How the heart works
The heart
is a muscular pump that ensures continuous movement of blood through the vessels.
Together, the heart and blood vessels make up the cardiovascular system. This system consists of the systemic and pulmonary circulation. From the left side of the heart, blood first moves through the aorta, then through large and small arteries, arterioles, and capillaries. In the capillaries, oxygen and other substances necessary for the body enter the organs and tissues, and from there carbon dioxide, metabolic products, are removed. After this, the blood turns from arterial to venous and again begins to move towards the heart. First along the venules, then through smaller and larger veins. Through the inferior and superior vena cava, blood again enters the heart, only this time into the right atrium. A large circle of blood circulation is formed. Venous blood from the right side of the heart is sent through the pulmonary arteries to the lungs, where it is enriched with oxygen, and returns to the heart again - this is the pulmonary circulation.
Inside, the heart is divided by partitions into four chambers. The two atria are divided by the interatrial septum into the left and right atria. The left and right ventricles of the heart are separated by the interventricular septum. Normally, the left and right parts of the heart are completely separate. The atria and ventricles have different functions. The atria store blood that flows into the heart. When the volume of this blood is sufficient, it is pushed into the ventricles. And the ventricles push blood into the arteries, through which it moves throughout the body. The ventricles have to do more hard work, so the muscle layer in the ventricles is much thicker than in the atria. The atria and ventricles on each side of the heart are connected by the atrioventricular orifice. Blood moves through the heart in only one direction. In the systemic circle of blood circulation from the left side of the heart (left atrium and left ventricle) to the right, and in the small circle from the right to the left.
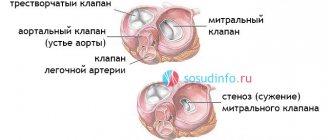
The correct direction of blood flow is ensured by the valve apparatus of the heart:
- tricuspid
- pulmonary
- mitral
- aortic
They open at the right time and close, preventing blood flow in the opposite direction.
Diseases
In patients in good health, the heart valve apparatus functions clearly and stably. When changes occur, the heart valves are subject to the following pathologies:
- narrowing of the valve snorts;
- reverse blood flow;
- a combination of both anomalies.
Due to the fact that the functions of the semilunar and atrioventricular snorts are carried out at different periods of time, narrowing and insufficiency manifest themselves in different ways.
Narrowing of the semilunar valves leads to the formation of murmurs. Atrioventricular narrowing manifests itself as a murmur in the bicuspid and 3-cuspid snort. Insufficiency in the first category is caused by diastole noise and is called aortic and pulmonary.
A disease such as insufficiency causes pathological changes in which the blood flow begins to return, despite the closure of the valve. Thus, the organ begins to work under increased stress and this is a stimulus for the development of diseases.
Aortic valve
Closes the entrance to the aorta. It also consists of three valves, which look like crescents. Opens when the left ventricle contracts. In this case, blood enters the aorta. When the left ventricle relaxes, it closes. Thus, venous blood (poor in oxygen) from the superior and inferior vena cava enters the right atrium. When the right atrium contracts, it moves through the tricuspid valve into the right ventricle. Contracting, the right ventricle ejects blood through the pulmonary valve into the pulmonary arteries (pulmonary circulation). Enriched with oxygen in the lungs, the blood turns into arterial blood and moves through the pulmonary veins to the left atrium, then to the left ventricle. When the left ventricle contracts, arterial blood enters the aorta through the aortic valve under high pressure and spreads throughout the body (systemic circulation).
The heart muscle is called the myocardium
There are contractile and conductive myocardium. The contractile myocardium is the actual muscle that contracts and produces the work of the heart. In order for the heart to contract in a certain rhythm, it has a unique conduction system. The electrical impulse to contract the heart muscle occurs in the sinoatrial node, which is located in the upper part of the right atrium and spreads through the conduction system of the heart, reaching every muscle fiber
Pulmonary snort
In the protected state of the tricuspid snort, the only route for blood is through the pulmonary trunk. This valve, in accordance with anatomy, is located at the inlet. Its structure is such that when pressure increases, it opens and allows blood flow into the arteries. Under the influence of the return flow, in a relaxed state of the ventricle, it is blocked, identical to the aortic one, protecting the pulmonary trunk from the reverse flow of blood.
The right chamber is a system in which the pressure is reduced. Therefore, the structure of the snort here is softer in comparison with the aortic one. When listening to a person in good health, the doctor hears the pulmonary and aortic valves of the heart.
Structure, localization, anatomy of the tricuspid heart valve
The tricuspid heart valve is located in the area of the atrium and ventricle on the right side.
Its structure is represented by the following elements:
| Name | Description |
| Fibrous ring | Consists of a large number of elastic fibers. Attached to the interatrial septum. A large number of cardiac conduction channels pass through the annulus fibrosus. Some part of this element is represented by muscle fibers and has a loose structure. As the annulus fibrosus moves away from the interatrial septum, its thickness gradually becomes smaller. |
| Doors | In most cases, the normal development of the heart valve involves 3 main leaflets (anterior, posterior and septal). Sometimes their number increases, according to experts, due to the bifurcation of the rear valve. They all vary in size. The largest door is the main one. The structure of these elements provides for the presence of a base, a body and a closure section. |
| Chordae tendons | There are 3 main elements, each of which performs its own specific functions. |
| Papillary musculature | The muscle is located on top of the interatrial septum. The chordae tendineae emerge from it. |
Tricuspid and other heart valves
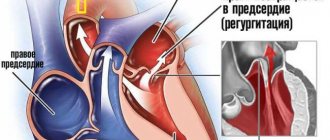
The condition and functioning of the tricuspid valve is important for normal blood circulation. It prevents the backflow of blood into the right atrium.
Work of the organ
The function of the tricuspid heart valve is to maintain normal blood circulation throughout the human body. Passing through all cells and internal organs, the blood gives off oxygen and receives carbon dioxide back. It is also saturated with decay products, due to which it acquires a dark color.
The work of the tricuspid valve is necessary for the separation of jointly functioning elements (left and right ventricles, atria). Venous blood returns through these sections to the pulmonary circulation. Saturated with oxygen through the left atrium, the ventricle flows into the systemic circulation.
Valve functions
The tricuspid heart valve is located on the right side and is an important element in the functioning of the pulmonary circulation.
It performs the following functions:
- During heart contractions, it prevents blood flow from the lower right ventricle to the atrium.
- Takes direct part in the blood circulation process. The tricuspid valve is responsible for the delivery of venous blood to the vessels located in the bronchi.
- Supports gas exchange in the alveoli of lung tissue and heat transfer.
Continuous blood circulation in the human body throughout his life is responsible for the rapid and timely delivery of oxygenated blood to all internal organs and tissues. Through venous outflow, carbon dioxide and decay products leave the body. In medicine, this mechanism is called the cardiovascular system.
The systemic circulation begins from the left ventricle and ends in the right atrium. The pulmonary circulation is directed from the right ventricle to the left atrium.
Recommendations
- "Anatomy of the tricuspid valve." e-echocardiography.com
. Received 2018-03-30. - Richard Van Prag: Anatomy of the Heart in A.C. Chang et al.: Pediatric Cardiac Intensive Care, Philadelphia, 1998.
- Healthline Editorial Team. "Right atrioventricular valve (tricuspid valve)." Health line
. - Reynertson, Sandra I.; Kundur, Ramesh; Mullen, J. Martin; Costanzo, Maria Rosa; McKiernan, Thomas L.; Louis, Eric K. (1999-08-03). "Asymmetry of right ventricular enlargement in response to tricuspid regurgitation." Circulation
.
100
(5):465–467. Doi:10.1161/01.CIR.100.5.465. ISSN 0009-7322. PMID 10430758. - "Enlarged heart - symptoms and causes." Mayo Clinic
. Received 2018-03-30. - Demin A.A., Drobysheva V.P., Welter O.Yu. (2000). "[Infective endocarditis in intravenous drug abusers]." Clinical medicine
(in Russian).
78
(8): 47–51. PMID 11019526. - Bhutani J, Dev V, Leong SV, Soor GS, Thangarupan M, Borger MA. (2006). "Infective endocarditis of the tricuspid valve." Journal of Cardiac Surgery
.
21
(6): 603–4. Doi:10.1111/j.1540-8191.2006.00313.x. PMID 17073968. - ^ a b
Mitchell R. S., Kumar V., Robbins S. L., Abbas A. K., Fausto N. (2007).
Robbins Basic Pathology
(8th ed.). Saunders/Elsevier. pp. 406–8. ISBN 1-4160-2973-7. - Tricuspid Valve Disease Mount Sinai Hospital, New York
- University Circle Inc. Archived 2008-06-17 on the Wayback Machine
Normal valve performance
The tricuspid heart valve is located between the right ventricle and the atrium. When the heart muscle relaxes, it opens. When the myocardium contracts, the valve closes. The valves close tightly due to the presence of chordae and muscles. During normal operation of the tricuspid valve, venous blood passes from the right side of the heart to the left. It is then sent to the lungs for gas exchange.
The backflow of blood into the heart cavity from large vessels is not observed due to the correct functioning of this valve. With the development of pathological processes, the entire mechanism is disrupted, as evidenced by the emerging clinical symptoms.
Pathologies of the 3-leaf heart valve, causes and symptoms
In most cases, pathological changes in the functioning of the tricuspid valve provoke heart failure or the development of stenosis. Degenerative processes impair blood circulation, resulting in characteristic clinical signs.
| Name | Description |
| Failure | A disease characterized by insufficient closure of the tricuspid valve. In this situation, the blood returns back to the atrium. |
| Stenosis | The pathological condition is characterized by narrowing of the holes located in the tricuspid heart valve. In most cases, problems arise against the background of rheumatism, congenital defects, or after prolonged mechanical impact on the chest. |
| Congenital defects | Pathological changes occur during the period of intrauterine development of the human body, often against the background of hereditary diseases in the mother’s body. |
Stages of dysfunction
The tricuspid heart valve is located on the right side of the organ. Pathological changes develop gradually. In medicine, disorders of the functioning of the tricuspid valve are distinguished, taking into account the complexity of pathological changes and the possibility of their reversal.
There are the following stages of development of organ damage:
| Name | Description |
| Stage I | At this stage of pathological processes, minor disturbances occur that affect the movement of the pulmonary and systemic circulation. |
| Stage II | Pathological processes progress, but if treatment is started in a timely manner, degenerative changes can be reversed. |
| Stage III | Significant problems in the functioning of the tricuspid valve provoke the appearance of specific symptoms, for example, increased blood pressure. |
| IV stage | At the last stage of the disease, the valve structure is affected. The person’s condition is deteriorating, progressive symptoms require urgent hospitalization and strict supervision by the attending physician. |
At the last stage of valve damage, pathological processes are irreversible. Properly selected therapy will improve the patient’s quality of life and prevent complications.
Disorders
Tricuspid regurgitation is not uncommon.
Infected valves can lead to endocarditis in intravenous drug users.[6][7] Patients who inject narcotics or other drugs intravenously may develop an infection that can spread to the right side of the heart and is most often caused by the bacteria S. aureus
.[8] In patients without a history of intravenous administration, endocarditis is more likely to be left-sided.[8]
The tricuspid valve may be affected by: rheumatic fever, which can cause tricuspid stenosis or tricuspid regurgitation.[9] Some people are born with a congenital abnormality of the tricuspid valve. Congenital apical displacement of the tricuspid valve is called Ebstein's anomaly and usually causes significant tricuspid regurgitation.
Certain carcinoid syndromes can affect the tricuspid valve, causing fibrosis due to the production of serotonin by these tumors.
The first endovascular tricuspid valve implant was performed by surgeons at the Cleveland Clinic.[10]
Diagnostic methods
A comprehensive examination will help the doctor establish the correct diagnosis and select the most effective treatment regimen.
Patients with damage to the tricuspid heart valve are prescribed the following diagnostic methods:
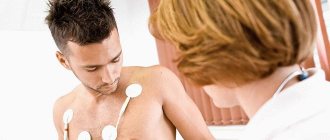
| Name | Description |
| Electrocardiogram (ECG) | An examination method that will assess the functioning of the heart and identify existing pathological abnormalities. Also determine the volume of the atria. |
| Phonocardiogram | During the examination, the specialist identifies systolic murmurs. |
| X-ray examination | Using the images, the doctor will determine the dilatation of the affected ventricle or atrium, the size of the organ itself, and identify congestion. |
| Echocardiography | The cardiac septa and their movements are observed. Deformation of the valves and the appearance of new formations are also revealed. |
| Spiral computed tomography (CT) | The specialist evaluates the functioning of the main muscle in the human body. |
| Catheterization | The diagnostic method is carried out to determine the pressure in the right side of the heart. |
An important determining point in the diagnosis is the systolic murmur during exhalation. Its presence indicates the development of tricuspid valve insufficiency. Additionally, or before surgery, patients are prescribed coronocardiography. A diagnostic method that will allow you to evaluate blood circulation and detect pathological changes.
Publications in the media
Isolated pulmonary valve stenosis is a pathological condition characterized by an obstruction to the flow of blood at the level of the pulmonary valve. Frequency : 10–12% of patients with congenital heart disease.
Etiology • Congenital anomalies (isolated stenosis or in combination with other congenital defects) • Secondary stenosis with carcinoid syndrome or infective endocarditis (massive vegetations) • Pseudostenosis of the pulmonary valve due to compression by tumors of the right ventricle and mediastinum, aneurysm of the aortic sinuses or saddle embolus.
Pathophysiology • Increased afterload • Right ventricular hypertrophy and prolongation of ejection period • Right ventricular failure • Subendocardial right ventricular ischemia • Secondary tricuspid valve regurgitation aggravates right ventricular failure • Decreased left ventricular filling and cardiac output.
Clinical picture and diagnosis
• Complaints •• Fatigue •• Fainting •• Symptoms of right ventricular failure •• With an open foramen ovale - cyanosis.
• Valve symptoms •• Systolic trembling in the second intercostal space to the right of the sternum •• Pulsation in the epigastric region (increased impulse of the right ventricle) •• Weakening of the pulmonary component of the 2nd tone, sometimes an ejection click •• Severe splitting of the 2nd tone •• Rough mesosystolic murmur ( the later the murmur reaches its peak, the more severe the obstruction), extending to the left clavicle and into the interscapular space •• As right ventricular failure develops, a pansystolic murmur of tricuspid regurgitation appears.
• Symptoms of circulatory failure in a large circle.
Special studies
• ECG •• Signs of hypertrophy and overload of the right sections •• Less commonly, ventricular arrhythmias.
• Jugular venogram: Increased amplitude of wave A, appearance of pronounced wave V (with tricuspid insufficiency).
• X-ray of the chest organs •• Bulging of the arches of the right parts of the heart without deviation of the contrasted esophagus •• Poststenotic dilatation of the pulmonary artery •• Depletion of the pulmonary vascular pattern.
• EchoCG •• Hypertrophy and dilatation of the right heart •• Detection of abnormalities in the number of valve leaflets •• Dome-shaped protrusion of the leaflets with congenital stenosis •• Vegetations with infective endocarditis •• Carcinoid plaques •• Compression of the pulmonary artery by a tumor or aneurysm of the aortic sinuses •• Thrombus in the proximal section of the pulmonary artery •• In Doppler mode, the pressure gradient between the right ventricle and the pulmonary artery is detected •• The severity of stenosis is determined by the degree of increase in systolic pressure in the right ventricle: I degree - less than 60 mm Hg, II degree - 60–100 mm Hg .st., III degree - more than 100 mm Hg.
• CT or MRI can help determine the presence and size of tumors causing compression.
• Right heart catheterization •• Right ventricular systolic pressure and pressure gradient between the right ventricle and the pulmonary artery are measured •• Increased right ventricular diastolic pressure •• Right atrial pressure is also usually elevated •• A wave amplitude is increased •• In secondary failure tricuspid valve - the appearance of a pronounced wave V.
• Right ventriculography and angiopulmonography •• Assessment of the degree of stenosis •• Test with aminophylline and oxygen is negative •• Detection of emboli in the proximal pulmonary artery.
TREATMENT
• Conservative therapy •• Thrombolysis for pulmonary embolism •• Radiation therapy for mediastinal tumors •• See also Heart failure.
• Surgical treatment •• Indications: II degree stenosis with clinical manifestations, III degree stenosis •• Contraindications ••• Dystrophic stage of circulatory failure ••• Severe concomitant pathology that threatens the patient’s life •• Methods of surgical treatment ••• For isolated congenital or acquired in case of valve stenosis, balloon valvuloplasty is used ••• Surgical decompression - in case of contraindications or ineffectiveness of radiation therapy for tumors compressing the pulmonary artery ••• Thrombectomy from the proximal pulmonary artery - in case of contraindications or ineffectiveness of thrombolysis.
Specific complications • Restenosis • Acute heart failure caused by inadequate elimination of stenosis.
Prognosis • Right ventricular failure due to pressure overload is usually reversible even with severe right ventricular depression and tricuspid valve regurgitation, but recovery of right ventricular function may take weeks or months, and the presence of right ventricular failure is associated with increased mortality • Postoperative in-hospital mortality - 0. 5–1.5% • 5-year survival rate – 91%.
Synonyms. Valvular pulmonary stenosis, Valvular pulmonary artery stenosis.
ICD-10 • I37 Pulmonary valve lesions
Methods of treating diseases
The tricuspid heart valve (located between the right atrium and the ventricle) is treated depending on the degree and stage of its damage, how extensively the pathological processes develop, and the area of their localization. During the period of therapy, it is important to strictly follow all the doctor’s recommendations, without exception, since the disease is life-threatening for the patient.
Drug therapy for damage to the tricuspid heart valve involves the use of the following drugs:
Indications and types of operations
The indication for surgical intervention is the second degree of pathological changes in the area of the tricuspid heart valve. The same applies to the lack of positive dynamics after drug therapy.
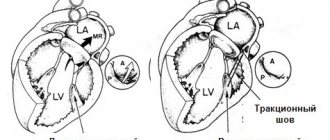
The tricuspid heart valve, depending on the location of the pathological foci, is operated on using the following methods:
| Name | Description |
| Annuloplasty | During medical procedures, the fibrous ring of the tricuspid valve is mobilized. U-shaped sutures are placed on the expanded commissures. |
| Semicircular annuloplasty | The operation is performed in case of severe expansion of the fibrous ring of the tricuspid valve. The surgeon uses a purse string suture. |
| Bicuspidization | Suture annuloplasty of the tricuspid valve, which involves placing U-shaped sutures on the fibrous ring in the area of the posterior leaflet. Teflon gaskets are used during this operation. |
| Prosthetics | A method of surgical treatment that is most often used for stenosis or insufficiency of the tricuspid valve. During surgery, the surgeon excises all the main valves and installs artificial prostheses in their place. |
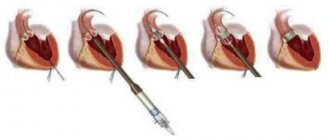
Surgical treatment is carried out using an artificial device that maintains blood circulation in the patient’s body. The surgical method is selected taking into account the anatomical characteristics of the patient’s body and the provoking factors that caused the development of the pathological mechanism.
Artificial heart valves
Recommendations for patients with a prosthetic heart valve 1.6 MB
Artificial heart valve: 2 main types
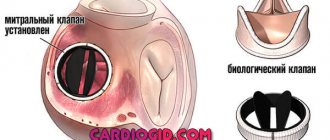
If any of the 4 heart valves malfunction - they are narrowed (stenosis) or excessively dilated (insufficiency) - it is possible to replace them or reconstruct them using artificial analogues. An artificial heart valve is a prosthesis that provides the required direction of blood flow by intermittently closing the mouths of venous and arterial vessels. The main indication for prosthetics is gross changes in the valve leaflets, leading to severe circulatory disorders.
There are two main types of artificial heart valves: mechanical and biological models, each of which has its own characteristics, advantages and disadvantages.
1. Butchart EG et al. Recommendations for the management of patients after heart valve surgery. European Heart Journal. 2005: 26(22); 2465-2471.
Figure 1. Two main types of artificial valves
Mechanical heart valve or biological prosthesis?
The mechanical heart valve is reliable, lasts a long time and does not need to be replaced, but requires constant use of special medications that reduce blood clotting.
2. Bonow RO, Carabello BA, Kanu C. et al.; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease) : developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation 2006; 114(5):e84-231; J Am Coll Cardiol 2006; 48(3):e1-148.
Biological valves may gradually deteriorate. Their service life largely depends on the age of the patient and concomitant diseases. With age, the process of destruction of biological valves slows down significantly.
The decision about which valve is most optimal should be made before surgery during a mandatory conversation between the surgeon and the patient.
Life with an artificial heart valve
People with prosthetic heart valves are at very high risk of thromboembolic complications. The fight against thrombosis is the basis of the management strategy for such patients, and it is its success that largely determines the prognosis for the patient.
The risk of thromboembolic complications is reduced with the use of biological valve prostheses, but they have their disadvantages. They are implanted infrequently and mainly in older people.
Living with an artificial heart valve requires a number of restrictions. The majority of patients with prosthetic valves are those with mechanical prostheses, who belong to a group at high risk of developing thrombotic complications. The patient is forced to constantly take antithrombotic drugs, in the vast majority of cases - indirect anticoagulants (warfarin). Almost all patients with mechanical heart valves should take them. The choice of a bioprosthesis also does not exclude the need to take warfarin, especially in patients with atrial fibrillation. To avoid dangerous bleeding, patients chronically taking warfarin should avoid daily activities and entertainment associated with an increased risk of injury (contact sports, working with cutting objects, or a high risk of falls, even from height).
The most important aspects of medical monitoring of a patient with an artificial heart valve today include:
- blood clotting control;
- active prevention of thromboembolic complications using anticoagulants (most often warfarin).
3. Bonow RO, Carabello BA, Chatterjee K. et al.; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2008;118(15):e523-661; J Am Coll Cardiol 2008; 52(13):e1-142.
It is important to note that European and American experts now consider the levels of antithrombotic therapy previously recommended for most patients to be too intense. Modern approaches to risk assessment make it possible to identify subgroups of people with the highest risk of thromboembolic complications and active antithrombotic therapy. For other patients with prosthetic heart valves, less aggressive antithrombotic therapy may be more effective.
Prevention of thrombosis in patients with mechanical heart valves
Prevention of thrombosis in patients with a mechanical heart valve requires lifelong antithrombotic therapy.
The intensity of warfarin therapy depends on the location of the prosthesis and its type. For example, in accordance with the ACC/AHA (2008) recommendations, a mechanical aortic valve prosthesis requires maintaining an INR in the range of 2.0-3.0 when using double-leaf (bicuspid) prostheses, as well as the Medtronic Hall valve (one of the most popular single-leaf artificial valves in the world). valves), or in the range of 2.5-3.5 for all other disc valves, as well as for the Starr-Edwards ball valve.
4. Salem DN, O'Gara PT, Madias C., Pauker SG; American College of Chest Physicians. Valvular and structural heart disease: American College of Chest Physicians Evidence
Mechanical mitral valve prosthesis requires maintaining the INR within 2.5-3.5 for all types of valves.
Table 1. Recommended INR value for mechanical heart valves
| Heart valve position | Risk factors for TE complications | |
| none | present | |
| Aortic | 2,0-3,0 | 2,5-3,5 |
| Mitral | 2,5-3,5 | 3,0-4,0 |
5. Methodological recommendations were reviewed and recommended by the scientific council of the Federal State Budgetary Institution “Research Institute for Complex Problems of Cardiovascular Diseases” of the Siberian Branch of the Russian Academy of Medical Sciences on July 1, 2011, updated on January 14, 2014.
However, even with recommended antithrombotic therapy, the risk of thromboembolic complications in patients undergoing heart valve replacement remains at 1-2%. The results of most clinical studies indicate that the risk of thrombosis is higher in patients with mitral valve prostheses (compared to aortic valve prostheses). If for patients with artificial aortic valves a less intensive anticoagulant regimen is possible (with a target INR of 2.0-3.0), then in the case of a mechanical mitral valve prosthesis, the anticoagulant regimen should be quite intensive (with a target INR of 2.5-3 ,5).
6. Vahanian A., Baumgartner H., Bax J. et al.; Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology; ESC Committee for Practice Guidelines. Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J 2007; 28 (2): 230-68.
Regardless of the type of artificial valve used, the risk of thrombosis is highest in the first few months after surgery - until the completion of epithelization processes at the site of implantation of the prosthesis. American experts consider it advisable to keep the INR within 2.5-3.5 in the first 3 months. after surgery, even for patients with an artificial aortic valve.
In addition, keeping the INR within a more stringent range (2.5-3.5) is recommended by the ACC/AHA in the presence of high-risk factors for thromboembolism, regardless of the type of prosthesis and its location. Such factors include atrial fibrillation, a history of thromboembolism, left ventricular (LV) dysfunction, and a hypercoagulable state.
Currently, there are portable devices for self-determination of INR (similar to systems for monitoring sugar levels in patients with diabetes), which help maintain INR levels in the required range. Among them, Coagucheck XS has proven itself for independent testing and immediate receipt of PTT/INR results. The device allows you to get accurate results in less than a minute, using only 8 µl (one drop of blood).
However, regardless of the chosen antithrombotic treatment strategy after heart valve replacement, regular patient monitoring, education, and close collaboration with the treating physician remain essential.
7. Butchart EG Antithrombotic management in patients with prosthetic valves: a comparison of American and European guidelines. Heart 2009;95: 430 436.
This allows for timely adjustment of drug doses, as well as changes in their thrombolytic activity, depending on the nutritional characteristics, state of the patient’s liver and kidney function.
Prevention of thrombosis in patients with bioprosthetic valves
In patients with bioprosthetic valves, less aggressive anticoagulant therapy is indicated, since in most studies the risk of thromboembolic complications in such patients, even in the absence of anticoagulant therapy, averaged only 0.7%.
According to American experts, the addition of warfarin may be useful in cases of increased risk of thromboembolism, but is not routinely recommended for all patients. When using warfarin, the INR should be kept within 2.0-3.0 if the aortic valve is replaced, and 2.5-3.5 if the mitral valve is replaced.
The use of warfarin with a target INR of 2.0-3.0 may also be appropriate in the first 3 months. after surgery and in patients with a mitral or aortic valve prosthesis without risk factors, given the increased tendency to thrombus formation in the early stages after valve replacement. Patients with a mitral valve prosthesis benefit particularly from this strategy.
Table 2. Recommended INR value for biological heart valves
| Heart valve position | Risk factors for TE complications | |
| none | present | |
| Aortic | 2,0-2,5 | 2,5-3,0 |
| Mitral | 2,5-3,0 | 3,0-3,5 |
| Tricuspid | 2,5-3,0 | 3,0-3,5 |
However, European ESC experts believe that there is currently insufficient evidence to support the need for long-term antithrombotic therapy in patients with bioprosthetic heart valves, unless these patients have any additional risk factors.
European guidelines recommend the use of warfarin in such patients only for the first 3 months. after surgery (target INR - 2.5).
Long-term (lifelong) anticoagulation therapy in patients with bioprosthetic valves may only be appropriate in the presence of high-risk factors (eg, atrial fibrillation; to a lesser extent, heart failure with LVEF <30% may be a risk factor), according to ESC guidelines6.
Thus, for patients with bioprosthetic heart valves, European experts recommend a more cautious tactic of antithrombotic therapy, while American experts consider a more aggressive approach justified. At the same time, in the United States, there is a more widespread tendency to minimize the patient’s time in hospital and the cost of his treatment, so American doctors prefer to prescribe acetylsalicylic acid to patients with bioprostheses. In Europe, they are still inclined to keep the patient in the hospital longer, if necessary, and to use warfarin in this category of patients, which is more demanding in monitoring blood coagulation parameters.
One of the most significant problems in the management of such patients in domestic healthcare settings is the inability to adequately control blood coagulation parameters against the background of constant use of anticoagulants.
It is the INR indicator that is recommended by all international guidelines as necessary to ensure the safety and effectiveness of therapy.
Recommendations for patients with a prosthetic heart valve 1.6 MB
Prognosis for pathologies
In most cases, progressive pathological processes with damage to the tricuspid valve lead to serious complications within 5-10 years.
Among the dangerous consequences it is necessary to highlight:
- pulmonary embolism;
- fatal atrial fibrillation;
- congestive heart failure;
- the occurrence of ascites;
- damage to the liver or blood vessels of internal organs;
- development of severe pneumonia;
- the occurrence of gastrointestinal bleeding due to portal hypertension.
As a result of secondary infectious damage to the human body against the background of defects, the risk of complete cardiac arrest increases. Life expectancy for patients with tricuspid heart valve disease is 20-30 years. It is important to constantly be under the supervision of a doctor.
Timely diagnosis of the location of the pathological focus and treatment of the tricuspid heart valve is the main prevention against complications and consequences.
At an early stage of dysfunction, drug therapy will help to cope with disorders. Late stages of disease require surgical intervention. Modern medicine offers enough methods to avoid death and prolong a person’s life.