after 10 years and a significantly increased mortality rate. The following clinical cardiac symptoms are characteristic of subclinical hyperthyroidism: tachycardia, shortening of systolic intervals, increased stroke volume of the left ventricle, diastolic disturbances (decreased diastolic filling).
Should subclinical hyperthyroidism be treated? Currently, there is no evidence-based medicine assessment yet. It has been empirically shown that the use of (-blockers improves pulse, reduces atrial fibrillation, diastolic dysfunction in patients treated with thyroxine. If subclinical hyperthyroidism is a variant of Graves' disease, then the effectiveness of (-blockers is currently questioned (M. Niels, HK Yde, N. Soren et al., 1998). The question of whether such patients should be treated with thyreostatics has not been resolved. “Wait and watch,” especially if there are no obvious disturbances in cardiac function and bone metabolism, is one point of view. But since subclinical hyperthyroidism in many cases can quickly progress to an obvious clinical form, then there are many supporters of the active use of thyreostatics.Obviously, the decision must be made individually.
Heart with manifest hypothyroidism and manifest thyrotoxicosis
The terms “myxedematous (hypothyroid) heart” and “thyrotoxic heart”, which are currently used to designate myocardial damage in manifest hypothyroidism or manifest thyrotoxicosis, were first proposed by H. Zondek at the beginning of the twentieth century. Let us consider the pathogenetic mechanisms of hypothyroid and thyrotoxic heart.
Pathogenesis of hypothyroid heart
1.Reducing oxidative phosphorylation and oxygen absorption by the myocardium, increasing the permeability of cell membranes; deficit of macroergs. 2. Slowing down of protein synthesis, fatty infiltration of muscle fibers, accumulation of mucopolysaccharides and glycoproteins in the myocardium 3. Accumulation of creatine phosphate. Myocardial pseudohypertrophy 4. Increased LPO; oxidative stress. Damage to cell membranes 5. Electrical instability of the myocardium. 6. Increased sodium and decreased potassium content in cardiomyocytes 7. Edema of muscle fibers and interstitial tissue of the heart; mucous edema of the myocardium 8. Decreased myocardial tone, myogenic dilatation. Impaired microcirculation 9. Mucous edema of the pericardium, effusion in the pericardial cavity. 10. Atherosclerosis of coronary vessels 11. Anemia
Pathogenesis of thyrotoxic heart
1. Increasing the need for oxygen in the myocardium and other tissues. Stimulation of oxidative processes by thyroid hormones. Oxidative stress 2. Increased tone of the sympathetic nervous system and increased tissue sensitivity to adrenaline. Pathological sensitivity of heart tissue to catecholamines 3. Persistent tachycardia. Shortening of diastole. Depletion of reserves 4. Decreased ATP synthesis. Deficiency of macroergs 5. Increased overall pulmonary resistance. Pulmonary hypertension 6. Protein catabolism (myocardial and enzymatic) 7. Enhanced glycolysis, including in cardiomyocytes 8. Hypocalygistia 9. Impaired permeability of cell membranes, impaired microcirculation. 10. PMK. 11. Anemia (in some cases severe).
The most significant complications that threaten the lives of patients with hypothyroidism and thyrotoxicosis are caused by pathological changes in the cardiovascular system: rhythm and conduction disturbances, cardialgia, arterial hypertension, myocardial dystrophy, circulatory failure.
Arrhythmias in thyroid pathology
The idea that bradycardia is inevitable with hypothyroidism has long been outdated. Indeed, sinus bradycardia is a characteristic, but not an absolute clinical sign of hypothyroidism, including myxedema: the tachysystolic form of atrial fibrillation and flutter is often observed, usually in the form of paroxysms. The alternation of such paroxysms with bradycardia is mistakenly taken for sick sinus syndrome as a consequence of coronary artery disease. A thorough examination of the patient is required, including electrophysiological and hormonal studies. Treatment with antiarrhythmic drugs in such cases is not only useless; Amiodarone, Sotalex and other antiarrhythmics aggravate hypothyroid arrhythmia. There is an interesting description in the literature of ventricular flutter-fibrillation in myxedema, eliminated by thyroid hormones without antiarrhythmic therapy (A. Gerhard et al., 1996). Conduction disturbances in different parts of the heart are also common in hypothyroidism.
In a thyrotoxic heart, persistent sinus tachycardia is observed. Heart rate does not depend on either emotional or physical stress. Tachycardia does not decrease during sleep. In severe cases of the disease, patients develop a tachysystolic form of atrial fibrillation. Treatment with amiodarone and saluretics provokes atrial fibrillation. Extrasystole in thyrotoxicosis is rare. Its appearance is associated not with thyrotoxicosis, but with a previous heart disease.
Thyroid diseases and arterial hypertension
Arterial hypertension is observed in both hypothyroidism and hyperthyroidism, but the pathogenetic mechanisms are different.
HYPOTHYROIDSIS: increased predominantly diastolic blood pressure
THYROTOXICOSIS: increased systolic blood pressure, decreased diastolic blood pressure
1. Retention of sodium and water in the body 2. Change in sensitivity to circulating catecholamines 3. Impaired secretion of natriuretic peptide
1. Increased cardiac output, increased cardiac output 2. Activation of the kinin-kallikrein system 3. Hypersecretion of adrenomedullin 4. Functional hypocortisolism
Arterial hypertension in hypothyroidism is aggravated by the associated atherosclerotic process. In this case, its course does not differ from the course of essential hypertension, but partial or complete refractoriness to antihypertensive drugs develops. Arterial hypertension in thyrotoxicosis is called high cardiac output syndrome, while left ventricular hypertrophy is usually absent. A recently discovered peptide, adrenomedullin, has very pronounced vasodilator activity. Its participation in reducing diastolic blood pressure in patients with thyrotoxicosis has been proven. High cardiac output syndrome can transform into hypertension. If arterial hypertension in a patient persists for several months after normalization of thyroid function, this case should be considered as a transition to essential hypertension and conventional antihypertensive therapy should be carried out.
Heart failure in hypothyroid and thyrotoxic heart
In hypothyroidism, despite pronounced dystrophic changes in the myocardium, heart failure occurs extremely rarely (in myxedema with a long history of the disease). This is explained primarily by a decrease in the need for peripheral tissues for oxygen, as well as vagotonia.
In thyrotoxic heart, a decrease in myocardial contractile function and the development of heart failure depends on the severity of the disease. Shortening of diastole leads to depletion of the reserve capacity of the myocardium. The contraction power of both ventricles decreases, which is the result of significant fatigue of the heart muscle due to developing myocardial dystrophy. At the same time, total peripheral resistance decreases and pulmonary resistance increases. An increase in pressure in the pulmonary artery occurs due to a reflex narrowing of the pulmonary arterioles (Kitaev reflex). Hemodynamic disorders in thyrotoxicosis lead to the fact that the left ventricle of the heart works under conditions of isotonic hyperfunction (load “volume”), and the right ventricle works under conditions of mixed type hyperfunction (load “volume and resistance”). Heart failure in thyrotoxicosis develops predominantly of the right ventricular type. At the same time, it can be aggravated by the developing insufficiency of the tricuspid valve with regurgitation of blood into the right atrium. MVP occurs frequently in thyrotoxicosis, but does not significantly affect hemodynamics, although in some cases signs of left atrial hypertrophy can be found on the ECG (S.B. Shustov et al., 2000).
ECG changes in thyrotoxicosis
An ECG in mild cases of the disease is characterized by:
1. An increase in the voltage of the P, QRS, and T waves (especially often in leads II and III). 2. Lengthening the PQ interval to 0.2″. 3. Sinus tachycardia. 4. Shortening the time of electrical ventricular systole.
ECG changes in thyrotoxicosis of moderate severity or with a long duration of the disease are characterized by:
1. Decrease in P wave voltage, appearance of P wave jaggedness. 2. Slowing of intraatrial conduction (P>0.1″). 3. Downward displacement of the ST segment. 4. Decrease in the T wave or the appearance of T (-+), or T (-) in a large number of leads, especially often in leads I, II, AVL, V4-V6; 5. Lengthening the electrical systole of the ventricles;
Characteristics of ECG in severe thyrotoxicosis.
1. Atrial fibrillation (tachysystolic form). 2. Signs of relative coronary insufficiency.
Differential diagnosis of thyrotoxic heart and rheumatic carditis
Experience shows that heart changes during the manifestation of thyrotoxicosis are often mistakenly interpreted as manifestations of primary rheumatic carditis, especially if the symptoms appeared after a tonsillar infection. Shortness of breath, palpitations, heart pain, weakness, low-grade fever, prolongation of the PQ interval on the ECG are characteristic of both diseases. It is clear that antirheumatic therapy will not only have no effect, but may worsen the condition of patients. The following clinical signs help in making the correct diagnosis: with thyrotoxicosis, patients are excitable, they have diffuse hyperhidrosis, warm palms, “Madonna” hand, persistent tachycardia, increased heart sounds, systolic arterial hypertension, and with rheumatic carditis, patients are lethargic, local sweating, hands the hands are cold, the tachycardia is inconsistent, intensifies after exercise, the 1st sound at the apex of the heart is weakened, blood pressure decreases.
Differential diagnosis of thyrotoxic heart and mitral valve disease
Diastolic murmur always indicates organic heart damage. Thyrotoxicosis is an exception: systolic and diastolic murmurs arise due to disruption of laminar blood flow in the cavities of the heart due to accelerated blood flow, decreased blood viscosity, and the addition of anemia. Auscultatory changes in the heart in patients with thyrotoxicosis are mistakenly interpreted as a sign of mitral disease. The mitral configuration of the heart, which appears in thyrotoxicosis due to increased pressure in the pulmonary artery (smoothness of the waist of the heart due to the bulging conus pulmonale) “confirms” the diagnosis. Of course, sonographic examination of the chambers, cavities, and valve apparatus of the heart helps to avoid this kind of diagnostic errors. But even in patients with heart defects, it may be necessary to monitor TSH in the blood to confirm the diagnosis.
Differential diagnosis of thyrotoxic cardiopathy and ischemic heart disease
Diagnosis of thyrotoxicosis can be difficult in the elderly due to clinical similarities with coronary artery disease and atherosclerosis. Fussiness in behavior, sleep disturbances, hand tremors, increased systolic and pulse blood pressure, paroxysmal or permanent form of atrial fibrillation can be observed in both thyrotoxicosis and atherosclerosis. However, with thyrotoxicosis, the tachycardia is persistent, the heart sounds are increased even with atrial fibrillation, the level of cholesterol and LDL in the blood decreases, diffuse hyperhidrosis is expressed, hand tremors are small, a goiter, shiny eyes and other symptoms of thyrotoxicosis can be detected. These signs are uncharacteristic of atherosclerotic heart disease, and weakening of the 1st tone and hyperlipidemia will suggest coronary artery disease. It should be taken into account that both diseases are often combined, thyrotoxicosis is layered on top of long-standing atherosclerosis. Since thyrotoxicosis can occur in elderly people without enlargement of the thyroid gland, it is necessary to monitor their TSH levels in the blood more often.
Treatment of hypothyroid heart
Eliminating hypothyroidism and achieving a euthyroid state gives undoubted success in the treatment of hypothyroid heart. The main drug for the treatment of hypothyroidism is T4. Its average dose is 10-15 mcg/kg in children and 1.6 mcg/kg in adults; Usually the daily dose for women is 75-100 mcg, for men 100-150 mcg. In young adult patients with hypothyroidism, the initial dose of T4 is 50 - 100 mcg/day. It is increased every 4-6 weeks by 50 mcg. In elderly patients, with ischemic heart disease and rhythm disturbances, the initial dose of T4 should not exceed 25 mcg/day. It is increased carefully under the control of general condition and ECG after 5-6 weeks. Treatment occurs under the control of TSH and thyroid hormones in the blood. It should be remembered that many drugs, such as (adrenergic blockers, tranquilizers, central sympatholytics, amiodarone and sotalol, etc., can themselves cause drug-induced hypothyroidism.
Treatment of thyrotoxic heart
Elimination of thyrotoxicosis is the first condition for successful treatment of thyrotoxic heart. There are three types of treatment for Graves' disease: medication, surgery, and radioactive iodine therapy. Among the methods of conservative therapy, thyreostatic drugs (mercazolyl, or its analogues thiamazole, methimazole) are still used. Propylthiouracil is increasingly entering practice in the treatment of thyrotoxicosis. Although its dose is approximately 10 times higher than the dose of Mercazolil, it nevertheless has a number of advantages. Propylthiuracil is able to bind tightly to blood proteins, which makes it suitable for the treatment of pregnant and lactating women. Its additional advantage is the ability to inhibit the conversion of T4 to T3. Compared to Mercazolil, a smaller amount of propylthiouracil penetrates into the placenta and breast milk. Along with the antithyroid effect, it also has an antioxidant effect, which is very important in the presence of oxidative stress in patients with thyrotoxicosis.
The question of the regimen of taking thyreostatics for Graves' disease must be resolved in two stages: first to achieve a euthyroid state, and then to carry out maintenance therapy to achieve long-term remission of this chronic autoimmune disease. The question remains debatable with what doses therapy with thyreostatic drugs should be started - with maximum doses, gradually decreasing, or with small ones. In recent years, there has been more and more support for the treatment of thyrotoxicosis with small doses of thyreostatics. Reducing the dose of thyreostatics reduces the number of side effects and does not weaken the antithyroid effect. It should be noted that in patients with large goiters and/or high levels of T3 in the blood serum, small doses of thyreostatics cannot achieve a euthyroid state even after a long (more than 6 weeks) course of drug treatment. Therefore, the tactics of drug treatment of thyrotoxicosis should be individual.
There is no single point of view on the tactics of maintenance therapy. There are fewer supporters of the use of high doses of thyreostatics in combination with thyroxine, according to the “block and replacement” principle, than supporters of minimal doses of thyreostatics sufficient to maintain a euthyroid state. Prospective studies, including a European multicenter study, have not shown any benefit from maintenance treatment with high doses of drugs. According to the results of surveys of both European and American specialists, 80-90% of endocrinologists believe that the course of maintenance therapy should be at least 12 months. The question of the optimal duration of treatment remains open. It is believed that treatment can be recommended for 18 months, especially in patients who have antibodies to TSH receptors in their blood. When carrying out treatment, it is necessary to remember the side effects of thyreostatics. Although hematological complications (agranulocytosis, aplastic anemia) develop rarely (in 0.17% - 2.8% of cases), they are serious and can be fatal. It should be noted that agranulocytosis can develop with a low dose of thyreostatic drugs, and after a long period (12 months) after the start of their use. Hepatotoxicity is often observed during treatment with thyreostatics, and the frequency of this pathology increases with increasing dosage of drugs. 10-25% of patients experience minor side effects of treatment, such as urticaria, skin itching, arthralgia, gastritis, etc. These effects are clearly dose-dependent and require selection of an individual dose of thyreostatic for each patient.
The frequency of relapses of Graves' disease after a long course of maintenance therapy with thyreostatics, according to the observations of various authors, ranges from 2 to 35%. Currently, the opinion that combined therapy with thyreostatics and thyroxine significantly reduces the frequency of disease relapses has been revised; Prospective studies have not confirmed this. However, another 78% of Japanese doctors continue to use antithyroid drugs in combination with thyroxine (M. Toru et al., 1997). There are no clear criteria yet to predict the onset of remission of Graves' disease. However, the following factors may indicate the possibility of an unfavorable outcome of the disease: large goiter sizes, an initial high level of thyroid hormones in the blood, or a high titer of antibodies to TSH receptors. A method for using thyreostatics in combination with cholestyramine has been developed. The latter reduces thyrotoxic intoxication by absorbing thyroid hormones in the stomach and intestines and preventing their reabsorption.
In many countries, combined treatment with thyreostatics and radioactive iodine is used for Graves' disease. This treatment is currently being tested because it is unclear whether thyreostatic therapy has an adverse effect on the effectiveness of subsequent radioactive iodine treatment. Transient hypothyroidism often develops after such treatment in patients with Graves' disease, and its development cannot be predicted in advance.
Surgery remains the fastest method of achieving a euthyroid state compared with thyroid drugs or radioactive iodine and, in addition, as shown by a randomized prospective study, this type of treatment is accompanied by the lowest relapse rate over the next two years. However, the risk of complications allows us to recommend performing strumectomies only in those surgical centers where there is sufficient experience. But even under this condition, the frequency of delayed development of severe hypothyroidism is at least 30% after 5 years, and subclinical hypothyroidism is even more common (up to 46% of cases), although in some patients it is cured spontaneously. The issue of the optimal volume of surgical intervention for Graves' disease is still being debated. After subtotal thyroidectomy, delayed (5-10 years after surgery) relapses of thyrotoxicosis may develop in at least 10% of cases. Therefore, there have been many supporters of radical treatment of Graves' disease - total thyroidectomy. The need for constant thyroid hormone replacement therapy in this case is a serious objection to this method of surgical treatment.
The use of cardiac glycosides in patients with thyrotoxicosis, even with severe shortness of breath, is a gross mistake. It is known that cardiac glycosides have a cardiotonic effect, cause an increase in cardiac systole, prolongation of diastole, a vagotropic effect, and a slowdown in conduction, in particular atrioventricular conduction. With thyrotoxicosis, there is a hyperkinetic type of hemodynamics, slowing of atrioventricular conduction, and therefore the use of cardiac glycosides is pointless. Resistance to these drugs in thyrotoxic hearts has long been noted. Refractoriness to antiarrhythmic drugs in patients with thyrotoxicosis is an indisputable fact. Amiodarone, which consists of 1/3 iodine, has a particularly harmful effect. There are descriptions in the literature of cases of the development of thyrotoxic crisis during treatment with amiodarone in patients with unrecognized thyrotoxicosis. Of course, with a thyrotoxic heart, it is necessary to prescribe drugs that improve metabolism in the myocardium: macroergs, vitamins, antioxidants, potassium and magnesium preparations.
Heart changes in thyroid cardiopathy are reversible if correction of thyroid function is started in a timely manner.
Professor Irina TERESCHENKO. Perm State Medical Academy.
Prevention of hyperthyroidism
People with a family history, women during menopause and older men need to be examined annually by an endocrinologist and take a blood test to check the level of thyroid hormones.
It is recommended to balance the diet according to the BZHU and introduce foods rich in vitamins A, B, C and minerals into the diet. Diet and hydrotherapy help not only in the prevention of hyperthyroidism, but also in the rehabilitation of patients after treatment. Regular visits to specialized sanatoriums help maintain and improve the health of the endocrine system.
Tachycardia - what tests should I take?
Among the laboratory tests necessary for correct diagnosis and the most effective treatment of tachycardia, the following are worth mentioning first:
- general blood analysis;
- lipid profile, which examines total cholesterol and triglycerides, as well as levels of “good” and “bad” cholesterol - high-density lipoproteins (HDL), low-density lipoproteins (LDL) and very low-density lipoproteins (VLDL);
- electrolyte analysis;
- tests for thyroid hormones - thyroid-stimulating hormone (TSH) and free T4, as well as a test for antibodies to thyroid peroxidase (ATPO).
In addition, to diagnose tachycardia, the cardiologist prescribes some other non-laboratory examinations: electrocardiography (including daily Holter ECG monitoring), echocardiography, etc.
If you “catch” the disease in the early stages and determine the cause of its development as accurately as possible, tachycardia can be cured completely . Therefore, under no circumstances should you be indifferent to any alarming symptoms associated with your heart rhythm, because otherwise the disease will progress, the danger will increase, and treatment will ultimately be much longer and more expensive.
Full treatment of tachycardia is not only taking medications, but also revising your own lifestyle and diet. Try to maintain normal physical activity (but without overwork), sleep at least 7–8 hours a day, reduce the consumption of animal fats, refined sugar, caffeine, and alcohol. The ideal basis of nutrition for tachycardia and other heart problems is dairy-vegetable. As prescribed by your doctor, you can take multivitamin and mineral supplements with potassium and magnesium, or take these elements separately.