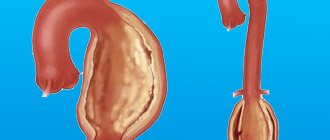
Normally, the pulmonary veins carry arterial blood from the lungs and drain into the left atrium. From there, the blood enters the left ventricle and carries oxygen throughout the body through the aorta and arteries.
If the pulmonary veins drain abnormally, they may drain into the right atrium or into the vena cava, which carries venous blood into the right atrium. This defect can be complete or partial.
- With complete anomalous pulmonary venous drainage, there is no direct communication between the pulmonary veins (pulmonary circulation) and the left atrium (systemic circulation). This state is incompatible with life. But if the child has other developmental defects (the presence of an atrial or ventricular septal defect, an unclosed foramen ovale), blood can still enter the left side of the heart and treatment is possible.
- With partial anomalous drainage of the pulmonary veins, one vein may flow into the left atrium and then the condition of the newborn depends on the volume of abnormal blood discharge. Complete anomalous pulmonary venous drainage occurs in approximately 3% of all congenital heart defects.
Four Types of Abnormal Pulmonary Vein Drainage
- The pulmonary veins can drain into the superior vena cava system, which collects venous blood from the upper extremities and head, occurring in 55% of cases.
- The pulmonary veins drain into the right atrium or into the coronary sinus, occurring in 30% of cases.
- The pulmonary veins can flow into the inferior vena cava system, which collects blood from the lower extremities and internal organs, occurring in 12% of cases.
- In 3% of cases there may be various combinations of this condition.
The condition of patients depends on whether it is a complete or partial defect. With complete anomalous drainage of the pulmonary veins and lack of communication between the right and left parts of the heart at the level of the atrium, newborns are in critical condition; emergency surgery is required to save their lives within the first weeks. With a partial defect, there is an overload of the right parts of the heart, an increase in pressure in the vessels of the lungs. Children of older age groups complain of shortness of breath and fatigue during physical activity. Then signs of right ventricular failure appear.
Currently, the etiological factors of many genetic syndromes and familial congenital heart defects (CHDs) have been identified, but the genetic basis of most “sporadic” CHDs still remains unknown. The leading causes of the development of congenital heart disease are presented in Fig. 1.
Rice. 1. Etiology of congenital heart disease [3].
Many genes associated with heart development have been identified. It has been established that a number of individual congenital heart diseases and genetic syndromes are associated with mutations in various single genes [1].
Many genes encoded by transcription factors or signaling molecules are currently associated with CHD in humans. Transcription factors are proteins that contain DNA-binding domains and play a fundamental regulatory role in controlling gene expression. Signaling molecules are proteins that enable cells to respond to their environment and are thus involved in the regulation of many important biological functions.
The phenotypes of congenital heart disease range from small septal defects that may remain undetected throughout life to hemodynamically significant abnormalities that manifest clinical symptoms. These include anomalies ranging from persistent fetal circulation (eg, patent ductus arteriosus) to complex defects such as transposition of the great vessels, single ventricle, hypoplastic left heart syndrome, and other heterotaxy variants. In accordance with the pathogenetic classification of congenital malformations of the cardiovascular system, there are 6 causal mechanisms: anomalies in the migration of ectomesenchymal tissue (anomalies of the aortic arch), defects in intracardiac blood flow (septal defects and obstructive defects of the left or right heart), anomalies associated with cell death (septal valve defects and anomalies), extracellular matrix abnormalities (atrioventricular canal defects), abnormal growth (partial or complete pulmonary venous return malformation and triatrial heart), and malposition and torsion, which includes left-right asymmetry [2].
For example, homeobox-containing proteins encoded by the NKX-2.5 group of genes play an important role in regulating the tissue-specific expression of genes required for tissue differentiation, as well as for determining temporal and spatial patterns of development. Research has recently shown that non-syndromic congenital heart disease may be the result of a single gene defect. Based on this, we can conclude that the predisposition to congenital heart disease is the result of single nucleotide polymorphisms or mutations of key genes, which, when interacting with environmental factors, disrupt the normal morphogenesis of the heart, which leads to the development of congenital cardiac anomalies (see table)
Genetic causes of congenital heart defects [3] [3].
Partial anomalous pulmonary venous drainage (PAPVD) is a congenital heart defect characterized by one or more (but not all) pulmonary veins draining into the right atrium, or into the vena cava, or into their main branches [4].
There are four anatomical forms of PAD, which are based on the location of the confluence of the pulmonary veins: supracardial, intracardial, infracardial and mixed. Anomalous drainage of the right pulmonary veins occurs 2 times more often than the left ones. The right pulmonary veins can drain into the superior vena cava, which is often combined with a defect of the venous sinus (Fig. 2, a),
Rice. 2. Scheme of options for CHADLV. a — drainage of the right pulmonary veins into the SVC with a venous sinus defect; b - in the IVC; c — confluence of the left pulmonary veins into the innominate vein; d - coronary sinus. a — right pulmonary veins drainage into SVC with sinus venosus ASD; b —into IVC; c — connection of left pulmonary veins with innominate vein; d - coronary sinus. or flow into the inferior vena cava (see Fig. 2, b) with an intact atrial septum and bronchopulmonary sequestration. The left pulmonary veins often drain into the innominate vein (see Fig. 2, c) or into the coronary sinus (see Fig. 2, d). Atrial septal defect is usually accompanied by abnormal drainage of the left pulmonary veins [5].
The defect was first described by Winslow in 1739. A pathological examination revealed abnormal drainage of the vein of the upper lobe of the right lung into the superior vena cava. Brody (1942) studied the anatomy and clinical picture of this defect most fully. R. Darling et al. systematized the anatomical variants (types) of the defect. [4] in 1957
The first successful operation to correct partial anomalous drainage using the closed “atrioseptopexy” method was performed by W. Neptune in 1953. In 1956, J. Kirklin et al. [4] reported 5 successful operations to correct the defect using the semi-open Gross method.
The specific causes of PAD are unknown. They may be associated with chromosomal abnormalities detected by karyotyping in more than 1/3 of patients with congenital heart disease. Most often this is trisomy on the 21st, 18th and 13th pairs of chromosomes. In addition to Down's disease, there are about 20 hereditary syndromes, in most cases accompanied by various congenital heart defects. In total, syndromic pathology is found in 6-36% of patients. The monogenic nature of congenital heart disease has been proven in 8% of cases; about 90% are inherited multifactorially, that is, they are the result of a combination of genetic predisposition and exposure to environmental factors. The latter act as provoking agents, revealing hereditary predisposition when the threshold of their joint action is exceeded [6, 7].
According to various studies [8–10], the incidence of PADVC among all congenital heart defects ranges from 0.7 to 9.4%. Of all cases of anomalous pulmonary venous drainage, about 2/3 of them occur in PAD. More often the right pulmonary veins are involved in the process. The superior vena cava is the most common site of entry for anomalous right pulmonary veins (35–57% of all cases of PAPV). Less commonly, the pulmonary veins flow into the azygos vein; an extremely rare option is the flow of one or all pulmonary veins into the innominate or accessory left vein.
The second most common type is the intracardial type of right-sided PAD. Anomalous drainage of the left pulmonary veins is extremely rare, with the pulmonary veins draining into the coronary sinus or directly into the right atrium.
With the infracardial type of defect, the veins (usually the middle and lower lobes of the right lung) flow into the inferior vena cava (IVC) immediately below the diaphragm [11].
PADLV may also be part of more complex congenital heart disease. About 20% of patients with PAD have associated heart defects, such as tetralogy of Fallot, atrial septal defect (ASD) or ventricular septal defect (VSD), common ventricle, common atrium, transposition of the great vessels, and hypoplastic left heart syndrome [12]. However, most often PAD is found in combination with ASD (more than 50% of cases) [9].
Between the 4th and 5th weeks of embryogenesis, pulmonary veins begin to form in the dorsal mesocardium, emanating from the midpharyngeal endothelial stalk [13]. The formation of PAD occurs as a result of atresia of a large branch of the common pulmonary vein. Once the right or left part of the common pulmonary vein becomes atretic, the persistence of the pulmonary-systemic venous connection on this side creates the etiological basis for PADV [12].
Risk factors for the development of PAD are maternal age over 40 years, toxicosis and threat of miscarriage in the first trimester, a history of stillbirth, and the presence of children with congenital malformations in parents and close relatives [14].
It should be noted that in the closest relatives of a patient with cardiac anomalies, the incidence of congenital heart disease is 2-5 times higher [14]. However, in the literature available to us, the familial form of PADLV has not been described.
Hemodynamics and clinical signs of PAD are consistent with those of ASD. X-ray examination may reveal dilation of the shadow of the superior vena cava (SVC) and the root of the right lung with abnormal drainage into the SVC. When the pulmonary veins flow into the SVC in the anteroposterior projection, against the background of the lower lobe of the right lung, the shadow of an abnormally running vessel in the form of a “Turkish saber” is revealed (S. Dolter, 1949). An increase in the vascular bundle to the left allows one to suspect an anomalous flow of the left pulmonary veins into the left innominate vein [15].
Currently, echocardiography (EchoCG) is the main diagnostic method of research in patients with PAD, however, only computed tomography (CT) with intravenous contrast allows one to establish a final diagnosis, clarify the anatomy of the congenital defect and determine further surgical tactics [16, 17].
There are several described techniques for correcting partial right anomalous pulmonary venous drainage: simple tunnelization (switching the pulmonary veins into the left atrium with a patch), tunnelization with expansion of the SVC with a patch, tunnelization with excision of the SVC and its movement into the right atrium (usually into the atrial appendage) [18 ].
There are no differences in the results of the three techniques. The choice of surgical intervention technique depends on the type of abnormal drainage and the clinical picture of the defect [18].
We present a clinical observation of patient A
., 18 years old, admitted to the cardiac surgery department with a diagnosis of congenital heart disease, ASD. Partial anomalous drainage of the right superior pulmonary veins into the superior vena cava. Pulmonary hypertension stage I Circulatory failure degree IIa, functional class II.
At the time of examination, the patient complained of shortness of breath and weakness during moderate physical activity. From the anamnesis it is known that the diagnosis of ASD was established in November 2021 at the age of 17 years according to echocardiography. In January 2021, a multislice CT (SCT) of the heart with intravenous contrast was performed, revealing a secondary ASD without an upper edge, partial anomalous drainage of the right pulmonary veins into the SVC, and dilation of the right chambers of the heart.
According to the SCT data, three veins from the upper, middle and partially lower right pulmonary veins flow into the lower third of the SVC in a single collector (diameter 17 and 11 mm) from the middle lobe (Fig. 3, 4).
Rice. 3. Patient A., 18 years old. SCT with IV contrast. 1 - ASD. CT-scan. 1 - atrial septal defect.
Rice. 4. Patient A., 18 years old. SKT. The collector of the upper, middle and partially lower right pulmonary veins, flowing into the SVC (indicated by an arrow). CT-scan. Partial anomalous pulmonary venous return into SVC (arrow). The distance from the upper edge of the collector to the level of the entry of the SVC into the right atrium was 17 mm. Also, a vein from the middle lobe of the right lung with a diameter of 5.5 mm flowed into the ASD area. An ASD without an upper edge measuring 25×18 mm was determined. The right inferior pulmonary vein, as well as the left pulmonary veins, drain into the left atrium.
The patient's condition upon examination was moderate. Blood pressure 110/70 mm Hg, heart rate 70 beats/min. Heart sounds are clear and rhythmic. A systolic murmur is heard in the second intercostal space on the left. The ECG showed 1st degree atrioventricular block (PQ interval 0.22 s), as well as incomplete right bundle branch block.
According to echocardiography, there was an enlargement of the right heart, dyskinesia of the interventricular septum, and pulmonary hypertension up to 40 mm Hg. In the area of the upper third of the MPP, a fault with a diameter of 12–13 mm was determined from left to right.
The patient admitted to our department was operated on. Intraoperatively: the heart is enlarged in size due to the right sections. A collector of three pulmonary veins from the upper and middle lobes of the right lung with a diameter of 4, 6 and 7 mm was visualized extrapericardially (Fig. 5),
Rice. 5. Intraoperative photo. The collector of the upper, middle and partially lower right pulmonary veins, flowing into the SVC (indicated by an arrow). Partial anomalous pulmonary venous return into SVC (arrow). flowing into the ERV at a distance of 17 mm from its mouth, the height of the collector is 14 mm. The vein from the lower lobe of the right lung flows into the left atrium. The left pulmonary veins also drain into the left atrium. During the revision of the MPP, a defect without an upper edge of 2.5×1.8 cm and an open oval window were noted. The lower edge of the defect is connected to the open oval window, forming an ASD of 3.5×2 cm (Fig. 6).
Rice. 6. Intraoperative photo. 1 - ASD without upper edge; 2 - open oval window. 1 — secundum atrial septal defect; 2 - primum atrial septal defect.
The defect was repaired with a tunnel-shaped patch made of xenopericardium (Fig. 7).
Rice. 7. Tunnel-shaped patch from the xenopericardium on the ASD with the relocation of the mouths of the right pulmonary veins to the left atrium. The MPP is sealed. IAS is sealed.
The postoperative period was uneventful and uneventful. The patient was discharged on the 8th day after surgery in satisfactory condition. Upon discharge, the ECG showed an inferior atrial, regular rhythm with a frequency of 75 beats/min, as well as incomplete blockade of the right bundle branch.
From the anamnesis it is known that the patient’s father, at the age of 15 years, was also diagnosed with PAD and ASD. He was operated on, and intraoperatively an anomalous drainage of the superior right pulmonary vein into the ostium of the SVC was discovered. The patient underwent ASD repair with relocation of the pulmonary vein from the upper lobe of the right lung to the left atrium.
In addition, the patient's cousin was operated on at the age of 8 months for congenital heart disease. A central atrial septal defect with a diameter of 15 mm and an anomalous drainage of the right superior pulmonary vein into the right atrium were identified; the defect was closed with a xenopericardial patch with relocation of the pulmonary vein orifice into the left atrium.
In our center, they underwent cardiac MRI to visualize the anatomical structure and monitor the long-term results of surgical treatment of patients (Fig. 8),
Rice. 8. Patient A. (father of the patient). MRI of the heart. The superior pulmonary veins flow into the left atrium at the mouth of the SVC (indicated by an arrow). MRI. Partial anomalous pulmonary venous return (arrow). According to the results of which, the collector of the right pulmonary veins flowing into the left atrium was visualized in the patient’s father. There was no evidence of ASD recanalization, other additional pathological discharges, or obstruction of blood flow through the pulmonary veins in both cases. A feature of these operations is the presence of an inferior atrial rhythm with an adequate frequency, which is associated with the placement of sutures in the projection of the sinus node.
Predisposition to congenital heart disease results from single nucleotide polymorphisms or mutations in key genes that, when interacting with environmental factors, disrupt normal cardiac morphogenesis, leading to the development of congenital cardiac anomalies. The specific causes of PAD are unknown. Deviations in embryogenesis and the presence of congenital heart disease in close relatives increase the likelihood of anomalies in the development of the heart. In the above clinical observation, the presence of PADLV was noted in the patient’s father and cousin. PAD is more common in combination with an ASD and is often represented by one or more abnormally draining right pulmonary veins. According to long-term results, surgical intervention in the form of tunnelization (moving the mouths of the pulmonary veins into the left atrium with a patch) is a reliable and effective method of treating PAD. In the available literature, we have not found a description of the familial form of PADLV.
The authors declare no conflict of interest.
The authors declare no conflicts of interest.
Information about authors
Ivanov A.S.
- Doctor of Medical Sciences, Prof.; https://orcid.org/0000-0001-9645-7192;
Glamazda S.V.
- Ph.D.; https://orcid.org/0000-0002-7623-2954;
Lugovsky M.K.
- Ph.D.; https://orcid.org/0000-0001-6461-7008, e-mail;
Lebedeva A.V.
- Ph.D.; https://orcid.org/0000-0001-7000-6254;
Govorova T.N.
— resident of the cardiac surgery department; https://orcid.org/0000-0001-6537-7173;
Satsyuk O.V.
- cardiovascular surgeon; https://orcid.org/0000-0001-8797-3283.
Ivanov A.S., Glamazda S.V., Lugovsky M.K., Lebedeva A.V., Abramova N.N., Govorova T.N., Satsyuk O.V. Familial form of partial anomalous pulmonary venous drainage. Cardiology and cardiovascular surgery
I. 2019;12(1):53-59. https://doi.org/10.17116/kardio20191201153
How is hemodynamics disrupted?
During intrauterine development, this heart defect does not manifest itself in any way, since the intracardiac circulation in the fetus is accompanied by communication between the left and right atria through the open foramen ovale. ADLV begins to make itself felt after the birth of a child, and the severity of hemodynamic disturbances will be determined by the type and variant of this anomaly. In addition, the severity of the symptoms of the defect may be influenced by the presence of concomitant defects in the development of the heart and blood vessels.
With total ADPV, oxygenated blood entering the right atrium mixes with venous blood. Next, some of it enters the right ventricle, and the other enters the left atrium through an existing atrial septal defect or patent foramen ovale. In such cases, total ADLV is compatible with life, since communication between the systemic and pulmonary circulation is not lost. Such hemodynamic disturbances lead to overload of the right chambers of the heart, increased pressure in the pulmonary vessels and a decrease in the oxygen content in the blood, which leads to oxygen starvation of organs and tissues.
With partial ADLV, hemodynamic disturbances occur in the same way as with interatrial defects. The severity of the patient's condition with this type of anomaly is determined by the volume of pathological arteriovenous discharge.
Diagnostics
One of the methods that helps detect and verify ADLV is cardiac ultrasound.
For the first time, ADLV can be detected in the fetus during an ultrasound examination in the third trimester of pregnancy.
To clarify all the data about such a congenital heart defect, the following instrumental research methods are prescribed:
- radiography;
- ECG;
- Echo-CG (for adults and older children, transesophageal echo-CG is prescribed);
- MRI;
- probing of the heart chambers and angiocardiography.
Symptoms
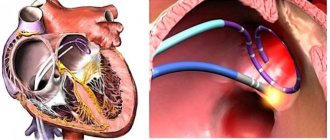
Signs of ADLV make themselves known immediately after the birth of the child. With a total defect, which is not accompanied by the presence of defects in interatrial communications, blood circulation between the small and large circles becomes completely impossible, and the newborn quickly dies. In such cases, only an emergency cardiac surgery using the Rashkind method, such as balloon endovascular atrioseptostomy, can save the child’s life in such cases.
In other cases, the severity of clinical manifestations of ADLV is determined by its anatomical variant, the size of atrial septal defects and the nature of hemodynamic disorders. Typically, parents of children with such a congenital defect notice the following manifestations of this pathology:
- fast fatiguability;
- the appearance of shortness of breath after physical activity (feeding, crying, etc.);
- pain in the heart area (the child sleeps poorly, is restless, cries loudly and stretches his legs);
- pale skin;
- mild cyanosis;
- cough;
- nausea and vomiting;
- slow weight gain;
- frequent ARVI and pneumonia.
By palpating the pulse, the doctor can detect its rapidity and arrhythmia. Upon examination, deformation of the chest and an increased heartbeat are determined.
Mild cyanosis that occurs in the first weeks of life may disappear or become less pronounced over time. As a rule, cyanosis is more pronounced during physical activity.
When listening to heart sounds, specific signs of this defect are not detected. Can be heard:
- loud I tone over the heart;
- splitting of the second tone with increased pulmonary component;
- heard III sound above the apex of the heart (in many patients);
- soft systolic murmur over the pulmonary artery with variable duration and intensity.
The ECG determines the overload of the right parts of the heart - high voltage of the P wave in the right leads and deviation of the electrical axis to the right in standard leads. Overload of the right atrium is indicated by a high P wave in the right chest and standard leads. Often there are signs of incomplete blockade of the right bundle branch.
If there is a large defect in the interatrial septum, the child may develop normally and then, at an older age, complaints arise of a significant decrease in exercise tolerance, shortness of breath, pallor and poor general health. Later, such children develop signs of right ventricular failure.
Treatment
The only way to eliminate ADLV is its cardiac surgical correction. Before surgery, the child is recommended to limit physical activity and is prescribed medications to prevent acute heart failure (diuretics, cardiac glycosides). Parents of a child should be aware that even crying or temperature discomfort can significantly worsen the state of health with such a heart defect. If the child is already an adult, then they must constantly monitor his leisure time - he should not run, lift weights and overwork.
Methods of surgical correction
The method of performing cardiac surgical correction for ADPV is determined by the type and variant of the defect.
With total drainage, a critically ill child under 3 months of age can undergo palliative surgery, which consists of increasing interatrial communication using balloon atrioseptomy using the Rashkind technique. This correction promotes blood flow into the left atrium and restoration of blood circulation in the systemic circle.
To radically eliminate ADLV, interventions are carried out aimed at creating a wide anastomosis between the left atrium and the pulmonary veins, stopping the pathological drainage of the pulmonary veins with other venous vessels and eliminating the atrial septal defect. It is preferable to perform such interventions at an early age. The radical correction technique is selected depending on the anatomical variant of the ADLV.
Corrective interventions are quite effective, however, despite the development of modern cardiac surgery, the percentage of deaths occurring during surgery or in the postoperative period remains high. The result of radical correction largely depends on the skill of the cardiac surgeon, technical equipment, and the type and variant of the defect. In addition, the highest risk of death is observed among newborns and young children suffering from severe pulmonary hypertension. Most often, this manifestation of the defect is observed among patients with the subcardial variant of the anomaly.
Long-term results among children who survived radical correction of ADLV are quite satisfactory. Only some patients may develop sinus node weakness and pulmonary hypertension, which are difficult to respond to drug therapy.
Causes
Heredity probably plays a certain role in the development of ADLV.
The probable causes of the development of ADLV may be the same external factors that provoke other congenital anomalies of the structure of the heart and blood vessels:
- heredity (chromosome mutations);
- taking certain teratogenic drugs;
- exposure to toxic substances on the body during pregnancy;
- bad habits of the expectant mother;
- infectious diseases suffered by the pregnant woman;
- endocrine disorders;
- toxicosis;
- unfavorable environment.
Disconnection of the pulmonary veins from the left atrium can be caused by the following factors:
- the absence of their connection with the left atrium is formed due to the fact that, under the influence of the external causes described above, the left atrial outgrowth cannot communicate with the venous plexuses of the future lung;
- early pulmonary vein atresia - occurs with the initial connection of the pulmonary vascular bed and the common pulmonary vein, the lumen of which is subsequently obliterated, and blood from the lungs begins to flow through other collateral pathways.
A little history
This rare congenital defect was first described by Wilson back in 1798, but its variants were examined in more detail only after sufficient cardiac surgical experience had been accumulated in the 50-60s of the 20th century. The first successful operation to correct this pathology was performed by Miller in 1951. Subsequently, cardiac surgical techniques were improved, and in 1956, radical correction of ADLV was carried out using superficial hypothermia. In the same year, a similar intervention was performed using artificial circulation.
On the territory of the USSR, the first successful operation to eliminate such a heart defect was performed by Nikolai Mikhailovich Amosov in 1978, and one of the leading Russian cardiac surgeons, Georgy Eduardovich Falkovsky, reported on a series of successful corrections in young children in 1984.