Treatment of acute ischemic stroke
In Russia, stroke ranks second in the structure of overall mortality and is the main cause of permanent disability: approximately 20% of patients who have had a stroke become severely disabled and require outside help. Among all types of stroke, ischemic brain damage predominates.
Ischemic stroke is a heterogeneous clinical syndrome. According to the international TOAST criteria, several pathogenetic variants of ischemic stroke are distinguished: stroke associated with damage to large arteries and developing as atherothrombosis or arterio-arterial embolism; cardioembolic stroke; microvascular (lacunar) stroke; rare forms (moya-moya syndrome, stroke due to inflammation of the vessel wall (vasculitis), dissection (dissection) of the arterial wall, etc.), as well as undifferentiated forms. Treatment and secondary prevention of ischemic stroke should be carried out taking into account its pathogenetic variant.
In the early 1990s. It has been shown that the development of infarction in the first minutes and hours of the disease occurs through rapid mechanisms of necrotic cell death. The trigger point is an energy deficiency, which initiates the so-called glutamate-calcium cascade, characterized by excessive release of excitatory aminacidergic neurotransmitters - aspartate and glutamate - and excessive intracellular accumulation of Ca2+ ions - the main trigger of the final mechanisms of the cascade, leading to cell death.
The formation of the nuclear zone (“core” of the infarction) is completed within 5–8 minutes from the moment of acute cerebrovascular accident. This area of the brain is surrounded by a potentially viable zone of the “ischemic penumbra” (penumbra), in which the level of blood flow is reduced, but energy metabolism is generally preserved and functional, but not structural, changes are present.
The formation of 50% of the total volume of the infarction occurs within the first 90 minutes from the moment of stroke development, 70-80% - within 360 minutes, and therefore the first 3-6 hours of the disease are called the “therapeutic window”, within which therapeutic measures can be taken most effective by saving the penumbra zone.
At the same time, the processes that began in the first hours of the disease retain their significance at a later date, especially when the area of ischemic damage is extensive. They induce and support other “long-term consequences of ischemia”: a genome reaction with the inclusion of genetically programmed molecular programs, dysfunction of the astrocytic and microglial cell pools with the development of immune changes and local inflammation at the site of ischemia, disruption of microcirculation and the blood-brain barrier. The time for “additional formation” of infarct changes in each case is individual and ranges from 3 to 7 days from the moment of cerebrovascular accident.
Therefore, in case of a stroke, it is very important to provide quick and pathogenetically based medical care, preferably within the first 2–3 hours from the moment of its development.
Modern understanding of the mechanisms of development of ischemic stroke has made it possible to identify two main directions of pathogenetic therapy: improving the perfusion of brain tissue (early recanalization of the vessel and reperfusion) and neuroprotective therapy.
When adequate perfusion of brain tissue is restored, clinical improvement in patients can be expected even if they do not have a visualized zone of diffusion-perfusion mismatch on MRI.
Carrying out therapeutic reperfusion is advisable within 3–6 hours, then its use significantly increases the risk of not only reperfusion damage, but also hemorrhagic complications. Thus, reperfusion should be early, active and short-term if possible.
The nature of reperfusion therapy is determined by the pathogenetic variant of stroke development. In case of occlusion of medium- and large-caliber arteries, the effectiveness of therapeutic measures is determined by the achievement of early recanalization of the vessel. With partial restoration of blood flow, such a “dramatic” improvement occurs in almost half of the patients, while in patients with no early recanalization of the affected vessel, significant clinical improvement does not occur within the first 24 hours. Moreover, in the long-term period, 3 months after a stroke, a significantly more complete restoration of impaired neurological functions is observed in patients with complete early recanalization of the occluded artery and rapid (within the first 24 hours) regression of focal symptoms.
It has been established that the severity of positive clinical dynamics depends on the speed of thrombus lysis: the best restoration of neurological functions occurs with rapid (almost instantaneous) thrombus lysis. At the same time, the rate of thrombus lysis varies with different pathogenetic variants of stroke. The most rapid and complete lysis occurs in cardioembolic stroke, which is accompanied by a significant improvement in stroke outcome and more complete functional recovery of the patient. Slow recanalization is more often observed with atherothrombotic artery disease and may not be accompanied by a significant improvement in clinical dynamics.
Spontaneous recanalization of an occluded artery occurs in approximately 10% of patients with ischemic stroke. Carrying out early ultrasound examination (transcranial ultrasound Dopplerography - devices Angiodin 3, Companion III, Sonovit SV-30, Biomed-2, Sonomed-500) increases the frequency of possible recanalization to 20%.
For most occlusions of medium and large arteries, the treatment of choice is thrombolysis, which provides early recanalization in 30-40% of cases. Currently, five generations of thrombolytics have been developed:
I generation - systemic thrombolytics: natural plasminogen activators (streptokinase, urokinase);
II generation - fibrinoselective thrombolytics: recombinant tissue plasminogen activator (rt-PA, alteplase, actylyse), recombinant prourokinase;
III generation - improved rt-PA and other plasminogen activators: fibrin-specific form of rt-PA - tenecteplase, non-glycosylated form of rt-PA - reteplase, rt-PA with a long half-life - lanoteplase, acylated streptokinase + plasminogen complex, ensuring targeted delivery to thrombus, fibrin-activated human plasminogen;
IV generation - improved plasminogen activators of the III generation (biosynthetic);
V generation - compositions of thrombolytics (rt-PA + + urokinase-plasminogen conjugate, etc.).
First generation thrombolytics are not used in clinical settings due to their systemic effect on hemostasis and the high incidence of hemorrhagic complications. Thrombolytics of III–V generations are still being tested in experimental preclinical work.
The main role in clinical practice is played by second generation thrombolytics: rt-PA and recombinant prourokinase, which have a low systemic thrombolytic effect, act predominantly on fresh thrombus and do not activate blood coagulation factors V and VII, which significantly reduces the risk of developing generalized hemorrhagic complications.
Recombinant tissue plasminogen activator is recommended for use in the first 180 minutes after the development of ischemic stroke caused by occlusion of an artery of medium and large diameter, in the absence of a hemorrhagic component in the ischemic focus and an area of extensive hypodensity on CT/MRI of the brain, exceeding 1/3 of the area of the middle cerebral artery , with systemic blood pressure values not higher than 180/110 mm Hg. Art. A dose of 0.9 mcg/kg should be used, maximum 90 mg/day; 10% of the dose is administered intravenously as a bolus, the remaining 90% is administered intravenously by drip over 60 minutes.
The use of recombinant prourokinase is accompanied by vessel recanalization in 40% of cases, but causes hemorrhagic complications in 10.2% of patients. The use of the drug is advisable for angiographically confirmed occlusion of a large artery (internal carotid, middle cerebral, main). Recombinant prourokinase is used intraarterially, accompanied by low doses of intravenous heparin. An improvement in the outcome of the disease is recorded within 3 months of observation, even with the administration of the drug delayed by 6 hours from the onset of acute ischemic stroke. This suggests that thrombolytic therapy may be effective beyond the three-hour window if patients are carefully selected.
One of the promising areas of recanalization is surgical removal of the thrombus - endovascular extraction or excision. The results of the completed Merci Retriever Study, evaluating the effectiveness of endovascular thrombus extraction using Concentric Medical Inc. technologies, showed that rapid (instant) recanalization of an occluded vessel occurs in 48% of cases, and recanalization within the first day in 81% of cases. No complications arising from the manipulation were identified.
Contraindications to early recanalization of an occluded artery are: late admission to the hospital (outside the “therapeutic window”); absence of occlusion of medium and large diameter confirmed by transcranial Dopplerography (hemodynamic, lacunar and other pathogenetic variants of stroke); hemorrhagic syndrome of any localization and etiology observed in the patient in the last 3 months before the stroke; tumors, injuries; surgeries undergone in the last 6 weeks before the stroke; treatment-resistant arterial hypertension with blood pressure above 180/110 mmHg. Art.
Theoretical data suggested that anticoagulants, in particular heparin, should be effective in ischemic stroke. However, international studies (International Stroke Trial Collaborative Group) have shown that when treating patients with ischemic stroke with heparin, the high risk of early hemorrhage exceeds the positive effect of therapy. Only a post-hoc subgroup analysis proved the feasibility of using anticoagulant therapy with heparin in the first days of progressive atherothrombotic stroke, as well as in cases of confirmed cardiogenic embolism and surgical interventions on cerebral vessels. Heparin is prescribed during the first 3–5 days of the disease in a daily dose of up to 10–15 thousand units. under the control of laboratory parameters, primarily APTT (which should not increase more than 2 times). 1–2 days before the end of the course of heparin treatment, it is advisable to gradually reduce its dose with the prescription of indirect anticoagulants (acenocoumarol, warfarin, ethyl biscoumacetate), which continue to be taken for the next 2–3 weeks. The most effective use of warfarin is at a dose of 2–5 mg/day, especially with long-term previous heparin therapy, in the presence of atrial fibrillation, after heart valve replacement or concomitant myocardial infarction. In the absence of concomitant cardiac pathology, it is possible to prescribe phenyline in a daily dose of 0.03–0.06 g. It should be remembered that treatment with indirect anticoagulants must also be carried out under strict laboratory monitoring of coagulogram parameters. The biological activity of heparin depends on the plasma protease inhibitor antithrombin-3. Therefore, in case of antithrombin-3 deficiency, patients with increasing thrombosis of the main or internal carotid artery are recommended to administer blood plasma (albumin, dextran - 100 ml 1-2 times a day) simultaneously with heparin.
Among the non-hemorrhagic complications of heparin therapy, transient thrombocytopenia should be noted (in 25% of patients, with 5% severe), as well as paradoxical thromboembolism (Reilly et al., 2001), due to heparin-induced platelet aggregation. Thromboembolic complications caused by the use of heparin are treated by stopping its administration and prescribing indirect anticoagulants.
Thus, the administration of heparin in the first days of ischemic stroke can be recommended only for a limited number of patients. However, it has now been shown that, in contrast to conventional heparin, low molecular weight heparins (LMWHs) with a molecular weight of 4000–5000 daltons (enoxaparin (Clexane), fraxiparin, fragmin, clevarin, etc.) have predominantly anti-factor Xa activity, and inhibit even those factor Xa molecules that have managed to contact the surface of platelets. The advantages of LMWH are also: less binding to the vascular endothelium and plasma proteins, which leads to better digestibility of these drugs and their rapid absorption from subcutaneous fat depots (after subcutaneous administration, 90% of LMWH is “absorbed” and only 15–30% of conventional heparin); a longer half-life (possibly their subcutaneous administration 1-2 times a day and less frequent laboratory monitoring); lower affinity for von Willebrand factor, which helps to reduce the effect of these heparins on the cellular component of hemostasis (platelets) and significantly reduce the risk of developing “heparin thrombocytopenia/thrombosis”, and also makes it possible to better predict anticoagulant effects even when using high doses of drugs. Hemorrhagic complications with the use of LMWH are generally rarer and less severe than with treatment with conventional heparin. It is important that these drugs prevent the risk of developing deep vein thrombosis and pulmonary embolism - one of the most dangerous complications of the acute period of stroke.
Hemodilution and antiplatelet therapy, without having a radical reperfusion effect, somewhat improve microcirculation in brain tissue, which serves as the basis for their traditional use in the first days of ischemic stroke under the control of hemorheological and cardiovascular parameters.
Hemodilution is carried out with low molecular weight dextrans (reopoliglucin, reomacrodex, longasteril, reochem - 250-500 ml intravenously). The main benchmark for the effectiveness of hemodilution is to reduce the hematocrit level to 30-35%.
A comparative comparison of the effects of various antiplatelet drugs showed the high effectiveness of acetylsalicylic acid (thrombo ACC, aspirin cardio) at a dose of 1 mg/kg/day in the absence of a sufficient antiaggregation effect of smaller doses of the drug, which is associated with their insufficient effect on cAMP and prostacyclin concentration. The effectiveness of pentoxifylline (trental, flexital, pentilin), which has a complex rheological effect aimed not only at reducing the aggregation ability of platelets, but also at improving the deformability of erythrocyte membranes and normalizing microcirculation in general, has also been established. In young patients with a hyperkinetic type of blood circulation (severe tachycardia, persistent increase in systolic blood pressure), it is preferable to choose small doses of β-blockers (obzidan, anaprilin, inderal), which have antiaggregation properties. In elderly patients, it is advisable to prescribe angioprotectors (anginin, prodectin, parmidin), which also have an antiplatelet effect.
Drugs with complex vascular-metabolic action, a prominent example of which is Cavinton (vinpocetine), have a positive effect on the state of cerebral hemodynamics. An analysis of 25 years of experience in using the drug has shown that Cavinton improves cerebral blood flow and microcirculation, exerting a selective vasodilating and antivasoconstrictor effect on cerebral vessels, inhibiting aggregation and adhesion of blood cells, and normalizing the deformability of erythrocyte membranes. Along with this, the drug helps improve energy metabolism, optimizing redox processes, activating the transport of oxygen and glucose, as well as their utilization in brain tissue. Cavinton has antioxidant and anti-excitotoxic properties, normalizes the ionic gradient of cell membranes. For ischemic stroke in the acute phase, it is effective to prescribe the drug at a dose of 10–20 mg/day intravenously (diluted in 500 ml of physiological solution) for 7–10 days (in some cases up to 21 days) with the subsequent transfer of the patient to tablet forms drug: Cavinton forte - 10 mg 3 times a day for 3-4 weeks, then Cavinton - 5 mg 3 times a day for 1-3 months.
Active reperfusion therapy is possible only in a hospital after a neuroimaging study (CT/MRI of the brain), which allows to exclude the hemorrhagic component of the lesion, assess the size of the ischemic area and the pathogenetic variant of the stroke. This highlights the advantages of another direction of therapy - neuroprotection (cytoprotection, metabolic protection of the brain), which can be used at the prehospital stage when the first symptoms of a stroke appear, even if it is possibly hemorrhagic.
Primary neuroprotection is aimed at interrupting the rapid mechanisms of necrotic cell death - reactions of the glutamate-calcium cascade. The use of this type of neuroprotection should begin from the first minutes of ischemia and continue treatment throughout the first 3 days of stroke, especially active in the first 12 hours. Secondary neuroprotection is aimed at reducing the severity of “long-term consequences of ischemia,” i.e., blocking proinflammatory cytokines, cellular molecules adhesion, inhibition of pro-oxidant enzymes, increased trophic supply, temporary inhibition of apoptosis. It can be started 3–6 hours after the onset of stroke and should continue for at least 7 days.
The discovery of the phenomenon of excitotoxicity resulted in the creation of new therapeutic strategies—drugs antagonists of glutamate NMDA and AMPA receptors and inhibitors of presynaptic glutamate release. Despite the fact that drugs from these groups experimentally demonstrated pronounced neuroprotective effects, clinical trials of most of them were discontinued due to a wide range of serious side effects (mental, locomotor, general toxic).
Currently, studies are ongoing on the effectiveness of remacemide, a low-affinity non-competitive antagonist of NMDA receptors that has the ability to inhibit voltage-gated calcium channels. In clinical trials of intravenous and oral forms of remacemide at a dose of up to 400 mg every 12 hours, no significant side effects were found.
Another drug that blocks NMDA-gated channels in a voltage-dependent manner is magnesium sulfate.
The attention of researchers is drawn to the role of the inhibitory neurotransmitter glycine in the mechanisms of acute cerebral ischemia. The role of glycine as an inhibitory neurotransmitter has been proven in almost all parts of the central nervous system. G. E. Fagg and A. C. Foster, F. Mayor et al. concluded that GABA and glycine are equivalent neurotransmitters that provide protective inhibition in the central nervous system, the role of which increases under conditions of increased glutamate release. Glycine exhibits its inhibitory properties through interaction not only with its own glycine receptors, but also with GABA receptors.
At the same time, JW Johnson and P. Ascher (1987) were the first to experimentally prove that glycine in submicromolecular concentrations is necessary for the normal functioning of glutamate NMDA receptors. The potentiating effect of glycine on NMDA receptors occurs at concentrations below 0.1 µM, and concentrations from 10 to 100 µM completely saturate the glycine site. Administration of high concentrations of glycine (100 µmol and 1 mlmol) to rats under conditions of oxygen deprivation did not cause long-term modulation of NMDA receptor activity in the hippocampus and did not increase excitotoxicity. Interestingly, administering high doses of glycine or some of its agonists to animals (1-amino-1-carboxycyclopropane, which is an almost complete agonist, and D-cycloserine, which has 40–60% of the effectiveness of glycine) has an anticonvulsant effect and also enhances the effects of antiepileptic drugs.
Along with the neurotransmitter, glycine also has a general metabolic effect and binds low molecular weight toxic products that are formed in large quantities during ischemia.
Being a natural brain metabolite, glycine does not exhibit toxicity even in doses of more than 10 g/day. The only side effect of the drug can be considered mild sedation. The use of glycine in a dose of 1–2 g/day for 5 days in patients with acute ischemic stroke (starting 6 hours after the development of the first symptoms) allows for anti-ischemic protection of the brain in patients with different locations of vascular lesions and different severity of the condition - it significantly accelerates regression neurological symptoms (p < 0.01), improves functional recovery of patients and reduces 30-day mortality compared to the placebo group. A significant reduction in the volume of cerebral infarction and inhibition of subsequent cystic transformation of the lesion with the use of glycine, as well as accelerated normalization of the electroencephalographic pattern, have been proven.
An important area of secondary neuroprotection is antioxidant therapy. In the 1980s It was found that in the very early period of acute focal cerebral ischemia it is advisable to use “traps” of free radicals and drugs that destroy peroxides (with sulfide and thiol groups): 2,3-dimercaptopropanesulfonate (unithiol, antaxone, dimercaprol, dicaptol, dithioglycerol), thiosulfate sodium, etc. Following this, the administration of tocopherols and carotenoids, which bind catalysts and inactivate singlet oxygen, was recommended. However, attempts to use unithiol and tocopherol (vitamin E, including in a combined form - aevit) in the complex of intensive therapy for ischemic stroke showed the insignificance of the “contribution” of these drugs to the overall result of treatment.
Currently, potential neuroprotectors used in cerebral ischemia also include the enzymes superoxide dismutase (SOD) and catalase, glutathione, lazaroids, iron chelates, and phenyl-t-butyl-nitrone. Experimental and clinical trials of selective blockers of neuronal NO synthase [7-nitroindazole and 1-(2-fluoromethylphenyl)-imidazole], which significantly reduced the size of the infarct zone after focal and global cerebral ischemia in animals, are ongoing. Relatively selective blockade of iNO synthase with aminoguanidines also had a powerful neuroprotective effect in experimental stroke. Aminoguanidines have protective properties even when treatment is delayed by 24 hours, which is of obvious interest in terms of their possible clinical use in the treatment of ischemic stroke.
Of great interest is the organoselenium compound ebselen, which has glutathione peroxidase-like activity. Ebselen is able to suppress oxidative stress and inflammatory reactions, inhibiting the mitochondrial component of apoptosis, the induction of which is associated with the release of cytochrome C.
Unlike many other organoselenium compounds, ebselen has low toxicity.
In the course of experimental and clinical studies, the domestic drug Mexidol showed high efficiency. When administered intravenously at a dose of 100 to 1000 mg/day, Mexidol has a pronounced antioxidant effect, increasing the activity of the endogenous antioxidant system and reducing the severity of free radical processes.
The domestic drug emoxypine, a derivative of 3-hydroxypyridine, has an antioxidant effect. The main effects of emoxypine are inhibition of lipid peroxidation and activation of the antioxidant system, changes in the activity of membrane-bound enzymes and modification of the metabolic, receptor and transport functions of cell membranes. The drug is safe and well tolerated by patients.
An important area of neuroprotective therapy is the use of drugs with neurotrophic and neuromodulatory properties.
Endogenous regulators of CNS functions—neuropeptides—play a very important role. Their molecules, which are short amino acid chains, are “cut” from larger protein precursor molecules by proteolysis enzymes (“processing”) only “in the right place and at the right time,” depending on the needs of the body. Neuropeptides last only a few seconds, but their duration of action can be measured in hours. Each of the regulatory peptides is capable of inducing or inhibiting the release of a number of other peptides. As a result, the primary effects of a particular peptide can develop over time in the form of chain and cascade processes.
The physiological activity of neuropeptides is many times greater than that of non-peptide compounds. Depending on the site of their release, neuropeptides can perform a mediator function (signal transmission from one cell to another), modulate the reactivity of certain groups of neurons, stimulate or inhibit the release of hormones, regulate tissue metabolism or function as effector physiologically active agents (vasomotor, Na+-uretic and other types of regulation). It is known that neuropeptides are able to regulate the activity of pro- and anti-inflammatory cytokines through modulation of the activity of their receptors. Many neuropeptides exhibit pronounced neurotrophic growth properties and easily penetrate the blood-brain barrier.
One of the most well-known drugs of the neurotrophic series is Cerebrolysin - a protein hydrolyzate of an extract from the brain of pigs, the active effect of which is due to the fraction of low molecular weight peptides. The drug optimizes brain energy metabolism and calcium homeostasis, stimulates intracellular protein synthesis, slows down the processes of the glutamate-calcium cascade and lipid peroxidation. The optimal daily dose for moderate ischemic stroke is 10 ml, for severe strokes - 20 ml intravenously for 7-10 days of illness (further continuation of the course in the form of intramuscular injections of 5 ml per day until 21 days of illness is possible). In the acute period of carotid ischemic stroke, doses of 30–50 ml are more effective compared to 10–20 ml.
According to our data, the use of Cerebrolysin in patients with ischemic stroke in the carotid system at a dose of 50 ml/day intravenously significantly inhibits the growth of the infarction zone (by the 3rd day of the disease), and also normalizes the electroencephalographic pattern, compared with a dose of 10 ml .
At the Research Institute of Molecular Genetics of the Russian Academy of Sciences, a synthetic analogue of the ACTH fragment was created - the drug Semax, which is a heptapeptide devoid of hormonal activity. Semax is the first Russian non-depleting nootropic drug from the group of neuropeptides, which has a number of important advantages over well-known analogues: complete absence of toxic and side effects, hormonal activity, an increase in the duration of action by more than 24 times compared to the natural analogue, the possibility of intranasal administration with real penetration into the brain.
According to the results of a randomized, double-blind, placebo-controlled study conducted at the neurological clinic of the Russian State Medical University, the use of the drug in daily doses of 12-18 mcg/kg for 5 days leads to a significant reduction in 30-day mortality and improved clinical outcome and functional recovery of patients with ischemic heart disease. stroke initially of varying severity.
The use of drugs that affect energy metabolism and redox processes in nervous tissue does not go unnoticed. It has been established that the use of antihypoxants (short-acting barbiturates, benzodiazepines) is advisable only for the most severe forms of ischemic strokes.
For limited cortical foci of ischemia, clinically manifested by disorders of higher mental functions (primarily speech) and moderate motor deficits, the administration of nootropic drugs (GABA derivatives) that activate energy metabolism and redox processes in the brain is effective. A study of the dose-dependent effectiveness of piracetam (nootropil, lucetam, memotropil) showed that the optimal doses of the drug in the first 10–15 days of ischemic stroke range from 6 to 12 g/day when administered intravenously. To achieve the maximum clinical effect, long-term use of the drug is recommended (from the 15th day - oral administration at a dose of 4.8 g / day for 1-1.5 months), given the delayed neurotransmitter effect of piracetam, which increases the plasticity of nervous tissue.
Starting from the first days of the disease, after the formation of morphological infarct changes in the brain, reparative therapy aimed at improving the plasticity of healthy tissue surrounding the infarction, activating the formation of polysynaptic connections, and increasing the density of receptors is becoming increasingly important. Secondary neuroprotectors with trophic and modulatory properties, as well as nootropics (GABA derivatives), choline derivatives (gliatilin) enhance regenerative and reparative processes, helping to restore impaired functions.
Gliatilin (a-glycerylphosphorylcholine) is a compound containing 40% choline and is converted in the body into the metabolically active form of phosphorylcholine, capable of penetrating the blood-brain barrier and activating acetylcholine biosynthesis in the presynaptic membranes of cholinergic neurons. Pilot clinical studies of gliatilin in the acute period of ischemic stroke (intravenous administration at a dose of 1 g 3–4 times a day for 5 days) revealed a beneficial effect of the drug on clinical dynamics, especially on the mental activity of patients, memory, and restoration of speech functions.
A study of the effectiveness of the domestic drug aplegin (carnitine chloride) in the acute period of carotid ischemic stroke showed that its administration at a daily dose of 7–15 mg/kg during the first 7–10 days of the disease significantly improves the clinical course and outcome of the stroke. The drug has an “awakening” effect in seriously ill patients, accelerating the regression of focal neurological symptoms and mental dysfunction.
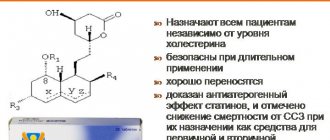
It is important to note that the treatment of acute ischemic stroke also includes components of its secondary prevention. Secondary prevention becomes especially relevant from the second week of the disease, when the risk of repeated vascular episodes increases significantly. The most significant preventive measures include monitoring blood pressure, glucose and blood lipids with correction of identified changes. In recent years, the importance of using antihypertensive drugs from the group of ACE inhibitors (captopril, enalapril, quinapril, lisinopril, moexipril, perindopril, ramipril, cilazapril, fosinopril) has been proven not only in patients suffering from severe arterial hypertension, but also in people with borderline and even normal hypertension. blood pressure values. This is due to the “additional” effects of ACE inhibitors - their normalizing effect on the structure and functional state of the vascular wall of arteries of different sizes (from the main arteries of the head to arterioles). The most important and proven area of secondary prevention is the long-term (often lifelong) use of true antiplatelet agents: acetylsalicylic acid (including in combination with dipyridamole (Curantil, Persantine)), clopidogrel (Plavix), ticlopidine (Aklotin, Tagren, Tiklid), high doses of dipyridamole. At the same time, in patients who have suffered a cardiogenic embolism against the background of atrial fibrillation, after heart valve replacement, after myocardial infarction, it is advisable to prescribe the indirect anticoagulant warfarin. In this group of patients, warfarin causes a reduction in the relative risk of recurrent stroke by 36–47% compared with aspirin, with a comparable frequency of hemorrhagic complications (1.3 and 1.0%). If hemodynamically significant stenoses of the carotid arteries are detected, as well as the presence of “embologenic” atherosclerotic plaques in them, a consultation with a vascular surgeon is necessary to decide on an endarterectomy or another method of surgical prevention of recurrent cerebrovascular accident. The presence of severe dyslipidemia, not correctable by diet, requires the prescription of lipid-lowering therapy (statins - lovastatin, simvastin, atorvastatin, pravastatin, fluvastatin, cerivastatin; fibrates - bezafibrate, fenofibrate, ciprofibrate).
Thus, improving the understanding of the causes and mechanisms of damage to brain tissue against the background of acute cerebrovascular accident determines the main strategic directions for the treatment of cerebral stroke. The results of clinical and experimental studies in recent years indicate the need for early (within the “therapeutic window”) combined pathogenetic therapy of ischemic stroke, including early recanalization of an occluded vessel and reperfusion of brain tissue, combined neuroprotection, stimulation of regenerative and reparative processes, as well as components of secondary prevention (prevention of (re-)embolism, secondary vascular and tissue damage).
The introduction of modern approaches to the treatment of ischemic stroke in the clinic of nervous diseases of the Russian State Medical University (Department of Fundamental and Clinical Neurology) made it possible to achieve significant success in the treatment of patients with ischemic stroke: reduce 30-day mortality from 32 to 10% within 5 years and increase the number of patients with good functional recovery (Barthel index >75; modified Rankin scale - 0–2) to 73.7% of the total number of surviving patients.
Increasing knowledge about ischemic brain damage and the regeneration of brain tissue allows us to more and more clearly imagine the endless complexity of understanding these processes. Fortunately, working in a clinic, we have the opportunity not to move away from the realities of life and daily evaluate the application of scientific hypotheses in practice. This helps to maintain optimism and hope.
V. I. Skvortsova , Doctor of Medical Sciences, Professor, Corresponding Member of the Russian Academy of Medical Sciences, Russian State Medical University, Moscow
Recovery in a boarding house
Rehabilitation of an elderly person after a stroke at home requires the full-time employment of one of the relatives for the next six months. While the patient is bedridden, he cannot be left at home alone for two reasons: he is helpless and it is necessary to deal with him several times a day. The volume of care is so large and multidirectional that soon the caregiver himself will need support and replacement.
In boarding houses for the elderly, professionals are involved in the rehabilitation of patients. Special equipment is used for treatment. An individual program is developed for each patient. Restoration methods that have been developed over the years produce positive results within a month.