Brugada syndrome has genetic roots. With this disease there is a risk of sudden cardiac death. The course of the syndrome can be observed on an electrocardiogram. This syndrome was first discovered in 1992 by Spanish specialists, but nowadays doctors know practically nothing about this disease. Let's talk about what Brugada syndrome is on an ECG: signs and treatment.
Every year in the world, especially in the southern and eastern regions of Asia, the number of people suffering from Brugada syndrome is growing rapidly. Most often, the disease affects middle-aged people. At the same time, the male population is at almost nine times greater risk of getting sick.
Today this syndrome is considered a clinical disease. The patients do not have organic heart pathologies, but the ECG shows RBBB (right bundle branch block). Also in some chest leads there is an increase in the ST segment.
Reasons for development
- Genetic syndrome is a mutation of some genes responsible for the development of this anomaly. Therefore, mutations in these genes may be the cause of this disease. However, many patients do not have genetic confirmation of the syndrome;
- Pathological failures in other genes that should be responsible for proteins;
- Inhibition of the autonomic nervous system, in which increased arrhythmogenesis is observed. This explains the fact that the syndrome causes attacks in most cases in the evening or at night.
What kind of pathology is this?
The peculiarity of the disease is that the signs of atrial fibrillation disappear against the background of transverse heart block. This makes it difficult to make a correct diagnosis.
The syndrome can be suspected if the patient has fainting conditions against the background of a permanent variant of atrial fibrillation, the pulse is rare but regular.
The insidiousness of Frederick's syndrome is that during the blockade the rhythm of contractions slows down, so a false impression is created that the condition is improving. Good health can remain for a long time.
But later, as the ventricular myocardium loses its ability to maintain rhythm, the patient’s condition deteriorates sharply. At a pulse rate of 20 to 30 contractions per minute, cerebral hypoxia develops, which can be fatal if there is a long pause between contractions.
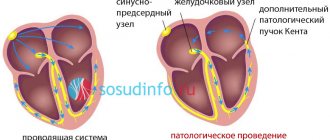
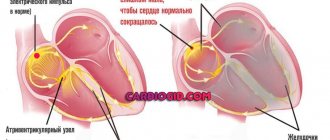
Each of these impulses gradually passes through the myocytes of all chambers of the heart, causing them to push blood through the body with a certain rhythm.
Characteristics of the disease
With arrhythmic disorders, one of which is Frederick's syndrome, the bioelectric impulse is looped in the atria and does not pass to the ventricles.
Whereas the ventricles function with a slow rhythm (less than 40 contractions in 60 seconds), synthesizing electrical impulses due to their own efforts.
Note! Frederick's pathology is a particularly dangerous disease for human life. According to statistics, 95% of patients who experienced the syndrome progressed to life-threatening complications (cardiogenic shock, acute heart failure, and even sudden death of heart tissue). The symptoms of the disease are similar to many heart pathologies, and an accurate diagnosis of the syndrome is possible only after an electrocardiogram (ECG), which clearly determines what characteristic disorders appear in a person
The symptoms of the disease are similar to many heart pathologies, and an accurate diagnosis of the syndrome is possible only after an electrocardiogram (ECG), which clearly determines what characteristic disorders are manifested in a person.
It will not be possible to completely get rid of the pathology, since it is a consequence of serious decompensated disorders in the heart. Implantation of an electrical pacemaker, which will constantly monitor attacks of arrhythmia, can prolong life and improve its quality.
In normal humans, the FXN gene encodes frataxin, a mitochondrial matrix protein. This globular protein consists of two α-helices and seven β-strands. It is highly conserved and is found in all eukaryotes and some prokaryotes.
Frataxin has many known functions. Promotes the synthesis of iron-sulfur in the electron transport chain. Leads to the formation of adenosine triphosphate (ATP), an energy molecule needed to carry out the metabolic functions of cells.
It regulates the transport of iron into mitochondria to ensure adequate amounts of reactive oxygen species (ROS) to maintain normal processes. Without frataxin, energy in mitochondria decreases, and excess iron causes the formation of additional ROS. The process leads to further cell damage.
With Federico's syndrome, there is usually a dysfunction of both atria at once.
They contract chaotically, independently of each other.
Often, such fluttering of the departments allows specialists to think about the failure of normal intracardiac geodynamics.
At first, this phenomenon does not frighten specialists and does not indicate any mortal danger. Nevertheless, after an ECG diagnosis after a certain period of time during the course of this disease, there remains very little chance of life, even with the most intensive and emergency treatment.
Signs
For Brugada syndrome, a characteristic symptom is a state of syncope, as well as SCD. Almost all patients who experienced sudden death had a history of syncope.
In severe cases of the disease, fainting occurs accompanied by seizures. In some patients, attacks occur with full consciousness, but severe weakness appears, the skin becomes pale, and the heart works intermittently.
Typically, the symptoms of the clinical condition of this pathology are characterized by the appearance of tachycardia and ventricular fibrillation. They most often appear in men under 40 years of age, but occur in children and people over 50 years of age.
Brugada syndrome, as mentioned above, occurs at night when heart rate is low. A small percentage of pathology occurs after hard work and alcohol consumption.
Symptoms
Typically, clinical manifestations of Brugada syndrome occur at rest—during sleep or rest—and include difficulty breathing, syncope, seizures, ventricular tachyarrhythmia, and cardiac arrest. Sudden cardiac arrest may be the initial symptom of SB in about a third of patients.
Brugada syndrome often occurs without obvious signs or symptoms (arrhythmias, difficulty breathing, loss of consciousness). The risk of sudden and complete cessation of cardiac activity in such patients is much lower.
Diagnostic methods
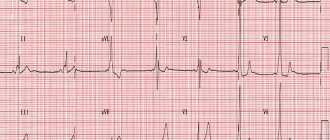
Electrocardiography is one of the main and most effective studies of various heart diseases, including Brugada syndrome. This procedure can identify the disease, as a result of which timely measures will be taken to combat it. An electrocardiogram in Moscow can detect cases of RBBB and ST-segment elevation in the precordial leads. And this makes it possible to determine and accurately diagnose this syndrome.
Brugada syndrome is a hereditary disorder. Signs of this disease appear on the ECG from the age of five. Nevertheless, there is a fact that the development of the disease cannot always be seen on electrocardiography.
It happens that signs of the disease appear on the ECG periodically, and this creates difficulties in determining an accurate diagnosis. However, in this case, the main determining factor will be the change in the ST segment, which rises in specific leads and has a completely different, dissimilar shape for a healthy cardiac organ. Thus, the doctor can see the form of SB in each specific case.
If, when undergoing an electrocardiogram, it is difficult for the doctor to determine the presence of the disease, then the patient is offered the same procedure, only with the use of a load. The patient is administered sympathomimetics, as a result of which all cases of SB previously detected will decrease. When novocainamide is administered, the manifestations, on the contrary, increase. In this case, the doctor has the opportunity to determine the correct diagnosis and prescribe effective treatment.
It is worth noting that the reason for the inability to detect Brugada syndrome on electrocardiography may be some medications that a person is taking at this moment. These can be psychotropic drugs, as well as blockers and various antiarrhythmic drugs. In this case, the specialist prescribes a repeat electrocardiogram after stopping taking these medications.
Brugada syndrome
What is Brugada syndrome? What is its prevalence? What medications are used for Brugada syndrome?
Sudden death is the most dangerous manifestation of diseases of the cardiovascular system. The main causes of sudden cardiac death in adults can be considered coronary heart disease and myocardial infarction, but in recent years the problem of sudden death in the absence of obvious diseases of the myocardium or coronary vessels has become increasingly acute, especially at a young age.
Today, a sufficient amount of data has been accumulated on the nature of diseases associated with the risk of sudden death. It has been determined that many of them are genetically determined, and this poses a particular danger, since not only the patient who has been diagnosed with the disease is at risk, but also his children and close relatives. These diseases are still extremely rarely detected in routine clinical practice. Patients die, as a rule, not in specialized hospitals, but at home or on the street, and the doctor at the clinic or the ambulance team is left to declare death. In this case, a rather vague diagnosis is made: acute cardiovascular failure. At autopsy, no lesions of the heart muscle or coronary vessels are detected. In children, paradoxically, most often posthumously they are diagnosed with an acute respiratory viral infection, the minimal manifestations of which are used to explain sudden death. All this gives grounds to assert that large Russian clinics do not have sufficient experience in monitoring and identifying these patients. The attention of cardiologists is often attracted only by the first symptoms of the disease, primarily syncope and palpitations. However, often the first and last manifestation of the disease is sudden death.
Modern clinical medicine has identified a number of diseases and syndromes that are closely associated with a high risk of sudden death at a young age. These include sudden infant death syndrome, long QT syndrome, sudden unexplained death syndrome, arrhythmogenic right ventricular dysplasia, idiopathic ventricular fibrillation and a number of others. One of the most “mysterious” diseases in this series is Brugada syndrome (BS). Despite the fact that hundreds of works devoted to this disease have been published all over the world, and thematic sections are regularly held at the largest international cardiology congresses, in the domestic literature there are only isolated descriptions of the syndrome, which do not always fully reflect the typical picture of the disease. At the same time, it is SB that, according to many experts, is “responsible” for more than 50% of sudden, non-coronary deaths at a young age.
The official discovery date of the syndrome is 1992. It was then that Spanish cardiologists, brothers P. and D. Brugada, currently working in various clinics around the world, first described a clinical electrocardiographic syndrome that combines frequent familial cases of syncope or sudden death due to polymorphic ventricular tachycardia, and the registration of a specific electrocardiographic pattern.
The predominant age of clinical manifestation of SB is 30-40 years, but this syndrome was first described in a three-year-old girl who had frequent episodes of loss of consciousness and subsequently died suddenly, despite active antiarrhythmic therapy and implantation of a pacemaker. The clinical picture of the disease is characterized by the frequent occurrence of syncope against the background of attacks of ventricular tachycardia and sudden death, mainly during sleep, as well as the absence of signs of organic myocardial damage at autopsy.
In addition to the typical clinical picture, SB has a specific electrocardiographic pattern. It includes right bundle branch block, specific ST segment elevation in leads V1-V3, periodic prolongation of the PR interval, and attacks of polymorphic ventricular tachycardia during syncope. The following clinical and electrocardiographic forms of Brugada syndrome are distinguished:
- Full form (typical electrocardiographic picture with syncope, perdsyncope, cases of clinical or sudden death due to polymorphic ventricular tachycardia).
- Clinical options: typical electrocardiographic picture in asymptomatic patients without a family history of sudden death or Brugada syndrome;
- typical electrocardiographic picture in asymptomatic patients, family members of patients with the full form of the syndrome;
- typical electrocardiographic picture after pharmacological tests in asymptomatic subjects, family members of patients with the full form of the syndrome;
- Typical electrocardiographic picture after pharmacological tests in patients with repeated syncope or idiopathic atrial fibrillation.
- typical electrocardiographic picture with obvious right bundle branch block, ST segment elevation and prolongation of the PR interval;
It is characteristic that a typical ECG pattern is more often recorded in patients in the period before the development of ventricular fibrillation, which indicates the need for dynamic monitoring of patients with suspected SB. During a test with dosed physical activity and a drug test with sympathomimetics (isadrin), ECG manifestations of SB decrease, while during a test with slow intravenous administration of antiarrhythmic drugs that block sodium flow, they increase. According to the standard protocol for examining patients with suspected SB, it is recommended to use the following antiarrhythmic drugs for testing: gilurythmal (ajmalin) at a dose of 1 mg/kg, procainamide (procainamide) at a dose of 10 mg/kg, or flecainide at a dose of 2 mg/kg. It must be taken into account that when these drugs are administered to patients with SB, dangerous ventricular tachyarrhythmias, even fibrillation, may develop, so such tests should be carried out under the condition of full readiness to provide emergency care. But despite this, tests today are the most reliable criterion for identifying a dangerous, life-threatening disease that requires constant monitoring and many years of antiarrhythmic therapy. When performing invasive electrophysiological studies (EPS) in patients with SB, ventricular arrhythmias are often induced, but EPS can hardly be considered the “gold standard” for diagnosing the full clinical form of the syndrome. Before 1992, cases of observation of young patients with a typical ECG pattern of SB, syncope and normal EPI values were often described. Subsequently, such patients, left without treatment, suddenly died (Mandell W., 1985).
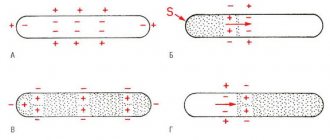
In recent years, the role of standard electrocardiography has increased significantly in the diagnosis of patients at risk of dangerous ventricular arrhythmias. Thus, in patients with SB, according to our observations, the epsilon wave - eW, which characterizes delayed depolarization in the area of the outflow tract of the right ventricle, is often recorded. This sign is a “major” diagnostic criterion for another disease associated with a high risk of sudden death - arrhythmogenic dysplasia of the right ventricle. However, given the single source of arrhythmia in both diseases—the right ventricular outflow tract—it can also be considered a diagnostically significant ECG manifestation of SB. In patients at risk of sudden death, much attention has been paid to prolongation of the QT interval as a risk factor for ventricular arrhythmias. However, a number of recent observations have shown that QT shortening, observed specifically in patients with SB and idiopathic ventricular fibrillation, also plays a proarrhythmogenic role. Even the term “short QT interval syndrome” is proposed (Gussak I., 2000). Our observations indicate that all patients with BS have QT interval values less than 50 percentile, and in the most severe patients - less than 5. These changes may be associated with the peculiarities of the electrophysiology of the cardiomyocyte in BS - a significant shortening of the 2nd phase of the action potential in epicardium of the right ventricle (with prolongation of the QT interval, the opposite electrophysiological mechanism is involved). Obviously, asynchronism of repolarization of any nature increases the arrhythmogenic readiness of the myocardium. With Holter monitoring, a high circadian index (CI - the ratio of the average daytime to average nighttime heart rate) - more than 1.45 (the norm is from 1.24 to 1.44) may be noted.
The prevalence of the syndrome is still unclear. Thus, in one of the regions of Belgium, the prevalence of SB was 1 per 100,000 inhabitants (Brugada P., 1999). According to Japanese researchers who analyzed 22,027 electrocardiograms from the population, the prevalence of the ECG pattern of SB in this country was 0.05-0.6% in adults and 0.0006% (analysis of 163,110 electrocardiograms) in children (Tohyou J. et al ., 1995; Hata Y. et al., 1997).
However, the actual incidence of the disease has not yet been determined, especially in some ethnic groups. Electrocardiographic changes similar to SB are described in sudden unexplained death syndrome, which is recorded mainly in people from Southeast Asian countries (Nademanee K., 1997). For the first time, this syndrome began to be identified as an independent disease in the 80s of the twentieth century, when the American Center for Disease Control in Atlanta (USA) recorded an unusually high (25 per 100,000 people) rate of sudden death in young people from the Southeast. Asia. Death occurred mainly at night; autopsy did not reveal damage to the heart muscle or coronary vessels. When comparing these data with statistical data accumulated in the countries of Southeast Asia and the Far East, it was noted that in this region, cases of sudden nocturnal death at a young age are significantly common (per year from 4 to 10 cases per 10,000 inhabitants, including including in Laos - 1 case per 10,000 inhabitants; in Thailand - 26-38 per 100,000). In these countries, there are even special names to refer to people who died suddenly in their sleep - bangungut in the Philippines, pokkuri in Japan, lai thai in Thailand. Often the ECG shows changes in the ST segment, similar to the pattern of SB or early ventricular repolarization. The extent to which these syndromes are related remains to be determined through further research. We observed several similar patients from similar ethnic groups (Buryats), in whose families there was a high concentration of cases of sudden death at a young age and frequent episodes of syncope or clinical death.
Another interesting feature of SB is that the disease is not reported in African-Americans; on the other hand, in Europe, SB is more often detected in representatives of the so-called “Caucasian” ethnic type, which, according to international gradations, also includes people from Eastern European countries. It is characteristic that the Brugada brothers identified the first of the described cases of the disease in a Polish girl. This indicates that the prevalence of SB in the Russian population may be quite high.
It is assumed that SB has an autosomal dominant mode of inheritance with damage to the SCN5a gene on chromosome 3. The same gene is affected in patients with the third molecular genetic variant of long QT interval syndrome (LQT3) and Lenegra syndrome - diseases also associated with a high risk of sudden arrhythmogenic death.
In 93.3% of cases, attacks during SB occur in the evening and at night (from 18 to 06 hours), and more often in the second half of the night. This undoubtedly confirms the role of increased vagal influences in the occurrence of ventricular fibrillation in SB. This circadian pattern also indicates differences in the pathogenesis of fatal arrhythmias in patients with SB and coronary heart disease, when the main circadian peak of sudden death occurs in the early morning hours (Deedwania P., 1998).
It is necessary to carry out a differential diagnosis of SB with a number of diseases that can cause similar electrocardiographic manifestations: arrhythmogenic dysplasia of the right ventricle, myocarditis, cardiomyopathies, Chagas disease (myocarditis), Steinert disease, mediastinal tumors.
To prevent ventricular fibrillation in SB, classical antiarrhythmic drugs are used, providing an effect in 60% of cases. Genetically determined damage to sodium channels theoretically suggests a lower effectiveness of drugs of the 1st group, as well as the possibility of a proarrhythmogenic effect when used. According to the algorithm for the formation of antiarrhythmic therapy, known as the “Sicilian Gambit” (Europ Heart J, 1991; 12), antiarrhythmic drugs that provide active blockade of sodium channels are procainamide, disopyramide, quinidine, rhythmonorm, gilurythmal, flecainide, encainide. A less pronounced blocking effect was observed with lidocaine, mexiletine, tocainide, bepridil, verapamil, cordarone and obsidan. It can be assumed that for SB it is safer to use drugs that do not block sodium channels - diltiazem, bretylium, sotalex, nadolol (Korgard). However, no targeted research has yet been carried out in this area. The most effective method to prevent the development of life-threatening arrhythmias in patients with SB today is the implantation of cardioverter defibrillators.
World statistics indicate the widespread prevalence of SB in the world. At the same time, its current low detection rate in Russia is obviously associated with the lesser focus of doctors on the entire clinical and electrocardiographic symptom complex, which often does not have features in individual components that allow a confident diagnosis. Therefore, in all patients with syncope of unknown etiology, nocturnal paroxysms of suffocation, cases of sudden death in the family (especially at a young age and at night), with a typical ECG pattern, it is necessary to exclude Brugada syndrome. To do this, such patients should undergo pharmacological tests, dynamic ECG examination of both the patient himself and his relatives, and Holter monitoring. In addition, one of the most reliable methods for diagnosing SB is molecular genetic research.
Since 1999, the Moscow Research Institute of Pediatrics and Pediatric Surgery of the Ministry of Health of the Russian Federation, together with the International Foundation for Brugada Syndrome and P. Brugada, has been conducting a study of the prevalence of Brugada syndrome in the Russian population. All Russian specialists who see patients with suspected Brugada syndrome can consult them free of charge in absentia based on ECG data and examinations performed. Identified patients will be included in a single International Register, which provides the opportunity to conduct molecular genetic studies.
For questions about literature, please contact the editor
Treatment
When determining this disease, the doctor first prescribes antiarrhythmic drugs. He prescribes medications for each patient individually. You should not take medications on your own, as they can be completely useless, and in some cases even harmful. Typically, medications such as amiodarone and quinidine are prescribed for the treatment of Brugada syndrome in Moscow.
However, ICD is considered the most effective method of combating this disease. In this case, the risk of an air force appearing is almost zero. The treatment method involves the patient undergoing surgery during which an antiarrhythmic drug is implanted into the body. This device is able to control the heart rhythm. The small computer works smoothly, controlling the operation of the organ.
This device may also be useful for ventricular fibrillation.
You can learn more about Brugada syndrome at a consultation with cardiologists at the ProfMedPomoshch clinic in Moscow.
Forecast
If possible, implantation of a defibrillator is relatively favorable. The probability of survival for 5-10 years is 90% or higher, depending on other factors.
Without treatment, the risk of premature death is in the range of 60-100%. Some clinical variants of the disease cannot be treated at all (based on the gene affected by the disorder, 5 or 6 types of process are distinguished).
Therefore, it is vitally important to conduct a test in order to, on the one hand, verify the diagnosis and, on the other, determine the prospects.
Brugada syndrome causes sudden cardiac death among young people. It cannot be cured, but there is a chance to prevent death in the medium term.
Causes of sudden cardiac death
The most common electrophysiological mechanisms leading to SCD are tachyarrhythmias, particularly ventricular fibrillation or ventricular tachycardia. Interrupting tachyarrhythmias using either an automated external defibrillator or an implantable cardioverter defibrillator is an effective treatment. The implantable defibrillator has become the main therapeutic modality in the prevention and treatment of sudden cardiac death. Patients with tachyarrhythmia have the best overall prognosis among patients with sudden cardiac arrest.
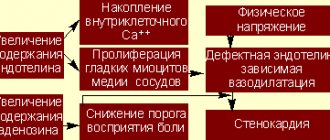
There are several factors at the organ level (eg, autonomic nervous system imbalance), tissue level (eg, re-entry mechanism, wave breaking, and alternative action potential effects), cellular level (eg, trigger activation and automaticity), and subcellular level (abnormal activation or deactivation of ion channels) causing the generation of ventricular fibrillation or ventricular tachycardia under different conditions. An anatomical or functional block in the process of impulse propagation can create a pattern with a circular wave front, resulting in ventricular tachycardia.
Other mechanisms, such as wave breaks and collisions, are involved in the generation of ventricular fibrillation or ventricular tachycardia. While at the tissue level the above-mentioned reentry and wave breaking mechanisms are the most important known mechanisms of these disorders, at the cellular level increased excitation or decreased repolarizing reserve of cardiomyocytes can lead to ectopic activity (eg, automaticity), contributing to the development of ventricular fibrillation or ventricular tachycardia .
At the subcellular level, altered intracellular Ca2+ currents, altered intracellular K+ currents (especially during ischemia), or mutations leading to sodium channel dysfunction (Na+ channelopathy) may increase the likelihood of developing disorders.
Approximately 20-30% of patients with documented cases of sudden death have bradyarrhythmia or asystole at an early stage. It is often difficult to determine with certainty the initiating event in a patient who has a bradyarrhythmia because asystole and pulsatile electrical activity may result from a sustained form of tachycardia. In rare cases, initial bradyarrhythmia causing myocardial ischemia may precipitate ventricular fibrillation or ventricular tachycardia.
Most cases of SCD occur in people with structural abnormalities of the heart. Myocardial infarction (MI) and cardiac remodeling are the most common structural abnormality in patients with SCD. In patients who have had myocardial infarction, the presence of premature ventricular contractions, especially complex forms, short coupling intervals (R-on-T) or tachycardia indicate an increased risk of sudden death. However, suppression of PVCs by antiarrhythmic drugs increases mortality due to the proarrhythmic risk of the drugs.
Hypertrophic cardiomyopathy and dilated cardiomyopathy are associated with an increased risk of SCD. Various valvular diseases, such as aortic stenosis, are associated with an increased risk of SCD. Acute diseases such as myocarditis can lead to the risk of SCD due to myocardial inflammation and fibrosis.
Less commonly, sudden cardiac death occurs in patients who do not have obvious structural heart disease. These conditions are usually inherited by arrhythmia syndromes.
Although many patients have anatomical and functional cardiac factors that predispose them to developing ventricular arrhythmias, only a small percentage develop SCD. Identification of patients at risk for developing SCD remains a challenge. The strongest known predictor of SCD is significant left ventricular dysfunction from any cause. An interaction between local ischemia, LV dysfunction, and transient events (eg, worsening CAD, acidosis, hypoxemia, wall stress, medications, metabolic disorders) has been suggested as a precipitator of sudden death.