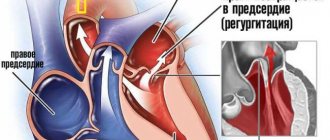
The feeling of rapid heartbeat, or tachycardia, accompanied by a very high heart rate (more than 100 per minute), can be caused by many diseases that lead to arrhythmia. Often such symptoms, together with specific changes in the electrocardiogram, are based on the anatomical features of the conduction system of the heart, which is responsible for the correct heart rhythm. The combination of such features constitutes clinical syndromes, generalized by the concept of shortening of the PQ interval.
So, shortened PQ interval syndrome is a group of electrocardiological symptoms, the basis of which is a decrease in the time it takes electrical excitation to reach the ventricles from the atria through the atrioventricular connection. This group includes Wolff-Parkinson-White syndromes (WPV syndrome)
, as well as
Clerc-Levy-Cristesco syndrome (CLC syndrome)
. These syndromes can occur at any age, even in the neonatal period, regardless of gender differences.
What happens with short PQ syndrome?
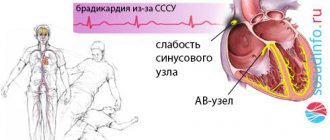
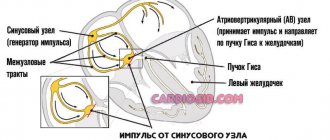
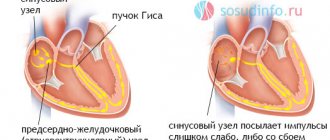
The PQ interval is a purely electrocardiographic criterion that allows one to evaluate the transmission time of an electrical impulse from the sinus node in the atrium to the contractile fibers located in the ventricles. In other words, it reflects the operation of the atrioventricular junction, a kind of “switch” that redirects electrical excitation from the atria to the ventricles. Normally, it is no less than 0.11 seconds and no more than 0.2 seconds:
example of shortening PQ to 0.03 s
- An increase in the interval beyond the specified time indicates a slowdown in conduction through the atrioventricular node,
- Shortening means that the excitation is carried out too quickly. In fact, there is more frequent impulse of the ventricles, with the so-called “reset” of excitation.
The shortening of this interval is due to the presence of additional conduction bundles in the conduction system of the heart.
It is through them that additional pulses are reset. Therefore, at certain moments the ventricles receive double impulses - physiological in a normal rhythm (60-80 per minute), and pathological, through bundles.
There may be several pathological bundles, and all of them are named after the names of the authors who first discovered them. Thus, the Kent and Maheim bundles are characteristic of the SVC syndrome, and the James bundles are characteristic of the CLC syndrome. In the first case, the pathological discharge of impulses goes from the atria directly to the ventricles, in the second, the James bundle passes through the atrioventricular node, that is, the node is stimulated first, and then the ventricles. Due to the “throughput” capacity of the AV node, part of the impulses conducted to the ventricles returns through the same bundle to the atria, therefore such patients have a high risk of developing paroxysmal supraventricular tachycardia.
main types of pathological pathways of additional conduction through the heart
Medical reference books
Pre-excitation syndrome
ICD-10: I45.6
Introduction
In 1930, L. Wolff, J. Parkinson and PD White published an article describing 11 patients who suffered attacks of tachycardia, and during sinus rhythm had a short PR interval and a wide QRS complex on the ECG, reminiscent of bundle branch block. After these authors, the syndrome they described was called Wolff-Parkinson-White (WPW) syndrome. WPW syndrome is one of the variants of “pre-excitation” of the ventricles. As defined by Durrer et al. (1970), the term “preexcitation” of the ventricles means that the ventricular myocardium is activated by the atrial impulse earlier than would be expected if the impulse entered the ventricles through the normal specialized conduction system. WPW syndrome is more common than other types of ventricular preexcitation. It is based on a congenital anomaly in the structure of the conduction system of the heart in the form of an additional bundle of Kent, connecting the myocardium of the atria and ventricles directly, bypassing the atrioventricular junction, and capable of rapidly conducting impulses. The conduction anomaly in WPW syndrome is manifested by characteristic ECG changes and a tendency to develop supraventricular tachycardias.
Pathophysiology
The bundles of Kent are formed in the embryonic period in the form of muscle bridges passing through the fibrous ring between the atria and ventricles. The electrophysiological characteristics of the Kent bundles (conduction velocity, refractoriness) differ from the properties of the atrioventricular connection, which creates the prerequisites for the development of arrhythmias through the mechanism of re-entry of the excitation wave (re-entry). The ability of the Kent bundles to rapidly conduct atrial impulses bypassing the atrioventricular junction determines the possibility of an unusually high heart rate during the development of atrial fibrillation/flutter, which in rare cases can cause ventricular fibrillation and sudden death.
Epidemiology
· Ventricular preexcitation occurs in the general population with a frequency of 0.1-0.3%. 60-70% of patients have no other signs of heart disease. · WPW syndrome is more common in men than in women. · The age of patients may vary. Typically, WPW syndrome is detected in childhood or adolescence when seeking emergency care for tachyarrhythmias. The speed of conduction along accessory pathways decreases with age. Cases have been described in which ECG signs of preexcitation completely disappeared over time. · Up to 80% of patients with WPW syndrome experience reciprocal (circular) tachycardia, 15-30% have atrial fibrillation, 5% have atrial flutter. Ventricular tachycardia is rare.\
Mortality
The risk of sudden arrhythmic death in patients with WPW syndrome is very low - about 0.15-0.39% over a follow-up period of 3 to 10 years. Sudden death as the first manifestation of the disease is relatively rare. On the contrary, in 1/2 of people with WPW syndrome who died suddenly, the cause of death was the first episode of tachyarrhythmia. The risk of sudden death increases when certain drugs (digoxin) are used to treat arrhythmias.
Clinical picture
History · Paroxysmal tachycardias in WPW syndrome often occur in childhood, but may first develop in adults. Having begun in childhood, arrhythmia can disappear for a while, then it recurs. If attacks of tachycardia have not stopped after the age of 5 years, the probability of their continuation in the future is 75%. · During an attack of tachycardia, various symptoms are possible - from minor discomfort in the chest, palpitations, dizziness to lightheadedness or loss of consciousness, severe hemodynamic disturbances and cardiac arrest. · Syncope can occur as a result of cerebral hypoperfusion against the background of tachyarrhythmia, or as a result of depression of the sinus node during tachyarrhythmia with the development of asystole after its cessation. · After the end of the paroxysm, polyuria may be observed (stretching of the atria during an attack leads to the release of atrial natriuretic peptide). · Signs of ventricular preexcitation can be detected by random ECG recording in individuals without rhythm disturbances (WPW phenomenon). · Asymptomatic patients rarely become symptomatic after age 40. With increasing age, there is a possibility of developing fibrosis in the area of attachment of the bundle, and therefore it loses the ability to conduct impulses from the atria to the ventricles. Physical findings WPW syndrome has no characteristic physical findings other than those associated with tachyarrhythmias. In young people, symptoms may be minimal even with a high heart rate. In other cases, during an attack there is coldness of the extremities, sweating, hypotension, and signs of congestion in the lungs may appear, especially with concomitant heart defects - congenital or acquired.
Diagnostics
1. Standard ECG WPW syndrome is characterized by the following ECG changes (
): · Short interval pQ < 0.12 s. - the result of accelerated entry of the impulse into the ventricles along the accessory pathway. · Delta wave – a gentle slope in the initial part of the QRS complex (the first 30-50 ms). - reflects the initial excitation of the ventricle through the accessory pathway, which runs away from the specialized conduction system and gives rise to a relatively slow transmission of impulse from fiber to fiber of the myocardium.
· Wide QRS complex > 0.10-0.12 s. - the result of excitation of the ventricles from two directions - leading through the accessory pathway, and immediately following this - through the AV connection. · Secondary changes in repolarization - the ST segment and T wave are usually directed in the direction opposite to the orientation of the delta wave and the QRS complex. Analysis of the polarity of the delta wave in various ECG leads allows us to determine the localization of the accessory pathway. An additional pathway can be manifest or hidden. — Manifest path - the ECG shows the above signs of pre-excitation, the bundle is capable of antegrade impulse conduction (from the atria to the ventricles). — Hidden path - there are no signs of pre-excitation on the ECG (the bundle conducts impulses only in the retrograde direction, from the ventricles to the atria, being the retrograde part of the re-entry circle in orthodromic tachycardia). In some patients, preexcitation may be intermittent (not constant), with a variable ECG pattern in the complexes of one recording or on cardiograms taken several days and even hours apart ().
2. Daily Holter ECG monitoring Allows you to record arrhythmias and detect intermittent ventricular preexcitation.
3. Echocardiography Allows you to evaluate the function of the left ventricle, myocardial contractility in various segments, exclude concomitant heart defects - valve defects, Ebstein's anomaly, corrected transposition of the great vessels, ventricular and atrial septal defects, hypertrophic cardiomyopathy, which can be combined with WPW syndrome.
4. Electrophysiological study (EPS) When examining some patients, a special electrophysiological study may be necessary. EPS can be non-invasive (method of transesophageal electrical stimulation of the heart - TEES) and invasive, endocardial. The last method is the most accurate. EPI allows you to clarify the mechanism of development of tachycardia, determine the localization of the accessory pathway and evaluate its electrophysiological properties (conductivity and duration of the refractory period). Beams with a short refractory period (less than 250-270 ms), and therefore capable of conducting pulses at high frequencies, are potentially dangerous.
Arrhythmias in WPW syndrome
In patients with WPW syndrome, various rhythm disturbances may occur, but the most common are 2 types of tachycardia: reciprocal (circular) tachycardia and atrial fibrillation/flutter.
1. Reciprocal atrioventricular tachycardia: orthodromic, antidromic · In sinus rhythm, impulses from the atria to the ventricles are carried out through both pathways - the atrioventricular connection and the bundle of Kent; there are no conditions for paroxysm of tachycardia. The triggering factor for circular tachycardia is usually an atrial extrasystole, which occurs at a critical moment in the cardiac cycle, namely when a premature impulse can be carried to the ventricles through only one path, due to the refractoriness of the second. · Most often, the Kent bundle is refractory (90-95% of cases), and the ventricles are excited through the atrioventricular connection (orthodromic). After the end of ventricular excitation, the impulse can return to the atria along the accessory pathway, moving in a retrograde direction, and re-enter the atrioventricular junction, closing the circle of tachycardia. With this direction of impulse movement, the tachycardia is called orthodromic reciprocal AV tachycardia. Its features: 1) narrow QRS complex; 2) strict regularity of rhythm; 3) signs of retrograde excitation of the atria behind the QRS complex (P wave - negative polarity in leads II, III and avF) (
).
· Attacks of orthodromic tachycardia can also be observed in patients with hidden bundles of Kent, due to the ability of the accessory pathway to conduct retrogradely. · Antidromic reciprocal AV tachycardia occurs 10-15 times less frequently than orthodromic tachycardia. In this case, the impulse enters the ventricle through the accessory pathway (antidromically) and returns to the atrium through the atrioventricular junction in a retrograde direction. Tachycardia is formed 1) with wide QRS complexes, 2) strictly regular, with a potentially higher rhythm frequency, since the beam has a short refractory period, 3) the retrograde P wave is located in front of the QRS complex, but is usually poorly distinguishable. The ventricular complex in this case represents a continuous delta wave, coinciding in polarity in each lead with the delta wave in sinus rhythm. Such tachycardia is difficult to distinguish from ventricular (
). If the diagnosis is in doubt, this form of supraventricular arrhythmia should be treated as ventricular arrhythmia. · Antidromic tachycardia is more dangerous than orthodromic tachycardia: it is less tolerated and more often transforms into ventricular fibrillation.
2. Atrial fibrillation · Atrial fibrillation occurs in patients with WPW syndrome much more often than in people of the same age in the general population - with a frequency of 11-38%. The predisposition to the development of atrial fibrillation in WPW syndrome is explained by changes in the electrophysiological properties of the atria under the influence of frequent paroxysms of circular tachycardia, and even by the very fact of attachment of the bundle to the atrial myocardium. It is also believed that atrial fibrillation may occur as a result of changes in the atria that accompany WPW syndrome, independent of the presence of the accessory pathway. · Atrial fibrillation is the most dangerous arrhythmia in WPW syndrome, which can degenerate into ventricular fibrillation. The appearance of paroxysms of atrial fibrillation in a patient with WPW syndrome means an unfavorable turn in the course of the disease. The time of onset of atrial fibrillation may vary: it may be the first attack of tachycardia in a patient with WPW syndrome, occur in patients with a long history of reentrant tachycardia, or develop during an attack of reentrant tachycardia. · Normally, the atrioventricular connection acts as a physiological filter on the path of frequent impulses from the atria, passing no more than 200 impulses per minute during atrial fibrillation into the ventricles. In patients with WPW syndrome, impulses flow from the atria to the ventricles using both the AV junction and the bundle of Kent. Due to the short refractory period and the high speed of conduction along the accessory pathway, the number of heart contractions can reach 250-300 or more per minute. In this regard, AF in WPW syndrome is often accompanied by hemodynamic impairment, which is clinically manifested by hypotension and syncope. · Impaired hemodynamics during a paroxysm of AF causes sympathetic activation, which further increases the frequency of conduction along the accessory pathway. Too high a heart rate can cause atrial fibrillation to transform into ventricular fibrillation. · On the ECG during atrial fibrillation in patients with ventricular preexcitation, a frequent irregular rhythm (heart rate more than 200/min) with wide polymorphic QRS complexes of unusual shape is recorded. The originality of the ventricular complexes in this arrhythmia is associated with their confluent nature (the shape of the QRS is determined by the relative participation of the AV junction and the accessory pathway in the excitation of the ventricles). Unlike polymorphic ventricular tachycardia, there is no “torsades de pointes” phenomenon (
).
· Measurement of the shortest RR interval on an ECG taken during an attack of atrial fibrillation in a patient with WPW syndrome is used to assess the degree of risk: the danger is maximum if RRmin ≤ 220-250 ms.
Risk stratification
The optimal treatment strategy for WPW syndrome is determined based on an individual assessment of the risk of sudden death. · Persons with signs of pre-excitation on the ECG who have not suffered tachycardia usually do not require either treatment or additional examination. The exception is professional athletes and representatives of high-risk professions (pilots, drivers, etc.): they are recommended to undergo an electrophysiological examination to determine the properties of the accessory pathway and the associated risk of sudden death. · The intermittent nature of ventricular preexcitation, the disappearance of the delta wave against the background of increased rhythm or during physical activity, as well as after intravenous administration of procainamide or ajmaline, are characteristic of accessory pathways with a long refractory period. Such accessory pathways are usually not capable of conducting impulses frequently if atrial fibrillation/flutter develops. The risk of sudden cardiac death is low. · High-risk markers for WPW syndrome, established in a retrospective analysis of cases of sudden death of patients: 1) the shortest RR interval for atrial fibrillation less than 250 ms; 2) history of symptomatic tachyarrhythmias; 3) multiple accessory pathways; 4) Ebstein's anomaly; 5) familial form of WPW syndrome, cases of sudden death in the family history. · The risk of sudden death can be assessed most accurately by intracardial EPI.
Treatment
Patients with WPW syndrome require treatment during paroxysms of tachycardia and to prevent relapses.
Relief of tachycardias 1. Orthodromic reciprocal tachycardia (narrow QRS complex, frequency - about 200/min, retrograde P is clearly visible behind the QRS complex): treatment is aimed at slowing down conduction in the AV node. · Vagal techniques (performing the Valsalva maneuver, unilateral carotid sinus massage, immersing the face in cold water, applying an ice pack to the face) are more effective at the onset of an attack. · ATP or adenosine intravenously (ATP for adults in a dose of 10-40 mg in the form of a bolus, intravenously quickly, over 3-5 seconds; adenosine 37.5 mcg/kg, intravenously quickly, if necessary - after 2-3 min at double dose – 75 mcg/kg). - do not use for sick sinus syndrome, bronchial asthma, vasospastic angina; — effective in 90% of cases of reciprocal tachycardia with a narrow QRS complex; ineffectiveness is usually associated with inadequate administration technique (rapid bolus administration is necessary due to the short half-life of the drug); - There should be a readiness to carry out defibrillation in the event of the development of another form of tachycardia (especially atrial fibrillation) after the administration of adenosine. · Verapamil intravenously (5 mg IV over 2 minutes, if the arrhythmia persists, repeat after 5 minutes at the same dose to a total dose of 15 mg). - used if tachycardia recurs, or ATP/adenosine is ineffective, or if the patient is taking theophylline; — verapamil is not used for WPW syndrome if the patient has already had episodes of atrial fibrillation.
2. Atrial fibrillation/flutter, antidromic reentrant tachycardia are tachycardias with ventricular preexcitation (wide QRS complex). They often occur with a high heart rate, severe symptoms, hemodynamic disturbances, and, therefore, require immediate cessation. In an urgent situation with unstable hemodynamics, electrical cardioversion is indicated (1st shock energy - 100 joules). If tachycardia is well tolerated, or electrical cardioversion is ineffective, drug therapy is performed. For the treatment of this group of arrhythmias, drugs that lengthen the refractory period of the accessory pathway (procainamide, cordarone) are indicated. Blocking the accessory pathway eliminates ventricular preexcitation, and with it the threat of sudden arrhythmic death. · Novocainamide – in patients without structural myocardial damage (intravenous infusion at a rate of 20 mg per minute until tachycardia is stopped; administration of the drug is stopped when hypotension develops, the QRS width increases by 50% of the original, or when the maximum dose of 17 mg/kg is reached). · Amiodarone – in patients with structural heart disease (loading dose – 5 mg/kg in a 5% glucose solution, IV drip over 20 minutes, then continue administration at a dose of 600-900 mg over 24 hours). NB: Digoxin, verapamil and beta blockers should not be used to treat atrial fibrillation/flutter in patients with WPW syndrome. By slowing down conduction through the atrioventricular junction, these drugs increase the conduction of impulses along the accessory pathway, heart rate and contribute to the transition of arrhythmia to ventricular fibrillation. NB: Lidocaine is also not used in this situation, since it does not prolong the refractory period of the accessory pathway. In patients with ventricular preexcitation, lidocaine may increase the rate of ventricular responses in atrial fibrillation.
Prevention
There are fundamentally two ways to prevent repeated episodes of arrhythmia in patients with WPW syndrome: pharmacological and non-pharmacological. The first approach involves long-term use of antiarrhythmic drugs. For a number of reasons, this route is not optimal: 1) arrhythmias in WPW syndrome are characterized by a high rate of rhythm and are associated with a potential threat to life, while the response to drug therapy is variable and unpredictable; 2) some drugs can paradoxically increase the frequency of paroxysms or the rhythm during episodes of tachycardia; 3) WPW syndrome often occurs in children and young people, in whom long-term use of antiarrhythmics is especially undesirable; 4) one cannot ignore the risk of developing systemic side effects, for example, thyroid dysfunction, photosensitivity or lung damage when taking amiodarone. · If drug therapy is necessary, preference is given to class ІC and III drugs that can block conduction along the accessory pathway (in international recommendations - propafenone and flecainide (IC), sotalol and amiodarone (III); recommendations of Russian authors also include etacizine (IC) and gilurythmal (IA)). Evaluation of the effectiveness of amiodarone in the treatment of patients suffering from tachycardias involving the accessory pathway did not show any advantages compared with class IC drugs and sotalol. These data, as well as the risk of systemic side effects, limit the long-term use of amiodarone in WPW syndrome to prevent paroxysmal tachycardias. The exception is patients with WPW syndrome and structural heart disease.
- Propafenone – 600-900 mg/day per day in 3 divided doses.
- Sotalol – 80-160 (maximum – 240) mg 2 times a day.
- Etatsizin – 25-50 mg 3 times a day.
Amiodarone - after reaching a saturating dose of 10-12 g - 200 mg 1 time per day, daily or with a break 2 days a week.
· Long-term use of beta blockers in WPW syndrome is acceptable, especially if, according to EPI, the accessory pathway is not capable of rapid completion. · Verapamil, diltiazem and digoxin should not be prescribed to patients with ventricular preexcitation, due to the risk of increased ventricular responses if atrial fibrillation develops.
The second way, non-drug, is associated with the destruction of the accessory pathway, either using a catheter (usually by electrical radiofrequency ablation - RFA), or surgically during open-heart surgery. Currently, RFA is becoming the first-line treatment for symptomatic patients with WPW syndrome, gradually replacing both medical and surgical approaches. The effectiveness of initial ablation of the accessory pathway reaches 95%, although in 5% of cases the arrhythmia may recur after elimination of inflammation and edema in the damaged area. In such cases, the accessory tract is usually successfully destroyed when the procedure is repeated. Catheter ablation can sometimes cause complications, and in rare cases, death (0 to 0.2%).
Indications for RFA · Symptomatic reciprocal atrioventricular tachycardia. · Atrial fibrillation (or other symptomatic atrial tachyarrhythmias) with a high frequency of conduction along the accessory pathway. · Asymptomatic patients with ventricular pre-excitation (WPW phenomenon), if the safety of the patient and those around him (pilots, deep-sea divers, etc.) may depend on their professional activities during the spontaneous development of tachycardia. · Family history of sudden death. Thus, for the treatment of patients with WPW syndrome (ECG signs of pre-excitation and symptomatic arrhythmias), especially with hemodynamic instability during arrhythmia paroxysms, RFA of the accessory pathway is the method of choice. In patients with hidden accessory pathways (without pre-excitation on the ECG), with rare low-symtom attacks of tachycardia, the risk is low; the treatment approach may be more conservative and take into account the patient's preferences.
Patient education Patients with WPW syndrome should be informed about the causes of the disease and its possible manifestations. This is especially important for young asymptomatic individuals, in case of detection of ventricular preexcitation for the first time during random ECG recording (WPW phenomenon). It is necessary to recommend that the patient undergo dynamic monitoring with mandatory consultation with a doctor if symptoms appear. It is advisable that a patient with ventricular preexcitation always have an ECG with him with a conclusion. Patients receiving drug therapy should know what medications they are taking. When counseling a patient with WPW syndrome, you need to explain the following: · How to recognize the manifestations of the disease. · How to apply vagal techniques, if necessary. · What may be the side effects of antiarrhythmic drugs if the patient takes them. · The advisability of refusing to engage in competitive sports. · Possibilities of RFA in the treatment of WPW syndrome and indications for its implementation, if any appear in the future. Relatives of a patient with WPW syndrome should be recommended a screening examination to exclude ventricular preexcitation.
What is the difference between a syndrome and a phenomenon?
Many patients, having seen the concepts of the phenomenon or CLC syndrome in the ECG conclusion, may be puzzled which of these diagnoses is worse. The CLC phenomenon, subject to a correct lifestyle and regular monitoring by a cardiologist, does not pose a great danger to health, since the phenomenon
– this is the presence of signs of PQ shortening on the cardiogram, but without clinical manifestations of paroxysmal tachycardia.
Syndrome
CLC, in turn, is an ECG criterion that is accompanied by paroxysmal tachycardia, most often supraventricular, and can cause sudden cardiac death (in relatively rare cases). Typically, patients with short PQ syndrome develop supraventricular tachycardia, which can be quite successfully stopped at the stage of emergency medical care.
ICD-10 code
Wolff-Parkinson-White syndrome is a combination of premature ventricular excitation and paroxysmal tachycardia. With age, the frequency of paroxysms of supraventricular tachycardia increases. In patients under 40 years of age, 10% of cases are observed, and in patients over 60 years of age – 36%. The syndrome in most cases is a precursor (if it is recorded in people under 40 years of age) of arrhythmia. In 30% of cases the disease is combined with congenital heart defects.
I45.6 Premature excitation syndrome
Why does short PQ syndrome occur?
As already indicated, the anatomical substrate of this syndrome in adults is a congenital feature, since additional conduction bundles are formed in the prenatal period
. People with such bundles differ from ordinary people only in that they have an additional tiny “thread” in the heart, which takes an active part in conducting the impulse. But how the heart behaves with this beam will be discovered as the person grows and matures. For example, in children, CLC syndrome may begin to manifest itself both in infancy and adolescence, that is, during the rapid growth of the body. Or it may not manifest itself at all, remaining only an electrocardiographic phenomenon throughout adult life until old age.
No one can name the reason why the syndrome begins to manifest itself as paroxysmal tachycardia. However, it is known that in patients with organic pathology of the myocardium (myocarditis, heart attack, hypertrophic cardiomyopathy, heart disease, etc.) attacks of tachycardia occur much more often and clinically occur with a more pronounced clinical picture and with a severe general condition of the patient.
But the provoking factors that can cause paroxysm can be listed:
- Physical activity that is significantly or not significantly greater than the patient’s usual physical activity,
- Psycho-emotional stress, stressful situation,
- Hypertensive crisis,
- Eating large amounts of food at one time, drinking very hot or very cold liquids,
- Visiting a bathhouse, sauna,
- Changes in external temperatures, for example, going out into severe frost from a very hot room,
- Increased intra-abdominal pressure, for example, during severe coughing, sneezing, defecation, pushing during childbirth, lifting heavy objects, etc.
Reasons for development
The phenomenon and syndrome of CLC are congenital diseases. Their exact cause is unknown. One can only assume that it is associated with a harmful effect on the fetus at that moment in pregnancy, when the heart and its pathways are formed. A genetic cause is also possible - a “breakdown” of a certain gene responsible for the development of intracardiac pathways.
A shortening of the PQ interval is observed in two out of a hundred healthy people, more often in middle-aged men. CLC syndrome can also be caused by coronary artery disease, hypertension, myocardial infarction, rheumatism, hyperthyroidism, hypovitaminosis B and other conditions affecting nerve cells and blood supply to the heart.
How does short PQ syndrome manifest?
The clinical picture of shortened PQ syndrome is caused by the occurrence of paroxysmal tachycardia, since during the interictal period the patient usually does not present any complaints from the cardiovascular system. Symptoms of tachycardia are the following:
- The sudden, sharp onset of an attack, caused by provoking factors or occurring without them, in itself,
- Feeling of a strong heartbeat, sometimes with a feeling of interruptions in the heart,
- Autonomic manifestations - severe weakness, hyperemia or paleness of the face, sweating, coldness of the extremities, fear of death,
- Feeling of suffocation or lack of oxygen, feeling of insufficient inspiration,
- Unpleasant discomfort in the heart area of a pressing or burning nature.
If the symptoms described above appear, you should definitely seek medical help by calling an ambulance or going to a clinic.
Symptoms
WPW syndrome can be asymptomatic or with minor clinical manifestations, without causing severe hemodynamic disorders. The main complaints that patients make are: sudden interruptions in the functioning of the heart and attacks of rapid heartbeat. You may also experience increased fatigue, decreased exercise tolerance, dizziness, and a feeling of general weakness. During an attack of palpitations, shortness of breath, loss of consciousness, and a decrease in blood pressure (arterial hypotension) may occur.
Diagnosis of shortened PQ
The diagnosis is established after recording an ECG and interpreting its data by a doctor. The main ECG signs of CLC syndrome:
- Increased heart rate - 100-120 per minute or more, sometimes reaching 200 beats per minute,
- Shortening of the PQ interval between the P wave and the ventricular QRST complex is less than 0.11-0.12 seconds,
- Unchanged ventricular complexes in case of supraventricular tachycardia, and dilated, deformed - in case of ventricular tachycardia, which is a life-threatening condition,
- Correct sinus rhythm in supraventricular tachycardia.
After the diagnosis has been established and the paroxysm has been relieved, the patient is prescribed an additional examination to exclude gross cardiac pathology (heart defects, myocarditis, heart attack, etc.). Of these, the use of the following is justified:
- Ultrasound of the heart,
- Installation of an ECG monitor during the day,
- Examination of the electrocardiogram after physical activity (stress tests using bicycle ergometry, treadmill, tests with a load of pharmacological drugs),
- TEE, or transesophageal electrophysiological study and electrical stimulation of the heart muscle by inserting a probe into the esophagus,
- In especially unclear clinical cases, endovascular or intravascular EPI (endoEPI).
The plan for further examination and treatment of the patient is determined only by the attending physician.
Diagnosis of WPW syndrome
Diagnosis of SVC syndrome in children and adults in the cardiology departments of NEARMEDIC is a complete clinical and instrumental examination. The duration of the entire complex of diagnostic measures is no more than 2 days.
The obvious type syndrome is easily diagnosed by performing an electrocardiogram, since it is endowed with standard ECG signs. The latent form of pathology is determined using EPI - an electrophysiological study, in which special catheter electrodes are installed in parts of the heart cavity.
To identify concomitant pathologies, a number of laboratory tests are prescribed, and differential diagnostics are also carried out to exclude bundle branch block.
Modern methods for detecting cardiac pathology used in cardiology:
- Holter monitoring and fragmentary ECG monitoring;
- EFI;
- endocardial mapping;
- CT;
- MRI;
- Ultrasound of the heart (ECHO CG);
- transthoracic echocardiography;
- transesophageal cardiac pacing (guarantees more accurate research results compared to traditional ECG).
Treatment of short PQ syndrome
- The shortened PQ ,
also called the CLC phenomenon, does not require treatment. It is quite enough to correct the lifestyle and undergo regular examinations with a doctor - a cardiologist or arrhythmologist, for a child - once every six months, for adults - once a year.- Treatment of shortened PQ syndrome
(CLC - Clerk-Levi-Christesco syndrome) consists of providing first aid at the time of tachycardia paroxysm and further taking prescribed medications.
First aid can be provided by the patient independently - this is the use of vagal tests.
These manipulations are based on a reflex effect on the vagus nerve, which slows down the heart rate. Vagal tests can be used at the time of paroxysm only if the patient has had an attack of tachycardia not for the first time, he has been diagnosed and has not previously had ventricular tachycardia. In addition, vagal tests should be explained to the patient in detail by the doctor. The most effective techniques include the following:
- Straining test (Valsalva maneuver),
- Simulating a cough or sneeze
- Lowering your face into a basin of cold water, holding your breath,
- Apply finger pressure with moderate force to closed eyeballs for three to five minutes.
Restoring the correct heart rhythm
is performed by a doctor or paramedic in an ambulance and is carried out through the administration of medications intravenously. As a rule, this is asparkam, verapamil or betaloc. After hospitalization of the patient in a cardiology hospital, treatment of the underlying heart disease, if any, is carried out.
“cauterization” of pathological pathways using RFA
In case of frequent attacks of tachyarrhythmia (several per month, per week), as well as a history of ventricular arrhythmias, a hereditary history of sudden cardiac death or death from cardiac causes in young people, the patient is indicated for surgical treatment.
The operation involves applying radio frequencies, a laser or a cold factor to an additional beam. Accordingly, radiofrequency ablation (RFA), laser destruction or cryo-destruction are performed. All indications and contraindications are determined by an arrhythmologist, cardiologist and cardiac surgeon.
Many patients are interested in the possibility of permanent pacing. An pacemaker can be installed if the patient has a tendency to paroxysmal ventricular tachycardia, ventricular fibrillation and there is a high risk of clinical death with cardiac arrest (asystole). Then you can consider installing a cardioverter-defibrillator, which, unlike an artificial pacemaker, does not impose the correct rhythm, but “restarts” the heart when such fatal arrhythmias occur.
Is it possible to develop complications when PQ is shortened?
The phenomenon of shortened PQ cannot lead to any complications. Due to the fact that the manifestation of PQ syndrome is an attack of tachyarrhythmia, there will be corresponding complications. These include the occurrence of sudden cardiac death, fatal arrhythmias (ventricular fibrillation), thromboembolism of the cerebral and pulmonary arteries, the development of myocardial infarction, arrhythmogenic shock and acute heart failure. Of course, not every patient develops such complications, but everyone needs to remember them. Prevention of complications is timely seeking medical help, as well as timely surgery if indications for it are discovered by a doctor.
Prevention
Patients whose disease is asymptomatic do not need any special treatment. If a patient suffers from shortness of breath, dizziness, nausea, then this is a reason to contact a cardiologist, who will competently prescribe treatment and find out the reasons. Methods for preventing all heart diseases are:
- lose extra pounds;
- proper and rational nutrition;
- give up all bad habits;
- healthy sleep of at least 8 hours a day;
- reduce salt intake;
- more fresh air;
- avoid stressful situations.
If the disease is asymptomatic, a person can live his whole life and not even suspect the presence of such a syndrome. In rare cases, this syndrome is fatal. In any case, at the first manifestations, you need to contact a competent doctor to receive treatment and then your life will be safe.
CLC syndrome can lead to a number of complications and is aggravated by heart disease: tachycardia, sudden cardiac death and death of the patient.
Forecast
Determining the prognosis for patients with CLC syndrome is always difficult, since it is not possible to predict in advance the occurrence of certain rhythm disturbances, the frequency and conditions of their occurrence, as well as the occurrence of their complications.
According to statistics, the life expectancy of patients with short PQ syndrome is quite high, and paroxysmal rhythm disturbances most often occur in the form of supraventricular rather than ventricular tachycardias. However, in patients with underlying cardiac pathology, the risk of sudden cardiac death remains quite high.
The prognosis for the phenomenon of shortened PQ remains favorable, and the quality and life expectancy of such patients do not suffer.
Features of the pathology
Pre-excitation of the cardiac ventricles can be asymptomatic, in which case we are talking about the “pre-excitation phenomenon”. When signs of pathology appear in a patient, the disease is classified as “pre-excitation syndrome.”
There are several types of disease:
- Breschenmash (atriofascicular) - here the right atrium is connected to the trunk of the His bundle;
- Maheima (nodoventricular) - in this case, the right side of the interventricular septum is connected to the atrioventricular node;
- Kent (atrioventricular) - here the atria and ventricles are connected bypassing the atrioventricular node;
- James (atrionodal) - impulses pass between the lower part of the atrioventricular and sinoatrial node.
Important! Sometimes there are several paths of abnormal conduction of impulses. The number of such patients is no more than 10% of all cases of the disease.
CLC syndrome is clearly monitored by performing an electrocardiogram