Coronary angiography
MITRAL VALVE SURGERY – MITRAL COMISSUROTOMY
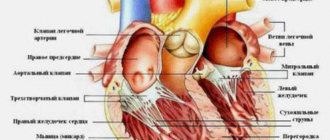
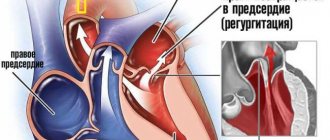
The mitral valve (bicuspid valve) of the heart is the valve between the left atrium and the left ventricle of the heart. It is represented by two connective tissue plates that prevent, during left ventricular systole, regurgitation (backflow) of blood into the left atrium.
Various pathological processes, both acquired and congenital, can cause valve dysfunction.
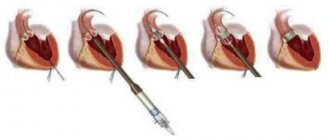
Mitral commissurotomy is a method of surgical treatment of congenital or acquired pathology of the mitral valve. MITRAL COMISSUROTOMY is a surgical operation whose purpose is to dissect the adhesions of the petals of the mitral heart valve (commissures).
Mitral commissurotomy is performed to treat mitral stenosis, a pathological narrowing of the mitral valve of the heart (between the left atrium and ventricle). The operation is performed on all patients with mitral stenosis, with the exception of the first stage of the disease, when the body’s own compensatory mechanisms allow it to easily cope with the resulting disorders.
There are two types of mitral commissurotomy:
Closed
Open
CLOSED MITRAL COMISSUROTOMY
Closed mitral commissurotomy is a surgical separation of the fusions of the left atrioventricular orifice in case of its stenosis.
This operation is performed on a closed heart without the use of artificial (extracorporeal) circulation from a left- or right-sided thoracotomy approach.
After transesophageal echocardiography has excluded the presence of a thrombus in the left atrial appendage, a purse-string suture is placed on it, the heart cavity is penetrated and a digital inspection is performed, thus assessing the condition of the mitral valve, determining the degree of its stenosis (narrowing) and the mobility of the leaflets.
Loose adhesions can be separated manually, dense adhesions - only with the help of special instruments (dilators, commissurotomes), which expand the narrowed mitral orifice to a diameter of 3.5-4 centimeters.
In order to reduce the risk of embolism of the arterial vessels of the brain, it is recommended to apply pressure on the carotid arteries at the time of performing this manipulation.
The inability to perform an adequate closed commissurotomy is considered a reason to switch to extracorporeal circulation and perform open surgery.
Since the 1950s, since the introduction of artificial blood circulation into practice, closed mitral commissurotomy has been replaced by prosthetics.
The use of closed commissurotomy is now considered justified only when it is not possible for one reason or another to perform artificial circulation. Closed mitral commissurotomy is sometimes performed in pregnant women with severe mitral stenosis.
With closed commissurotomy, there is a high probability of mitral regurgitation and embolism, especially in the case of atrial thrombosis and the presence of valve calcification.
OPEN MITRAL COMISSUROTOMY
Open mitral commissurotomy is an operation that involves performing a median sternotomy and switching to extracorporeal circulation with so-called bicaval cannulation.
Open mitral commissurotomy is used when there is mild damage to the patient’s valve apparatus in the absence of massive calcification.
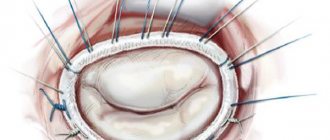
The surgeon enters the left atrium from its posterior surface in the area of the interventricular groove. After the mitral valve is exposed, they begin the commissurotomy itself, first of all identifying the area of fusion of the leaflets, and then cutting this area in the direction from its free end to the fibrous ring. Then the chordae tendineae are carefully separated and the incision continues longitudinally to the papillary muscles.
In this case, small calcium deposits can be locally removed, while being careful not to damage the valve apparatus in any way.
Finally, the left atrium incision is sutured and artificial circulation is stopped.
Unlike closed commissurotomy, the open technique allows for more reliable removal of blood clots from the atrium and dissection of existing adhesions.
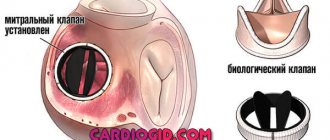
Open mitral commissurotomy gives excellent long-term results, but is associated with the risk of developing restenosis or valve insufficiency. Only a small proportion (no more than 7%) of all patients subsequently require mitral valve replacement.
Indications for surgery for mitral insufficiency
The annual mortality rate for drug treatment of symptomatic mitral valve prolapse is 6.3%. In these patients, a strategy of early surgery after diagnosis is associated with a better long-term prognosis, resulting in improved contractility and regression of left ventricular hypertrophy, and a decrease in left heart volumes. On the other hand, in patients with significantly damaged contractile function of the left ventricle, improvement in the latter may not occur. In this regard, surgical treatment of mitral regurgitation should be carried out before the CSI index reaches 40-50 ml/m2, since its increase ≥60 ml/m2 suggests an unfavorable prognosis. Other good prognostic markers for adverse effects of surgery are high NYHA functional class, concomitant coronary pathology, older age, and low right ventricular ejection fraction. Mitral valve plastic surgery for myxomatous lesions suggests good early and long-term functional results. Because fewer complications and lower in-hospital mortality are observed with valve repair compared with valve replacement, surgery should be performed early after diagnosis. The appearance of left ventricular systolic dysfunction dictates the need to maintain anullopapillary continuity during surgical correction. Surgical correction of ischemic mitral regurgitation is associated with a higher risk (9-30%), which is due to a decrease in the contractile function of the left ventricular myocardium. Mitral valve plasty is indicated for grade III-IV mitral valve insufficiency and preserved contractility of the left ventricle (co-option height ≤ 11 mm). In patients with reduced contractility (EF≤40%), grade II mitral insufficiency is also an indication for correction. In case of rupture of the papillary muscle or cooptation height ≥ 12 mm, bioprosthetic replacement of the mitral valve with preservation of the subvalvular apparatus is indicated. Patients who have undergone plastic surgery on the mitral valve, due to the preservation of the integrity of the fibrous framework of the left ventricle, have better survival and long-term prognosis. The 5-year survival rate after mitral valve repair compared to mitral valve replacement is 58-64% and 36-47%, respectively. According to the Mayo Clinic, the 5-year mortality rate in patients with ischemic mitral regurgitation with medical treatment was 62%, while with surgical treatment it was 39%. Patient survival is inversely proportional to the size of the effective regurgitant opening and the volume of the regurgitant jet. The 5-year survival rate in patients with an effective regurgitation orifice area of ≤20 mm2 and ≥ 20 mm2 was 47% and 29%, and in patients with a regurgitation volume of ≤30 ml and ≥ 30 ml, 44% and 35%, respectively. At the same time, in patients with organic mitral regurgitation, the area of the effective regurgitation orifice ≥40 mm2 is a risk factor that determines the same long-term survival. If surgical correction of mitral regurgitation is performed before volume overload myopathy reaches an irreversible stage, left ventricular function returns to normal. On the other hand, a delay in surgery, even with a favorable course of the postoperative period, leads to persistence of signs of congestive heart failure 5, 10, and 14 years after surgery in 23%, 33%, and 37% of patients. III/IV preoperative NYHA functional class is a prognostic sign that determines the long-term prognosis of patient survival. A decrease in ejection fraction after mitral replacement for mitral regurgitation is the result of a postoperative increase in afterload. Activation of the neurohumoral system may contribute to the deterioration of heart failure patients by limiting vasodilation. The persistence of excessive neurohumoral activation probably reflects incomplete restoration of left ventricular contractility after surgical treatment.
Preoperative preparation
In patients with signs of congestive heart failure, aggressive diuretic therapy and sodium restriction are required before surgery. For atrial fibrillation, patients need digoxin, beta blockers, and calcium antagonists to slow the heart rate. Patients with acute mitral regurgitation are often in cardiogenic shock; their condition is stabilized with the help of inotropes, arterial vasodilators, and intra-aortic balloon counterpulsation. Vasodilators reduce peripheral vascular resistance and accelerate the rhythm, reducing the amount of regurgitation into the left atrium.
Papillary muscle translocation
In ischemic mitral regurgitation, regurgitation is caused by displacement of the posterior papillary muscle towards the apex of the left ventricle, which impairs co-option of the mitral valve leaflets. In this regard, moving the posterior papillary muscle closer to the mitral valve annulus reduces posterior leaflet tension and improves cooptation. The technique for performing this procedure involves placing a traction suture through the papillary muscle and fixing it to the posterior part of the mitral valve annulus.
Posterior papillary muscle translocation technique
Operations on chordae and papillary muscles of the mitral valve
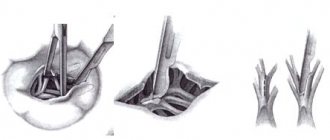
Surgeries on the chordae and papillary muscles of the mitral valve are used, as a rule, for prolapse of the anterior leaflet. The most commonly used types of operations are shortening, translocation and creation of artificial chords. Methods for shortening the chordae were first proposed by A. Carpentier for the correction of prolapse of the anterior leaflet of the mitral valve. Pathologically elongated chords are shortened by tucking them into the previously dissected papillary muscle, or by fixing the excess part of the chords to the edge of the anterior leaflet of the mitral valve. The durability of these methods is questionable, since in the long term there is a possibility of rupture of shortened chords.
Technique for shortening chords Translocation of chords involves moving the chords of the posterior valve that are normal in length to the prolapsing area of the anterior valve. To do this, a quadriangular resection of the posterior part and fixation to the prolapsed part of the anterior mitral valve leaflet is performed. The advantage for this procedure is that precise measurement of the length of the translocated chordae is unnecessary, since they always have a natural length to ensure normal co-option of the valves. The defect in the posterior leaflet is restored using the method described above.
Technique for translocation of posterior chords to the anterior mitral valve leaflet The creation of artificial chords is also used to correct prolapse of the anterior mitral valve leaflet. For this purpose, a thread made of polytetrafluoroethylene (4\0-5\0) is used, which has sufficient strength to ensure acceptable durability. The neochordae pass through the head of the papillary muscle and the free edge of the anterior leaflet of the mitral valve. The length is carefully measured to create a good co-option of the valves.
Creation of artificial chords
Related operations
Coronary artery bypass grafting is the most common procedure performed to correct mitral heart disease, especially of ischemic origin. Distal anastomoses on the posterior and inferior surfaces of the heart must be performed before valve replacement to prevent injury to the posterior wall of the left ventricle during cardiac enucleation. Correction of tricuspid valve disease is performed after completion of manipulations on the mitral valve. For this, additional access is used through the wall of the right atrium, if it was not opened when accessing the mitral valve. If it is necessary to correct concomitant aortic heart disease, the sequence of actions is as follows: first, the aortic valve is excised, then the mitral valve is corrected, and the aortic prosthesis is implanted last. Before removing the aortic clamp, careful prophylaxis of air embolism through the left ventricular apex, ascending aorta, and left atrium drainage is performed using volume loading and increased pulmonary excursion. Postoperative arrhythmias are a fairly common occurrence. Drug correction is carried out with amiodarone, β-blockers, digoxin. In patients with tachyform atrial fibrillation, if there is no effect of therapy, cardioversion may be performed. Bradyarrhythmias require more frequent pacing. Anticoagulant therapy is indicated in all patients with mechanical or biological valves. Warfarin is prescribed on the second postoperative day under the control of the international normalized ratio (INR), which should be 2.5-3.5. For patients with bioprostheses and sinus prostheses, therapy lasts 6-12 weeks, for patients with atrial fibrillation and mechanical prostheses - for life.
Mitral valve repair
A. Carpentier in 1983 theoretically substantiated and first performed plastic surgery on the mitral valve. He proposed three main types of correction: type I - remodeling of the mitral valve ring using the implantation of a rigid or flexible plastic ring, ensuring restoration of its normal area and shape; Type II – elimination of excess mobility of the leaflets by resection of the corresponding segment of the leaflets, shortening and translocation (from the posterior to the anterior leaflet) of the mitral valve chords; Type III – restoration of mobility of chords and valves (fenestration of primary chords and papillary muscles, resection of secondary chords). To simplify the understanding of plastic procedures on the mitral valve, A. Carpentier proposed a surgical anatomical classification of the mitral valve: the anterior leaflet is divided into three portions (AI, A2, A3) and the posterior leaflet is also divided into three portions (PI, P2, P3). S. Duran (1994) divides the anterior leaflet into two portions (AI, A2), the posterior leaflet into three portions (PI, PM, P2) and identified commissural portions (CI, C2)
Surgical anatomy of the mitral valve. 1 - according to A. Carpentier, 2 - according to S. Duran