Low hemoglobin - why it is dangerous
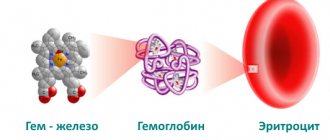
Hemoglobin is a complex chemical component of blood, which consists of protein and iron molecules. This constituent component is part of red blood cells and circulates through the bloodstream of the human body. The main task of hemoglobin is to transport oxygen molecules from the lungs to tissues, cells and internal organs.
With low hemoglobin, oxygen saturation of the body's cells decreases, which causes a decrease in immunity, a person loses vitality, organs do not receive proper nutrition, as a result of which their functionality is significantly reduced. Tissue cells suffer from oxygen depletion.
The most important complication of low hemoglobin is iron deficiency anemia. Its severe forms can cause loss of consciousness due to a high degree of brain hypoxia.
Iron deficiency conditions in gynecological diseases and methods for their correction
Iron deficiency anemia (IDA) is a hematological syndrome characterized by impaired hemoglobin synthesis due to iron deficiency. Anemia is based on tissue hypoxia, which develops as a result of a decrease in the amount of hemoglobin due to blood loss, impaired formation of red blood cells, their destruction, or a combination of these reasons [1, 2].
Iron deficiency occurs in almost 1/3 of the world's inhabitants, and iron deficiency anemia accounts for 80–90% of all anemias. The prevalence of anemia varies depending on gender, age, climatic-geographical and environmental-industrial causes [1, 3, 4].
A corresponding deficiency develops when iron losses exceed iron intake of 2 mg/day, and is observed in various physiological conditions and diseases [5, 6].
Iron deficiency (hypochromic, microcytic) anemia usually occurs due to a decrease in iron resources in the body due to chronic blood loss or insufficient external iron supply.
The most vulnerable to the development of iron deficiency are women of reproductive age due to monthly physiological blood loss during menstruation, pregnant women due to their high need for microelements, children and adolescents due to the high needs of a growing body, elderly people suffering from chronic diseases and having poor nutrition.
Iron is an essential microelement that plays an important role in the functioning of cells in many body systems, the main of which is the participation of iron in the processes of tissue respiration. The total amount of iron in a woman’s body reaches 2–3 g, and its concentration is 40–50 mg per kg of body weight.
There are two types of iron: heme and non-heme. Heme iron is part of hemoglobin, is found only in meat products, is easily absorbed, and its absorption is practically not affected by the composition of food.
Non-heme iron is found in free ionic form - ferrous or ferric iron. The absorption of non-heme iron (found primarily in vegetables), which accounts for up to 90% of the iron in the total diet, depends on a number of factors. The main part of iron is included in hemoglobin and myoglobin - 70%; iron depot - 18% (intracellular accumulation in the form of ferritin and hemosiderin); functioning iron - 12% (myoglobin and iron-containing enzymes); transported iron - 0.1% (iron bound to transferrin) [7–9].
The most important iron-containing compounds include: hemoproteins, the structural component of which is heme (hemoglobin, myoglobin, cytochromes, catalase, peroxidase), non-heme enzymes (succinate dehydrogenase, acetyl-CoA dehydrogenase, xanthine oxidase), ferritin, hemosiderin, transferrin.
In the mucous membrane of the small intestine, only divalent non-heme iron is absorbed from food. In order for iron to contact proteins and enter the cell, ferric iron is reduced to divalent iron in the presence of an acidic environment; in most cases, ascorbic acid plays the main role in this process [10–13]. Then, to enter the blood plasma and bind to transferrin, iron is again oxidized to trivalent. In the transferrin-bound state, iron is delivered to tissues through endocytosis, where it is either utilized by the cell or deposited in ferritin. Ferritin stores iron in an easily accessible and non-toxic form. Ferritin level is the “gold” standard indicator of the amount of stored iron in the body. Each microgram of ferritin corresponds to 8 mg of deposited iron and for women is normally 15–150 mg/ml [2, 14–16]. The concentration of serum iron is subject to significant daily fluctuations and averages 6.6–26 µmol/l in women. Therefore, its definition is not of paramount importance [2].
Hemoglobin is an oxygen transport protein containing heme iron. The hemes are combined into a protoporphyrin framework, in the center of which there is one divalent iron atom. One hemoglobin molecule can carry a maximum of 4 oxygen molecules. Thus, the number of red blood cells and hemoglobin plays an important role in oxygen transport [2].
During iron deficiency, successive stages are distinguished: latent iron deficiency, in which iron losses exceed iron intake without changes in hemoglobin concentration, and iron deficiency anemia itself, which, depending on the severity, is divided into compensated, subcompensated and decompensated forms [3].
Hemoglobin concentration is expressed in grams per liter. The normal hemoglobin level in women is considered to be 115-145 g/l (MCHC, Mean Corpuscular Hemoglobin Concentration - the average concentration of hemoglobin in a cell is 320-360 g/l); anemia is indicated when hemoglobin decreases below 110 g/l. The severity of anemia is judged by the level of hemoglobin. According to the severity of anemia, there is a mild degree - 90-109 g/l, a moderate degree - 70-89 g/l and a severe degree - less than 70 g/l [3, 14, 15].
In addition, iron deficiency anemia is characterized by a decrease in hematocrit, hypochromia (MCH, Mean Corpuscular Hemoglobin - the average absolute content of hemoglobin in one erythrocyte), microcytosis (MCV, Mean Corpuscular Volume - the average volume of an erythrocyte), hyposiderosis (a decrease in the amount of serum iron, an increase in total iron-binding ability of blood serum, decrease in ferritin level in blood serum and in hemolysate) [3, 5]. The number of red blood cells, hematocrit and hemoglobin constitute the measured values, and MCV, MCH, MCHC are derivatives of these values [2].
The clinical picture of anemia is caused by oxygen starvation of tissues, progressive hemic hypoxia with the subsequent development of secondary metabolic disorders. Clinical symptoms appear as the severity of the disease increases: general weakness, dizziness, headache, palpitations, shortness of breath, fainting, decreased performance, insomnia. Symptoms characteristic of IDA include changes in the skin, nails, hair, muscle weakness, and distortion of the sense of taste. The skin becomes dry and cracks appear on it. Due to impaired carotene metabolism, iron deficiency causes yellowness of the skin. The shape of the nails changes, they flatten, become concave and brittle. Hair becomes thinner, breaks and falls out profusely, and gray hair appears. With IDA, symptoms of damage to the cardiovascular system are also possible: palpitations, shortness of breath, chest pain and sometimes swelling in the legs [6, 14].
The main compensatory mechanisms are circulatory and ventilatory adaptation, including an increase in cardiac output, vasodilation, a decrease in vascular resistance, an increase in tissue perfusion, blood redistribution, an increase in minute volume of respiration, and an increase in erythropoietin activity. Acute, severe and uncompensated anemia can lead to circulatory collapse and shock [2].
The main cause of the development of iron deficiency anemia in women is blood loss of various etiologies. Iron deficiency is 6 times more common in women than in men. A significant amount of blood is lost during menstruation, pregnancy, and childbirth. With heavy menstruation, iron loss of 50–250 mg of iron is possible [2]. Most often, hyperpolymenorrhea is associated with the presence of uterine fibroids, adenomyosis, hyperplastic processes of the endometrium, and dysfunctional uterine bleeding. Metrorrhagia with uterine fibroids is most often associated with submucosal localization of nodes, in which the menstrual surface increases, the permeability of blood vessels supplying the fibroids increases, and the contractility of the uterus decreases. With fibroids, the regeneration of the bleeding surface of the uterus slows down after desquamation of the endometrium. With adenomyosis, the uterine myometrium is affected, which leads to prolonged heavy menstruation and secondary iron deficiency anemia.
Menstrual irregularities occur in women at different ages. Various reasons can lead to the development of hypermenstrual syndrome - severe emotional shocks, malnutrition, vitamin deficiencies, obesity, occupational hazards, infectious and septic diseases, immaturity of hypothalamic structures in puberty and involutive changes in premenopause. In a significant proportion of cases of menometrorrhagia in women, they are accompanied by anemia of varying severity, which contributes to the development of trophic disorders in various organs and tissues. Prevention and treatment of anemia in women with menstrual irregularities and organic gynecological diseases are the most important factors in restoring their health [1, 4, 17].
Regardless of the cause of menometrorrhagia (fibroids, endometriosis, ovarian dysfunction) and the need to influence the corresponding factor, long-term therapy with iron preparations for oral administration is required. The dose, dosage regimen and specific drug are selected individually, taking into account the iron content in the drug, its tolerability, etc.
When choosing a specific drug and the optimal dosage regimen, it is necessary to keep in mind that an adequate increase in hemoglobin levels in the presence of IDA can be ensured by the intake of 30 to 100 mg of ferrous iron into the body [18]. Dietary measures alone cannot compensate for iron deficiency and achieve a therapeutic effect.
Antianemic therapy should be carried out with oral medications and should not be stopped after hemoglobin normalization. Indications for parenteral administration are quite limited: intestinal pathology with malabsorption, intolerance to oral drugs, social reasons (use in patients with personality changes, the mentally ill). According to many studies, parenteral forms do not have advantages over oral ones, and a large number of serious side effects are noted [4, 10, 19]. Blood transfusions for IDA should be carried out only for health reasons.
Modern oral ferrous preparations are salts of ferrous iron (ferric ions are not absorbed in the digestive tract) or compounds consisting of a hydroxide-polymaltose complex of ferric iron (the absorption mechanism differs from that of ionic preparations) [20].
Ferric sulfate salt has the highest bioavailability, therefore ferrous sulfate is mainly contained in ferrous preparations [19, 21]. Ferrous sulfate is the most studied, proven form, which has proven itself with long-term use, therefore it is most often included in preparations for oral administration. It has the highest degree of absorption of all iron preparations.
In the process of absorption of divalent iron in the intestine, ascorbic acid is of great importance, which helps maintain iron in divalent form, so its presence in the preparation is very important [22]. In hematopoiesis, folic acid plays a significant role, enhancing nucleic acid metabolism. For normal folic acid metabolism, cyanocobalamin is necessary, which promotes the formation of its active form. Deficiency of these substances, which often occurs in anemia associated with blood loss, leads to disruption of DNA synthesis in hematopoietic cells, while the inclusion of these components in the drug increases the active absorption of iron in the intestine and its further utilization. The presence of ascorbic and folic acids, as well as cyanocobalamin in the drug significantly increases the rate of hemoglobin synthesis and increases the effectiveness of therapy for iron deficiency conditions and iron deficiency anemia [14, 23, 24].
The listed components that increase the bioavailability of iron are part of the complex antianemic drug Ferro-Folgamma® [24], which was developed and produced in accordance with the recommendations of the World Health Organization (1998).
1 capsule contains 112.6 mg of ferrous sulfate (elemental iron 37 mg), which is the optimal dose for therapy and reduces the incidence of side effects.
Absorption of iron from salt preparations (Fe2+) occurs in the form of passive diffusion according to a concentration gradient, does not depend on pH and motor activity of the gastrointestinal tract (GIT), which ensures rapid saturation.
Ascorbic acid improves the absorption of iron in the intestine, prevents its transition from divalent to trivalent form, accelerates the transport of iron and its inclusion in heme, and also participates in the process of releasing iron from the depot.
Ascorbic acid is necessary for the formation and preservation of the reduced form of folic acid - tetrahydrofolic acid.
In turn, folic acid is an essential factor for the synthesis of DNA and RNA, protein metabolism and the formation of red blood cells, and also acts as an additional stimulator of erythropoiesis and hematopoiesis [21, 23, 25].
Cyanocobolamine is necessary for the formation of hemoglobin, red blood cells, metabolism of proteins, fats, carbohydrates, and energy production.
Ferro-Folgamma® is highly effective, providing an average increase in hemoglobin of 2.5 g/l/day (the highest rate of increase in hemoglobin among ferrous sulfate preparations). When using the drug Ferro-Folgamma®, a weakening of the clinical symptoms of posthemorrhagic anemia is observed during the first 10 days of use [6].
Ferro-Folgamma® is prescribed 1 capsule 3 times a day after meals for 3–4 weeks for mild forms of anemia, for moderate forms — 1 capsule 3 times a day for 8–12 weeks, and for severe forms of anemia — 2 capsules 3 times a day for 16 weeks or more. During pregnancy, it is prescribed to prevent folic acid and iron deficiency, 1 capsule 3 times a day in the II and III trimesters, in the postpartum period during breastfeeding.
After cessation of treatment with Ferro-Folgamma®, the positive effect and stabilization of blood serum parameters (hemoglobin level, red blood cells, serum iron, total iron-binding capacity of blood serum) are guaranteed to persist for at least one month [4].
The active components of Ferro-Folgamma® are in a special neutral shell, which ensures their absorption in the upper part of the small intestine, which eliminates irritating effects on the stomach. The components are dissolved in rapeseed oil, which improves the absorption of iron and at the same time reduces the irritating effect of iron on the gastric mucosa, promoting good tolerability of the drug in the digestive tract [1, 13]. Due to the absence of an aggressive effect on the gastrointestinal mucosa, Ferro-Folgamma® can be successfully used to correct anemic syndrome, with a deficiency of vitamin B12 and folic acid against the background of impaired absorption in the gastrointestinal tract (atrophy of the gastric and duodenal mucosa).
In addition, Ferro-Folgamma® is effectively used for combined iron-folate-B12-deficiency anemia caused by chronic blood loss, chronic alcoholism, infections, taking anticonvulsants and oral contraceptives, anemia during pregnancy and breastfeeding.
The effectiveness of Ferro-Folgamma® for the treatment and prevention of iron deficiency anemia with good tolerability and favorable pharmacoeconomic characteristics (cost/iron dose/efficacy) was proven in several Russian studies that included a wide range of patients, including pregnant women and women with gynecological diseases [4, 18, 19]. Indications for the use of the drug Ferro-Folgamma® are anemia caused by a combined deficiency of iron, folic acid and vitamin B12, occurring against the background of chronic blood loss (menorrhagia and metrorrhagia, etc.), as well as with chronic alcoholism, infectious diseases, taking anticonvulsants and oral contraceptives. The drug is approved for the prevention and treatment of iron and folic acid deficiency in the second and third trimesters of pregnancy, in the postpartum period and during lactation.
Thus, anti-anemic therapy using the drug Ferro-Folgamma® with optimal iron content is highly effective with a rapid increase in hemoglobin (complete clinical and hematological remission in 93% of patients after 3 weeks of therapy). There is good tolerability - the absence of side effects in 95% of patients, allergic reactions and negative effects on the body of women, as well as the stability of the results achieved and the maintenance of a positive effect for a month after treatment, which makes it possible to recommend the drug Ferro-Folgamma® to a wide range of patients.
Literature
- Transcript of the scientific symposium “Iron deficiency conditions in obstetrics and gynecology.” III Russian Forum “Mother and Child”. M., 2001, 29.
- Hook R., Breiman K. Anemia during pregnancy and the postpartum period. M., 2007, 74.
- Gorodetsky V.V., Godulyan O.V. Iron deficiency conditions and iron deficiency anemia: diagnosis and treatment. Guidelines. M.: Medpraktika-M, 2005; 28.
- Konovodova E. N., Dokueva R. S.-E., Yakunina N. A. Iron deficiency conditions in obstetric and gynecological practice // Breast Cancer. 2011; 20: 1228–1231.
- Dolgov V.V., Lugovskaya S.A., Morozova V.T., Pochtar M.E. Laboratory diagnosis of anemia. M., 2001. P. 84.
- Kozlovskaya L.V. Hypochromic anemia: differential diagnosis and treatment // New Med. magazine 1996; 56:8–12.
- Shekhtman M. M. Guide to extragenital pathology in pregnant women. M., 2005, 816, 373–399.
- Johnson-Wimbley TD, Graham DY Diagnosis and management of iron deficiency anemia in the 21st century // Therap. Adv. Gastroenterol. 2011; 4 (3): 177–184.
- UNICEF/UNU/WHO. Iron Deficiency Anemia: Assessment, Prevention, and Control. A Guide for Program Managers. Geneva: WHO/NHD, 2001.
- Arkadyeva G.V. Diagnosis and treatment of iron deficiency conditions. Educational and methodological manual. M.: 1999: 22–25.
- Burlev V. A., Gasparov A. S. et al. Epocrine in the treatment of iron deficiency anemia in patients with uterine fibroids after hysterectomy // Problems of reproduction. 2003; 6:59–64.
- Kasabulatov N.M. Iron deficiency anemia in pregnant women // Breast cancer. 2003; 11, 1: 18–20.
- Lebedev V. A., Pashkov V. M. Principles of treatment of iron deficiency anemia in gynecological patients // Difficult patient. 2013, 11, 11: 3–7.
- Dvoretsky L.I., Zaspa E.A. Iron deficiency anemia in the practice of an obstetrician-gynecologist // Breastfeeding. 2008; 29.
- Kazyukova T.V., Samsygina G.A., Kalashnikova G.V. et al. New possibilities of ferrotherapy for iron deficiency anemia // Klin. pharmacology and therapy. 2000; No. 9 (2): 88–91.
- Pasricha SR, Flecknoe-Brown SC, Allen KJ et al. Diagnosis and management of iron deficiency anemia: a clinical update // Med. J. Aust. 2010; 193(9):525–532.
- Fernandez-Gaxiola AC, De-Regil LM Intermittent iron supplementation for reducing anemia and its associated impairments in menstruating women // Cochrane Database Syst. Rev. 2011. 12. CD009218.
- Burlev V.A., Konovodova E.N., Ordzhonikidze N.V., Serov V.N., Elohina T.B., Ilyasova N.A. Treatment of latent iron deficiency and iron deficiency anemia in pregnant women // Russian Bulletin of Obstetrics- gynecologist. 2006. No. 1. P. 64–68.
- Vertkin A.L., Godulyan O.V., Gorodetsky V.V., Skotnikov A.S. Iron deficiency anemia and the choice of drug for its correction // Russian Medical Journal. 2010. No. 5.
- Gratsianskaya A.N. Iron deficiency anemia: Ferro-Folgamma // Breast cancer. 2013; No. 29.
- Arvas A., Gur E. Are ferric compounds useful in the treatment of iron deficiency anemia? // Turk J Pediatr. 2000. Vol. 42(4). R. 352–354.
- Teucher B., Olivares M., Cori H. Enhancers of iron absorption: ascorbic acid and other organic acids // Int J Vitam Nutr Res. 2004. Vol. 74(6). R. 403–419.
- Konovodova E. N., Burlev V. A. Ferro-Folgamma + Erythropoietin - new possibilities for the treatment of anemia in patients with uterine fibroids // Farmateka. 2004. No. 15 (92). pp. 70–73.
- Ferro-Folgamma. Therapy with iron, folic acid, vitamin B12 and ascorbic acid. Scientific review. Werwag Pharma. M., 2001.
- Ghinea MM Treatment of iron deficiency anemia with Ferro-Folgamma // Rom J Intern Med. 2004. Vol. 42(1). R. 225–230.
A. Z. Khashukoeva1, Doctor of Medical Sciences, Professor S. A. Khlynova, Candidate of Medical Sciences M. V. Burdenko, Candidate of Medical Sciences M. R. Narimanova O. V. Kozlova, Candidate of Medical Sciences, Associate Professor
GBOU VPO RNIMU im. N. I. Pirogova Ministry of Health of the Russian Federation, Moscow
1 Contact information
Abstract. The role of Ferrum in the human organism has been analyzed, mechanisms of its digestion from food, clinical presentations of hypoferric conditions and women's hypoferric anemia, as well as approaches to therapy and prophylaxis of hypoferric anemia with modern oral preparations.
Low hemoglobin symptoms
Hemoglobin levels can be easily measured using a laboratory blood test, which can be prescribed by the local physician observing the patient. If you suspect a decrease in hemoglobin, and there is no time to wait for an appointment at a city clinic, you can contact any private laboratory. Such institutions often offer discounts on comprehensive examinations. You can donate blood to such an institution any day. The laboratory will not require you to buy your own syringe and container.
Symptoms to suspect low hemoglobin levels:
- daily weakness that does not disappear even on rest days;
- pale skin;
- there is a decrease in memory;
- tremor of the limbs appeared;
- hair lost its shine and began to fall out;
- shortness of breath appeared;
- Heart rate increased even at rest.
At the first signs of a decrease in hemoglobin, you should consult a doctor or donate blood yourself to check the iron level in your blood. However, detecting a decrease in hemoglobin is not enough. It is important to determine the reason why the body lacks it.
What is the norm for hemoglobin
As is already known, the protein is found in red blood cells and is responsible for the transport of oxygen in the body.
To maintain a normal level of health, eliminate fatigue, malaise and other characteristic signs of low hemoglobin, it is recommended to regularly take a blood test to determine protein.
The hemoglobin level is different for everyone, depending on age, gender and physiological characteristics of the body. So, for men this figure is 125-160 g/l, for women 115-140 g/l. In newborn babies, the protein level is in the range of 140-195 g/l; for children one year of age, hemoglobin is 110 g/l. And at school age, the blood protein level is at least 150 g/l.
In women, during pregnancy, hemoglobin drops and this is considered normal. At this time its value is about 110 g/l.
As a rule, a low rate is associated with iron deficiency anemia, which occurs in most people, regardless of age and gender.
Reasons for decreased hemoglobin
Hemoglobin appears in the blood thanks to a balanced diet rich in iron. First of all, the attending physician must find out which diet predominates in the patient. If the patient eats meals that exclude meat, animal proteins and legumes, the first step is to restore a varied diet.
If the patient is eating a fairly healthy and nutrient-dense diet but still has low hemoglobin, a number of other tests should be performed.
If we are talking about a female patient of reproductive age, then first of all it is necessary to conduct an ultrasound examination of the pelvic organs to detect pathologies that cause heavy bleeding. In addition, it is important to understand how much a woman experiences monthly blood loss associated with the menstrual cycle. Often they become the physiological cause of decreases in hemoglobin. In this case, the gynecologist should suspect the presence of fibroids, which forces the walls of the uterus to contract intensively, causing increased blood loss.
If we are talking about a male patient or examination of the female reproductive system did not produce results, the next stage of examination should be gastroscopy and colonoscopy. These diagnostic methods will help identify internal bleeding that is not so heavy as to become noticeable, but its regularity causes a significant amount of blood lost.
The third, no less important reason why hemoglobin may decrease is leukemia. This disease is associated with a gene mutation and causes disturbances in the functioning of the hematopoietic system.
How to compensate for iron deficiency?
A doctor should prescribe iron supplements; you should not buy them yourself at the pharmacy. “It is important to strictly follow the doctor’s recommendations. Hemoglobin and ferritin reserves are replenished slowly, up to 6 months, explains Irina Dobretsova. “Therefore, you should not stop taking medications before the end of the course or reduce the dose, even if you feel better. Sometimes, when taking iron supplements, there are side effects, such as belching and bloating. Then you can switch to intravenous administration of the drug, depending on the severity of the situation and the degree of intolerance.”
It is important to remember that preparations containing iron cannot be combined with dairy products. Calcium makes it difficult to absorb iron. It is also undesirable to drink iron supplements with black tea. But orange juice will give excellent results: vitamin C, on the contrary, helps iron absorption.
Who is at risk of iron deficiency or anemia?
- Pregnant women, unless taking special multivitamins
- Breastfed infants if the mother was anemic during pregnancy or while breastfeeding
- Babies whose diet was introduced to pureed meat too late (this is usually recommended from 6 months of age)
- Children who actively engage in sports and do not take additional vitamin and mineral complexes
- Teenagers during hormonal changes in the body
- Vegetarians
- Fans of strict diets and fasting.