Publications in the media
Aortic valve stenosis is a heart defect in the form of narrowing of the aortic opening due to pathology of the aortic valve and perivalvular structures.
Frequency • Isolated aortic valve stenosis - 1.5–2% of acquired valve defects; Aortic valve stenosis in combination with other defects - 23% • Congenital aortic stenosis - 3–5.5% of all congenital heart defects; in combination with other congenital disorders - 13% • The predominant gender is male.
Genetic aspects. Elastin gene defects (130160) in Williams syndrome (see Miscellaneous syndromes) and Eisenberg syndrome (*185500, supravalvular stenosis of the aorta, pulmonary arteries, peripheral arteries).
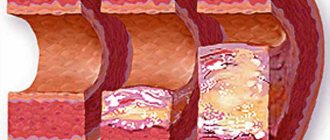
Etiology • Congenital anomalies, including cusp number anomalies and congenital isolated aortic valve calcification syndrome (61.3%) • Rheumatism (12.6%) • Atherosclerosis (24.2%) • Adult idiopathic calcification (1.9%).
Pathophysiology • Increased afterload ® increased left ventricular (LV) wall tension ® concentric LV hypertrophy ® decreased muscle fiber tension [according to Laplace's law: wall tension = (pressure´ radius)/(2 ´ wall thickness)] • This mechanism maintains systolic function, despite an increase in systolic pressure in the left ventricle • Further myogenic dilatation leads to decompensation and rapid progression of circulatory failure • A sharp decrease in cardiac output leads to insufficient blood supply to the brain, which is manifested by transient or permanent ischemia • If aortic valve insufficiency is associated with aortic valve stenosis, to the increased afterload, an increase in preload is added, which leads to an even greater increase in LV wall tension and a decrease in effective ejection • As a result of the discrepancy between perfusion pressure in the coronary bed and, accordingly, the volume of coronary blood flow to the needs of the hypertrophied myocardium, relative coronary insufficiency develops • With aortic atherosclerosis, the frequency of concomitant IHD, which makes diagnosis difficult • All of these factors lead to a high risk of sudden death • Late manifestations of the defect - hypertension in the pulmonary circulation.
Classification . Depending on the severity (determined by the systolic pressure gradient [SPG] between the LV and the aorta and the area of the valve orifice), four degrees of stenosis are distinguished: degree I - moderate stenosis, GSD <50 mm Hg, valve orifice area >1 cm2 ( norm 2.5–3.5 cm2); II degree - severe stenosis, GSD 50–80 mm Hg, valve opening area 1–0.7 cm2; III degree - severe stenosis, GSD >80 mm Hg; IV degree - critical stenosis, GSD above 80 mm Hg, valve opening area 0.7–0.5 cm2.
Clinical picture and diagnosis
• Complaints: pain in the heart (angina), fainting and shortness of breath are classic symptoms of aortic stenosis.
• Peripheral symptoms are caused by small output syndrome •• Pallor of the skin •• Tachycardia •• Low slow pulse •• “Heart hump” (with the development of the defect in childhood).
• Valvular symptoms •• Systolic trembling in the sternum, in the jugular and supraclavicular fossae •• Weakening of the aortic component of the II tone with severe calcification and decreased mobility of the valves •• With preserved mobility of the valves - increased II tone or click •• With relative mobility of the valves following I tone is used to listen to the systolic ejection sound, which occurs when the movement of the valves stops •• A rough, loud, high-frequency fusiform ejection noise, very well carried to the shoulders, shoulder blades, back of the head and, especially, to the great vessels (the volume of the noise is sometimes so high that it is even carried out on the chair on which the patient is sitting) •• The duration of the noise reflects the severity of the obstruction to a greater extent than its intensity •• Sirotinin-Kukoverov symptom - increased noise when raising the arms up •• With severe calcification of the valves, high-frequency components of the noise can be carried out in the axillary region or to the apex of the heart in the form of a soft mid-systolic murmur, simulating the murmur of mitral regurgitation - Galawarden's symptom.
• Left ventricular symptoms are caused by hypertrophy, dilatation and insufficiency of the pumping and contractile functions of the LV •• Long-lasting, but not diffuse apical impulse, shifted to the left, and a palpable IV tone are almost always present •• An increase in the area of relative dullness of the heart to the left •• Auscultatory signs of congestion in the lungs - diffuse moist rales of various sizes, best heard in the basal regions.
• Symptoms of the underlying disease: rheumatism, atherosclerosis, congenital heart disease.
Special studies
• ECG: signs of hypertrophy and overload of the left sections, primarily the LV; signs of myocardial ischemia.
• Sphygmography: a prolonged low pulse waveform with a difficult-to-define dicrotic notch and jagged apex—a symptom of a “cockscomb.”
• X-ray of the chest organs •• Cardiomegaly (cardiothoracic index exceeds 50%) due to expansion of the LV arch •• Signs of pulmonary congestion •• Expansion of the aortic arch due to its poststenotic dilatation •• Calcification of the leaflets and fibrous ring of the aortic valve.
• EchoCG •• Expansion of the cavity and hypertrophy of the LV myocardium •• Violation of local and global systolic, as well as diastolic functions of the LV •• Poststenotic expansion of the ascending aorta •• Damage to the cusps and apparatus of the aortic valve (defects, vegetations, abnormalities in the number of cusps, increased echogenicity and acoustic shadow with calcification, decrease in the amplitude of movement of the valves) •• In Doppler mode, high-speed flow is recorded through the narrowed opening of the aortic valve and the peak systolic and average pressure gradients between the LV and the aorta are determined •• The area of the aortic valve opening is measured in Doppler and B-modes (as a basis For diagnosis, the worst indicator is taken).
• Catheterization of the left and right ventricles and aorta: direct pressure measurement; see also Aortic valve insufficiency.
• Left ventriculography, ascending aortography •• The presence and degree of stenosis is determined by the diameter of the jet of contrast agent expelled from the LV •• The presence of zones of hypo- and akinesia of the LV indicates myocardial ischemia •• Combined valvular lesions are also diagnosed.
• Coronary angiography: see Aortic valve insufficiency.
TREATMENT
• Drug therapy •• The possibilities of conservative treatment are limited, since it has little effect on the functional class and mortality •• Regardless of the severity of aortic stenosis, infective endocarditis is prevented (see Infectious endocarditis) •• LV systolic dysfunction with congestive heart failure - cardiac glycosides, diuretics , treatment of atrial fibrillation, dual-chamber pacemaker, vasodilators - in small doses and under monitor control (can increase GDM) •• For refractory heart failure - IV inotropes •• Intra-aortic balloon counterpulsation is used as a backup method of hemodynamic stabilization in preparation for surgery •• Termination of pregnancy is indicated.
• Surgical treatment •• Indications ••• Aortic valve stenosis grade III–IV, accompanied by clinical symptoms ••• Asymptomatic aortic stenosis with LV dysfunction ••• There is no consensus on whether surgical treatment is indicated for severe asymptomatic aortic stenosis with normal function LV •• Contraindications: see Aortic valve insufficiency •• Methods of surgical treatment ••• Balloon valvuloplasty - radical treatment for a congenital unicuspid or bicuspid aortic valve, preparation for replacement in case of cardiogenic shock, patient refusal or impossibility of replacement in the near future (for example, with pregnancy, in elderly and debilitated patients) ••• Aortic valve replacement with a mechanical artificial heart valve under conditions of artificial circulation ••• Biological prostheses are not used.
Specific postoperative complications • Restenosis • Angina pectoris • Hemolytic anemia • Secondary infective endocarditis of the prosthesis • Thromboembolism • Aneurysms of the ascending aorta when using disc prostheses with a small opening angle.
Course and prognosis • In the natural course, the 5-year survival rate does not exceed 25%, and the 10-year survival rate does not exceed 10% • The average life expectancy after the onset of angina attacks is 3.5 years, after the onset of syncope episodes - 2 years, after the onset of LV failure - 1.5 years, after the onset of generalized heart failure - 7 months • Sudden death is recorded in 15–20% of patients with clinical manifestations • With GDM above 50 mm Hg. 17% of patients survived 3 years • With surgical treatment, hospital mortality is 3–8%, 9-year survival exceeds 85% with initially preserved function and 10–25% with LV dysfunction • In general, the prognosis after aortic valve replacement with its stenosis is better, than with insufficiency • After valvuloplasty for isolated calcification and rheumatism, despite a decrease in GDM and the area of the aortic valve orifice, in most cases severe aortic valve stenosis persists, postoperative mortality reaches 6%, mortality within a year is 25%, restenosis rate is 60% within 6 months.
Synonym. Aortic stenosis, aortic stenosis.
Abbreviations • GSD - systolic pressure gradient • LV - left ventricle.
ICD-10 • I06 Rheumatic diseases of the aortic valve • I35 Non-rheumatic lesions of the aortic valve
Congenital aortic valve stenosis
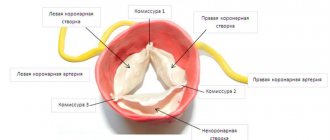
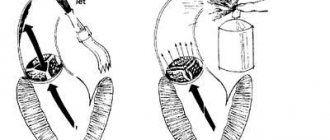
The essence of the violations in this quite common defect is as follows. The aortic valve, which regulates the flow of blood from the left ventricle to its ascending section, consists of three thin semilunar valves, which with each contraction of the left ventricle are pressed against the wall of the aorta, opening the way for the free flow of blood, and with each relaxation they close, blocking this path.
During one cardiac cycle, they thus perform two actions: opening and closing the “gate”. One can only imagine how ideal the condition of the doors themselves must be - their elasticity, mobility, density - in order to ensure such long-term, full-fledged and continuous operation. After all, their incomplete contact is enough to create an obstruction to the flow or reverse flow of blood towards the ventricle.
Stenosis (as applied to valves or vessels) means narrowing
With a congenital defect, the narrowing can be due to improper development of the valves, their fusion with each other (more precisely, their “non-division” into three), the absence of one of the valves (when there are two instead of three, but they still completely cover the valve opening) and finally, due to the narrowness of the ring itself to which these doors are attached. More often there is a combination of these structural changes, expressed to a greater or lesser extent.
The obstruction to blood flow creates a pressure difference between the left ventricle and the aorta, and the extent of this difference depends on the size of the opening and the state of development of the leaflets themselves. In any case, the load on the left ventricle is greater than during normal blood circulation: with a sharp narrowing, the ventricle may not be able to cope with it at all, but with a small one, it is relatively easy to bear everything, gradually thickening its wall and increasing in size.
In approximately 10% of cases of this defect, the valve leaflets are so disfigured that instead of them there is a membrane with a hole of 1-2 mm with small grooves. It is interesting that in a fetus located in the uterus, such a gross violation of the normal development of the heart almost does not manifest itself at all. This is understandable, since the bulk of the fetal blood bypasses the aortic valve through the pulmonary trunk and patent ductus arteriosus into the aorta. However, it can be diagnosed using echocardiography starting from 6-7 months of pregnancy, which is very important, since with such stenoses the child is in critical condition immediately after birth. Events will develop dramatically quickly, and if he is not helped urgently, he will die within the first weeks. This will happen, first of all, due to the fact that the left ventricle will constantly work with a load exceeding its capabilities, and it can “give up” very quickly. Moreover, the process of blood supply to the wall of the left ventricle itself, its nutrition, is disrupted. Urgent surgery is required.
It would seem - what could be simpler? Take it, “open” the valve - and that’s it. Fast and easy, especially with all the modern capabilities.
Unfortunately, it is not. Or rather, this is not always the case. In “ideal” cases, it is really not difficult to cut the fused valves and widen the valve opening. This is done in the X-ray surgery room. The procedure is short and quite safe. But, if the valves are poorly developed and the valve ring is narrow, then the effect may not be achieved, and in addition, the intervention itself may be too traumatic. Therefore, even today, treatment of children with critical aortic stenosis is associated with a very high degree of risk. Rupture of undeveloped valves in no way restores them, but only eliminates the narrowing, saving the child from imminent death. Even with a successful immediate outcome at this stage, in the future he will have to undergo surgery on the aortic valve to completely restore its normal function.
Interestingly, in recent years, a technique has been developed to eliminate aortic stenosis in the fetus before it is born, allowing the heart to function normally in the last months of intrauterine life and be better prepared for the stress that awaits it after the first independent breath. These are still the first steps, but it is quite possible that the day is approaching when critical aortic valve stenosis will become a completely treatable heart defect.
Fortunately, in most cases, the valve ring is moderately narrowed, two or only one valve leaf is fused with its neighbors, but not completely, and in general, the device functions well. Not bad - but not perfect. There is a load on the left ventricle, and its degree depends on the size of the obstacle. What happens to the ventricle? Its wall thickens, its mass increases, the cavity of its chamber decreases and the supply of the wall with arterial blood worsens due to the fact that the thickening is much faster than the development of the vascular network in the thickness of the heart muscle. Moreover, in the first operation, if the valve was represented by a poorly divided membrane, the result was achieved only by widening the narrow opening. The doors themselves remained disfigured. They do not close the hole completely, because... do not close in the diastole phase. As a result, some of the blood thrown into the aorta flows back into the left ventricle, increasing the already excessive load. This is valve insufficiency, which in combination with its stenosis significantly worsens the clinical picture, and will accelerate the need for complete corrective surgery.
Aortic valve stenosis in older children is a completely different and much less dramatic story. There may be no signs of heart failure for a long time, and the diagnosis is made on the basis of a typical murmur on the valve and according to echocardiography. A slow but steady increase in heart size, rhythm disturbances, and sometimes complaints of chest pain may be a sign that intervention is necessary. At the same time, the course of the disease allows you to monitor it over time and choose the time and method of correcting the defect.
In the best case scenario, it’s all about the doors themselves and simply separating them will be all that matters. In others, they are so altered that the entire valve will have to be replaced with an artificial prosthesis. In the third - the most severe - the aortic valve ring itself can be so narrow that it is impossible to sew a prosthesis into it, even the smallest in diameter. Then various methods of expanding this ring are used. These are already large and traumatic interventions with significant risks, and an excellent result is no longer so natural.
We won't go into technical details. The specifics of your child’s operation, as well as the degree of risk and consequences, should be explained to you in detail. Let's just say that a long (meaning 10-20 years) life with aortic valve stenosis is dangerous, because can lead to serious complications, including sudden death, in an otherwise apparently healthy teenager. In any case, the sword of Damocles of the necessary operation hangs until it is done, and this fact is also unfavorable for the normal psychological state of both the child and yourself.
The results of operations, including long-term ones, are quite good if they are performed against a favorable (in all other respects) background.
It is interesting that in a very common variant of a normally functioning aortic valve, when instead of three leaflets there are two (and they are discovered by chance in an “asymptomatic” person), the processes of “aging” of the leaflets associated with age proceed much faster than with a tricuspid structure. The destruction of the valves themselves and the deposition of calcium in them can be observed at the age of 40-50, when the first clinical symptoms appear: shortness of breath, enlarged heart, chest pain, noticeable rhythm disturbances. It is clear that this is a completely different scenario than the one described above, but it is caused by the same aortic valve stenosis. And, if the bicuspid valve is undoubtedly congenital, then its consequences can be attributed to congenital heart defects, no matter at what age they were first identified.
The indication for valve replacement when symptoms appear is absolute because the next symptom may be sudden cardiac arrest and death. The operation of aortic valve replacement today should not be accompanied by virtually any risk, and the designs of the valves themselves are so advanced and durable that they allow you to lead a normal life for many years.
How to get treatment at the Scientific Center named after. A.N. Bakuleva?
Online consultations
Minimally invasive aortic valve replacement surgery
Return to section:
Cardiology department
The blood expelled by the heart enters the aorta. The aorta is the largest vessel in the human body, through which blood is directed to organs and tissues. The aorta is connected to the heart by the aortic valve. By opening, this valve facilitates the outflow of blood from the heart; when closing, it prevents blood from penetrating back into the heart chambers.
Surgery to replace (prosthetic) the aortic valve may be required if dysfunction (malfunction) of the valve occurs. This, in turn, may be due to two reasons:
- The aortic valve narrows and does not allow the required amount of blood to pass through, i.e. Blood flow to the body decreases - this is called “aortic stenosis.” Among the main causes are age-related degenerative changes and rheumatism.
- The valve “leaks”, does not hold blood, and blood leaks back into the heart cavity - this is called “aortic regurgitation”. The main causes: congenital genetic defects, age-related enlargement of the aorta and valve ring, aortic diseases and bacterial damage to the valve.
Aortic valve replacement surgery involves removing the failed valve and replacing it with an artificial one. The operation is performed under conditions of artificial circulation, i.e. in a stopped heart, when the function of blood supply to organs and tissues is taken over by a special device. During the operation, the patient is under anesthesia and does not feel pain.
The first operation of aortic valve replacement with an artificial valve was performed in the early 1960s. Traditionally, the operation is performed through a 20 cm long midline incision in the sternum. To reach the heart, it is necessary to cut through the skin, soft tissue, the bone located in the middle of the chest, called the sternum, and the special lining of the heart, the pericardium. The next step is to connect the heart-lung machine by sequentially connecting the aorta and cardiac veins to it, and then temporarily stop the heart. Next, the surgeon dissects the aorta in a transverse direction to see the aortic valve. If the valve is severely damaged, it is removed and a new, artificial heart valve is sewn in its place. Artificial valves are divided into two types:
- Mechanical (metal-carbon): durable, can work effectively for decades, but requires lifelong use of drugs that reduce blood clotting (anticoagulants), which increases the risk of bleeding;
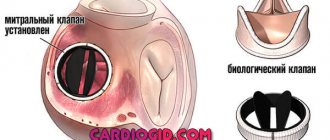
- Biological (created from animal or human tissue): more physiological, but less durable, lasting no more than 20 years, but does not require lifelong use of anticoagulants.
| Modern types of artificial heart valves: on the left – mechanical; on the right is biological. | |
After installing a new valve, the integrity of the aorta, sternum, soft tissue of the chest and skin is restored in turn. The duration of the operation is 2.5 – 3 hours.
| Operational access scheme. Full median sternotomy (left), J-shaped ministernotomy (right). | |
Modern knowledge and experience make it possible to reduce the size of the chest wound to perform aortic valve replacement surgery. Such operations are called minimally invasive, i.e. low-traumatic - through reduced access. The use of small accesses was proposed in the mid-1990s.
The technique of surgical intervention remains the same, but the length of the incision is only 7 centimeters, and you can do without a complete dissection of the sternum (full median sternotomy), which can significantly reduce the trauma of the operation, the severity of pain in the postoperative period, preserve the skeletal function of the chest, and speed up the recovery of the respiratory system. functions and return to normal life while maintaining high effectiveness of treatment.
The undoubted advantage for the patient with this operation is the cosmetic result.
Unlike traditional surgery, minimally invasive surgeries take slightly longer (3 – 3.5 hours).
Rice. 3 . Appearance of the chest wound after traditional (left) and minimally invasive surgery (right).
Over the past decade, a new, unique method of aortic valve replacement has been developed: Transcatheter Aortic Valve Implantation (TAVI).
This procedure is performed in a cath lab with a team consisting of cardiologists, cardiac surgeons, and x-ray surgery specialists. Most importantly, this procedure is performed on a beating heart, thus avoiding the risks associated with cardiopulmonary bypass.
An incision 3 cm long is made on the side of the chest as shown in Figure 4. In this case, the intervention is called transapical catheter valve implantation.
Rice. 4 . Access scheme for transapical catheter implantation of the aortic valve (Cheung, Lichtenstein, Annals of Cardiothoracic Surgery, 2012).
Two special hemostatic sutures are placed in the area near the apex of the heart, through which a high-tech device is passed - a delivery system, the diameter of which is only 8 mm. This system contains a biological valve in a “collapsed” state.
The valve is positioned at the required level under the control of an X-ray machine, which can rotate around the patient on a special arc, making it possible to obtain X-ray images from different angles.
Next, the artificial valve opens, occupying a predetermined position in the aortic root. The valve can be opened either using a balloon inflated with helium (see Fig. 5), or independently - for valves with a frame made of shape memory metal called “Nitinol” (an alloy of nickel and titanium).
| Opening an artificial valve using a balloon | View of the valve installed in the aortic position during transapical catheter implantation |
| (Cheung, Lichtenstein, Annals of Cardiothoracic Surgery, 2012). | |
Rice. 6 . Biological valve for implantation in the aortic position using a transcatheter method.
It should be noted, however, that there are strict indications for such an operation. Transcatheter valve implantation is performed for those patients who are contraindicated for traditional surgical intervention due to high risk (they may not tolerate traditional surgery): these are patients with critical aortic valve stenosis, usually elderly (over 80 years old), patients who have undergone coronary artery bypass surgery, patients with diseases of the aorta, which create technical difficulties when accessing the heart and valve from the classical approach.
The transcatheter implantation operation takes about 40 - 60 minutes, and the recovery period after it is much shorter compared to traditional surgery.
At the Department of Cardiac Surgery of City Hospital No. 40, all considered types of correction of acquired aortic valve defects are used.
← Back