Palpitations (causes and consequences)
Every year, an increasing number of people complain of palpitations, the causes of which can only be determined by a comprehensive examination. What is this phenomenon and how to deal with it?
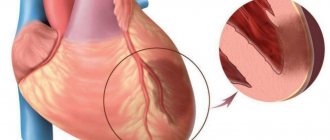
Palpitations can be caused by a variety of factors. The most common causes of its occurrence are malfunctions of the nervous autonomic system, which is responsible for the functioning of the heart. Heart rhythm disturbances such as tachycardia and arrhythmia are the result of malfunctions of this system.
In addition, the cause of rapid heartbeat can be thyroid dysfunction, anemia, myocarditis, and heart defects. Often, strong heart palpitations are caused by disorders of the functioning of the nervous system, which are accompanied by stress, internal unrest, fear, intense joy, and insomnia. Even the joyful state of falling in love adversely affects the functioning of the heart. If a person often experiences strong heart palpitations, the causes of which he cannot understand, he should definitely consult a cardiologist. Like any other malfunction of the heart, an increase in its rhythm requires a thorough examination.
A very unpleasant phenomenon is a strong heartbeat at night, which occurs after a sudden awakening. This condition can be caused by a significant increase in diastolic and systolic pressure against the background of pronounced tachycardia. The cardiovascular system is “to blame” for this phenomenon, or the patient has experienced a vegetative crisis. Such cases of rapid heartbeat require consultation with a cardiologist and an EchoCG and ECG.
Strong heartbeat is observed after physical exertion, but if it returns to normal within a short time, there is no cause for concern. If the frequency of contractions of the heart muscle exceeds one hundred beats per minute for a long time, doctors use the term “tachycardia.” In this case, the specialist establishes two necessary facts:
1. Whether the patient's heart rhythm is irregular or regular.
2. How does tachycardia develop: abruptly or gradually.
The gradual appearance of tachycardia most often occurs as a result of the action of physiological regulators that manifest themselves during strong emotions and physical exercise. Sometimes they can also appear if the patient has a high fever, anemia, or an overactive thyroid gland. Tachycardia that occurs unevenly may indicate that the patient is developing an abnormal rhythm coming from the atria (upper chambers of the heart). This condition is called atrial fibrillation.
Tachycardia, which begins abruptly, most often occurs due to a short circuit in the internal connections of the heart muscle. During the occurrence of an attack of this tachycardia, it is necessary to make an electrical recording (ECG) of the heart, since such a recording allows the doctor to accurately determine the causes of the short circuit and prescribe the necessary treatment methods.
In many clinical cases, palpitations, identified based on the patient's understanding of the causes of its occurrence and indicating that the person is experiencing a normal increase in heart rate, will not require therapeutic treatment. In such situations, you need simple reassurance and the belief that there is nothing wrong with it.
At the same time, the signs of rapid heartbeat, manifested as a result of atrial fibrillation and electrical short circuit, require a whole course of treatment, since in order to control these symptoms it is necessary to reduce the existing risk of adverse complications. There are a number of treatment options available that begin with teaching the individual how to stop an episode on their own, and end with prescribing specific medications to help prevent and stop these episodes.
The problem of tachycardia in patients with post-Covid syndrome, also known as “long COVID,” is discussed in an article by authors from Karolinska University Hospital (Stockholm, Sweden), published in The American Journal of Medicine.
Long-term symptoms (4-12 weeks or more) associated with COVID are more likely to occur in patients who have had severe illness. Due to differences in definitions, the exact prevalence of this syndrome is difficult to determine. In the longest follow-up of patients hospitalized for COVID, more than 60% reported increased fatigue and muscle weakness within 6 months of hospitalization. The article states that “long COVID” should not be considered as one syndrome; this term includes various subsyndromes and phenotypes. Typical symptoms include tachycardia, headache, weakness, shortness of breath, brain fog and others.
In his preview, the lead author, M. Ståhlberg, notes that in patients who have had COVID-19, the main interest of specialists is related to the issues of thrombus formation, peri- and myocarditis, while practically no attention is paid to the problem of long-term tachycardia, although clinical practice shows that 25-50% of these patients have tachycardia and/or complaints of palpitations for 12 weeks or more.
According to the data presented in the article, palpitations are detected in 9% of patients with post-Covid syndrome, which, according to the authors of the study, as with pain syndrome, requires a standard cardiological examination, including ECG, echocardiography and Holter monitoring of the ECG, MRI of the heart in cases of suspected peri- and myocarditis, as well as a tilt test or test with active orthostasis in the presence of symptoms of orthostatic instability to exclude postural orthostatic tachycaria (POT).
According to the authors of the article, the mechanism of tachycardia in this case is most likely of an autoimmune nature - the resulting autoantibodies can activate receptors that regulate blood pressure and heart rate (HR), as has previously been described in patients after other viral infections. Whether the same mechanisms underlie COVID-associated tachycardia is a question that requires further study. Other factors that cause an increase in heart rate may also play a role in the development of tachycardia as part of the post-Covid syndrome: hyperinflammation, hypercoagulation with thrombosis, RAAS dysfunction, desaturation due to lung damage, persistent or intermittent hyperthermia, pain, anxiety and depression, neuroinflammation and hypovolemia.
“Tachycardia can be considered a more universal and easily determined marker of post-Covid syndrome and its severity than patient complaints, blood tests or CT findings,” write the authors. Currently, it is necessary to study this issue in more detail to better understand the pathophysiological processes underlying the protracted post-Covid syndrome and to find a new target for action in the treatment of this disease.
Based on materials:
1. M. St˚ahlberg et alPost-Covid-19 Tachycardia Syndrome: A distinct phenotype of Post-acute Covid-19 Syndrome, The American Journal of Medicine (2021), doi: https://doi.org/10.1016/j .amjmed.2021.07.004 https://www.amjmed.com/
2. M. Frellick Tachycardia Syndrome May Be a Distinct Marker for Long COVID. Medscape Medical https://www.medscape.com
Text: Kozlova E.V.
Heartbeat
Some time ago I started having palpitations.
This mainly happens in the morning after, due to circumstances, I have had little sleep. At 6 am I get up normally, after which I have the opportunity to take a nap for another hour. After waking up this time, literally the moment I open my eyes, my heart starts beating so fast and so hard that I literally can’t get up!
After some time everything calms down.
Answered: 15
29/11/2007 17:32
since the conversation is not theoretical, I would refrain from describing in detail all possible options
Release form, composition and packaging
Excipients: L-arginine, sodium bicarbonate, crospovidone, magnesium stearate.
Shell composition: hypromellose, sucrose, titanium dioxide, macrogol 4000.
6 pcs. - blisters (1) - cardboard packs.
pharmachologic effect
NSAIDs. Ibuprofen, the active substance of the drug Faspik, is a derivative of propionic acid and has analgesic, antipyretic and anti-inflammatory effects due to the non-selective blockade of COX-1 and COX-2, as well as an inhibitory effect on the synthesis of prostaglandins.
The analgesic effect is most pronounced for inflammatory pain. The analgesic activity of the drug is not of the opioid type.
Like other NSAIDs, ibuprofen has antiplatelet activity.
The analgesic effect when using the drug Faspik develops 10-45 minutes after its administration.
Ankylosing spondylitis, or ankylosing spondylitis, is a term that describes a form of arthritis that primarily affects the joints of the spine. This disease usually affects the small joints between the vertebrae and reduces the mobility of these joints until the formation of ankylosis (bones fused to each other).
Pharmacokinetics
Suction
After oral administration, the drug is well absorbed from the gastrointestinal tract. After taking Faspik on an empty stomach in the form of tablets at a dose of 200 mg and 400 mg, the Cmax of ibuprofen in plasma is approximately 25 mcg/ml and 40 mcg/ml, respectively, and is achieved within 20-30 minutes.
After taking Faspik on an empty stomach in the form of a solution at a dose of 200 mg and 400 mg, the Cmax of ibuprofen in plasma is approximately 26 mcg/ml and 54 mcg/ml, respectively, and is achieved within 15-25 minutes.
Distribution
Plasma protein binding is about 99%. It is slowly distributed in and cleared from synovial fluid more slowly than from plasma.
Metabolism
Ibuprofen is metabolized in the liver primarily by hydroxylation and carboxylation of the isobutyl group. Metabolites are pharmacologically inactive.
Removal
It is characterized by two-phase elimination kinetics. T1/2 from plasma is 1-2 hours. Up to 90% of the dose can be found in the urine in the form of metabolites and their conjugates. Less than 1% is excreted unchanged in urine and, to a lesser extent, in bile.
Dosage
The drug is taken orally.
For adults and children over 12 years of age, the drug is prescribed in tablet at an initial dose of 400 mg; if necessary, 400 mg every 4-6 hours. The maximum daily dose is 1200 mg. Take the tablet with a glass of water (200 ml). To reduce the risk of developing dyspeptic side effects, it is recommended to take the drug with meals.
The drug should not be taken for more than 7 days or in higher doses. If it is necessary to use it for a longer period or in higher doses, consult a doctor.
In the form of an oral solution, the drug is prescribed to adults and children over 12 years of age, the drug is prescribed in a daily dose of 800-1200 mg (4-6 sachets of 200 mg or 2-3 sachets of 400 mg). The maximum daily dose is 1200 mg.
Before use, the contents of the sachet are dissolved in water (50-100 ml) and taken orally immediately after preparing the solution during or after meals.
To overcome morning stiffness in patients with arthritis, it is recommended to take the first dose of the drug immediately after waking up.
In patients with impaired renal, liver or cardiac function, the dose of the drug should be reduced.
Overdose
Symptoms: abdominal pain, nausea, vomiting, lethargy, drowsiness, depression, headache, tinnitus, metabolic acidosis, coma, acute renal failure, decreased blood pressure, bradycardia, tachycardia, atrial fibrillation, respiratory arrest.
Treatment: gastric lavage (only within 1 hour after administration), activated charcoal, alkaline drinking, forced diuresis, symptomatic therapy (correction of acid-base status, blood pressure).
Drug interactions
The effectiveness of furosemide and thiazide diuretics may be reduced due to sodium retention associated with inhibition of prostaglandin synthesis in the kidneys.
Ibuprofen may enhance the effect of indirect anticoagulants, antiplatelet agents, fibrinolytics (increasing the risk of hemorrhagic complications).
When administered simultaneously with acetylsalicylic acid, ibuprofen reduces its antiplatelet effect (an increase in the incidence of acute coronary insufficiency in patients receiving small doses of acetylsalicylic acid as an antiplatelet agent is possible).
Ibuprofen may reduce the effectiveness of antihypertensive drugs (including slow calcium channel blockers and ACE inhibitors).
Isolated cases of increased plasma concentrations of digoxin, phenytoin and lithium while taking ibuprofen have been described in the literature. Agents that block tubular secretion reduce excretion and increase plasma concentrations of ibuprofen.
Faspik, like other NSAIDs, should be used with caution in combination with acetylsalicylic acid or other NSAIDs, because this increases the risk of adverse effects of the drug on the gastrointestinal tract.
Ibuprofen may increase plasma concentrations of methotrexate.
Combination therapy with zidovudine and Faspic may increase the risk of hemarthrosis and hematoma in HIV-infected patients with hemophilia.
The combined use of Faspik and tacrolimus may increase the risk of nephrotoxicity due to impaired synthesis of prostaglandins in the kidneys.
Ibuprofen enhances the hypoglycemic effect of oral hypoglycemic agents and insulin; Dose adjustment may be necessary.
An ulcerogenic effect with bleeding has been described when Faspic is combined with colchicine, estrogens, ethanol, and corticosteroids.
Antacids and cholestyramine reduce the absorption of ibuprofen.
Caffeine enhances the analgesic effect of Faspik.
When Faspik is used simultaneously with thrombolytic agents (alteplase, streptokinase, urokinase), the risk of bleeding increases.
Cefamandole, cefoperazone, cefotetan, valproic acid, plicamycin, when used simultaneously with Faspic, increase the incidence of hypoprothrombinemia.
Myelotoxic drugs, when used simultaneously, increase the manifestations of Faspik's hematotoxicity.
Cyclosporine and gold preparations enhance the effect of ibuprofen on prostaglandin synthesis in the kidneys, which is manifested by increased nephrotoxicity.
Ibuprofen increases the plasma concentration of cyclosporine and the likelihood of developing its hepatotoxic effects.
Inducers of microsomal oxidation (phenytoin, ethanol, barbiturates, rifampicin, phenylbutazone, tricyclic antidepressants) increase the production of hydroxylated active metabolites of ibuprofen, increasing the risk of severe hepatotoxic reactions.
Microsomal oxidation inhibitors reduce the risk of hepatotoxicity from ibuprofen.
Pregnancy and lactation
The drug is contraindicated for use during pregnancy and lactation (breastfeeding).
The use of Faspik may adversely affect female fertility and is not recommended for women planning pregnancy.
Side effects
From the digestive system: NSAID gastropathy - abdominal pain, nausea, vomiting, heartburn, loss of appetite, diarrhea, flatulence, constipation; rarely - ulceration of the gastrointestinal mucosa (in some cases complicated by perforation and bleeding); possible - irritation or dryness of the oral mucosa, pain in the mouth, ulceration of the gum mucosa, aphthous stomatitis, pancreatitis, hepatitis.
From the respiratory system: shortness of breath, bronchospasm.
From the senses: hearing loss, ringing or noise in the ears, toxic damage to the optic nerve, blurred vision or double vision, scotoma, dryness and irritation of the eyes, swelling of the conjunctiva and eyelids (allergic origin).
From the central nervous system and peripheral nervous system: headache, dizziness, insomnia, anxiety, nervousness and irritability, psychomotor agitation, drowsiness, depression, confusion, hallucinations; rarely - aseptic meningitis (more often in patients with autoimmune diseases).
From the cardiovascular system: heart failure, tachycardia, increased blood pressure.
From the urinary system: acute renal failure, allergic nephritis, nephrotic syndrome (edema), polyuria, cystitis.
From the hematopoietic system: anemia (including hemolytic, aplastic), thrombocytopenia, thrombocytopenic purpura, agranulocytosis, leukopenia.
From laboratory parameters: possible increase in bleeding time, decrease in serum glucose concentration, decrease in creatinine clearance, decrease in hematocrit or hemoglobin, increase in serum creatinine concentration, increase in the activity of liver transaminases.
Allergic reactions: skin rash (usually erythematous or urticarial), skin itching, angioedema, anaphylactoid reactions, anaphylactic shock, bronchospasm, dyspnea, fever, erythema multiforme exudative (including Stevens-Johnson syndrome), toxic epidermal necrolysis (syndrome) Lyell), eosinophilia, allergic rhinitis.
With long-term use of the drug in high doses, the risk of developing ulcerations of the gastrointestinal mucosa, bleeding (gastrointestinal, gingival, uterine, hemorrhoidal), and visual impairment (impaired color vision, scotoma, damage to the optic nerve) increases.
Storage conditions and periods
The drug should be stored out of the reach of children at a temperature not exceeding 40°C. The shelf life of tablets is 2 years, granules are 3 years.
Indications
— febrile syndrome of various origins;
— pain syndrome of various etiologies (including sore throat, headache, migraine, toothache, neuralgia, postoperative pain, post-traumatic pain, primary algodismenorrhea);
- inflammatory and degenerative diseases of the joints and spine (including rheumatoid arthritis, ankylosing spondylitis).
Contraindications
- erosive and ulcerative diseases of the gastrointestinal tract (including peptic ulcer of the stomach and duodenum in the acute phase, Crohn's disease, UC);
- “aspirin” asthma;
- hemophilia and other bleeding disorders (including hypocoagulation), hemorrhagic diathesis;
- bleeding of various etiologies;
- diseases of the optic nerve;
- pregnancy;
- lactation period;
- children under 12 years of age;
- hypersensitivity to the components of the drug;
- history of hypersensitivity to acetylsalicylic acid or other NSAIDs.
The drug should be used with caution in patients with heart failure, arterial hypertension, liver cirrhosis with portal hypertension, hepatic and/or renal failure, nephrotic syndrome, hyperbilirubinemia, gastric and duodenal ulcers (history), gastritis, enteritis, colitis, diseases blood of unknown etiology (leukopenia and anemia), as well as in elderly patients.
special instructions
If signs of bleeding from the gastrointestinal tract occur, Faspik should be discontinued.
The use of Faspik may mask objective and subjective symptoms of infection, so the drug should be prescribed with caution to patients with infectious diseases.
The occurrence of bronchospasm is possible in patients suffering from bronchial asthma or allergic reactions in history or in the present.
Side effects can be reduced when using the drug in the minimum effective dose. With long-term use of analgesics, there is a possible risk of developing analgesic nephropathy.
Patients who experience visual disturbances during Faspik therapy should discontinue treatment and undergo an ophthalmological examination.
Ibuprofen may increase liver enzyme activity.
During treatment, monitoring of the peripheral blood picture and the functional state of the liver and kidneys is necessary.
When symptoms of gastropathy appear, careful monitoring is indicated, including esophagogastroduodenoscopy, a blood test to determine hemoglobin, hematocrit, and a stool test for occult blood.
To prevent the development of NSAID gastropathy, it is recommended to combine ibuprofen with prostaglandin E drugs (misoprostol).
If it is necessary to determine 17-ketosteroids, the drug should be discontinued 48 hours before the study.
During the treatment period, ethanol intake is not recommended.
The drug contains sucrose (1 tablet - 16.7 mg, 1 bag of granules - 1.84 g), which should be taken into account if patients have corresponding hereditary fructose intolerance, glucose-galactose malabsorption syndrome or sucrase-isomaltase deficiency.
Impact on the ability to drive vehicles and operate machinery
Patients should refrain from all activities that require increased attention and speed of psychomotor reactions.
For impaired renal function
In patients with impaired renal function, the dose of the drug should be reduced.
During treatment, monitoring of the functional state of the kidneys is necessary.
For liver dysfunction
In patients with impaired liver function, the dose of the drug should be reduced.