Publications in the media
Tricuspid valve stenosis is a narrowing of the tricuspid valve opening that prevents blood from flowing from the right atrium (RA) into the right ventricle (RV). Frequency • Severe tricuspid valve stenosis is observed in 14–15% of patients with heart defects • The ratio of men to women is 4:1.
Etiology • Rheumatism • Infectious endocarditis • Subendocardial fibroelastosis • Congenital anomalies (Ebstein's anomaly, etc.) • Idiopathic calcification • Myxoma of the right atrium • Secondary tumors of the right atrium (nephroblastoma sprouting from the inferior vena cava, metastases).
Pathophysiology • Hemodynamic abnormalities are similar to those of tricuspid regurgitation. The differences are that there are no changes in the pancreas, and stagnation in the systemic circulation occurs and progresses much faster • Since in the vast majority of cases, tricuspid stenosis is accompanied by insufficiency and defects of other valves, the general pathogenesis consists of the corresponding individual disorders • Based on the degree of narrowing of the valve opening, they are distinguished moderate (2.5–3 cm2), severe (1.5–2.5 cm2) and severe (less than 1.5 cm2) stenoses.
Clinical picture and diagnosis
• For complaints, see Tricuspid valve insufficiency.
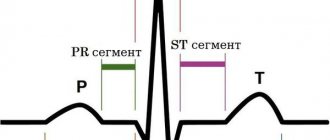
• Valvular symptoms •• Strengthening of the first tone, the opening click of the tricuspid valve •• With sinus rhythm - fusiform low-frequency diastolic murmur (increases on inspiration) •• The intensity and duration of the murmur depend on the diastolic transtricuspid pressure gradient •• Auscultatory diagnosis of the defect is difficult due to sound phenomena associated with concomitant anomalies of other valves.
• For symptoms of circulatory failure in the systemic circle, see Tricuspid valve insufficiency.
• Symptoms of the underlying disease (rheumatism, myxoma of the heart).
Special studies
• ECG see Tricuspid valve insufficiency.
• Jugular venogram: increase in A-wave amplitude and gentle Y-decline.
• X-ray of the chest organs •• Bulging of the arch of the right atrium (with an isolated defect - without deviation of the contrasted esophagus) •• Dilation of the shadows of the vena cava •• Signs of concomitant lesions of other valves •• With a moderate isolated defect, there may be a normal x-ray picture.
• EchoCG •• Expansion of the cavity of the right atrium •• Expansion of the superior vena cava •• Fusion, deformation and thickening of the tricuspid valve leaflets, a decrease in the amplitude of their opening and dome-shaped protrusion in diastole •• In pulsed wave Doppler mode, the diastolic pressure gradient is determined •• Reduction in the area of the tricuspid orifice valves are calculated in Doppler mode (pressure gradient) and B-mode (the smallest value is taken as the basis for diagnosis) •• For candidates for surgical treatment, the internal diameter of the annulus fibrosus is measured •• Signs of other valvular or congenital anomalies •• Displacement of the annulus fibrosus and atrialization RV, as well as an increase in the amplitude of movement of the valves with Ebstein's anomaly •• Detection of space-occupying formations in RA tumors •• Transesophageal echocardiography is performed in all patients to exclude RA thrombosis and vegetations, even with a normal rhythm.
• Right heart catheterization •• Increased RA pressure, flattened Y-slope, steep X-slope, increased A-wave amplitude and transtricuspid diastolic pressure gradient •• In severe tricuspid stenosis, especially in combination with mitral stenosis and pulmonary hypertension, cardiac output low •• For tumors of the right heart, catheterization is contraindicated.
• Right atriography and coronary angiography. Performed when tricuspid stenosis is suspected, when echocardiography data is insufficient, as well as for diagnosing other valve defects and coronary artery disease.
TREATMENT
• Conservative therapy. Treatment of right ventricular failure: diuretics and venous vasodilators (use with caution as higher than normal CVP is required to maintain preload).
• Surgical treatment •• Indications ••• III degree stenosis with pronounced clinical manifestations ••• II degree stenosis when simultaneous cardiac intervention is necessary for other diseases •• Contraindications ••• Severe concomitant pathology that threatens the patient’s life ••• Terminal stage circulatory failure ••• The activity of the rheumatic process is not considered a contraindication to surgical treatment •• Methods of surgical treatment ••• Open commissurotomy or balloon valvuloplasty is performed if the valve apparatus is intact and with concomitant tricuspid insufficiency no higher than grade I ••• In other cases, valve replacement is performed biological prosthesis or, less commonly, mechanical ••• See also Tricuspid valve insufficiency.
For specific complications , see Tricuspid valve insufficiency.
For prognosis , see Tricuspid valve insufficiency.
Synonyms • Tricuspid valve stenosis • Right atrioventricular orifice stenosis • Tricuspid stenosis
Abbreviations • RV - right ventricle • RA - right atrium.
ICD-10 • I07 Rheumatic diseases of the tricuspid valve
The tricuspid valve (TV) and its pathology, compared with the aortic and mitral valves (MV), do not receive due attention from cardiologists and cardiac surgeons. As a result, there is a lack of research and scientific publications in this direction, including in our country. An accurate understanding of the mechanisms of development of functional insufficiency of the heart valve and the determination of treatment tactics for correcting the defect remain a completely unresolved problem in valvular heart surgery.
Functional insufficiency of the valve, as a rule, is understood as failure without structural changes in the elements of the valve against the background of dilatation and dysfunction of the right ventricle (RV), dysfunction of the left ventricle (LV), pulmonary hypertension, which occur with pathology of the valve, less often the aortic valve.
According to various authors [10, 22, 37], functional insufficiency of the ventricle occurs in 8–35% of those operated for MV or aortic valve defects.
As is known, tricuspid regurgitation (TR) serves as an independent prognostic factor for the progression of chronic heart failure and death after adequate correction of MV and/or aortic valve defects [11, 21, 25]. Thus, according to J. Nath et al. [47], the one-year survival rate for patients with grades II and III regurgitation was 78.9 and 63.9%, respectively, regardless of LV function, degree of pulmonary hypertension and age. At the same time, according to A. Calafiore et al. [16, 18], the 5-year survival rate of patients with grades II and III TR after isolated correction of mitral valve disease was 46%.
The etiological factor in the occurrence of functional tricuspid insufficiency (TN) is pathological processes causing defects of the left heart or pulmonary hypertension [10, 41, 51, 53, 55]. Most often these are rheumatic heart disease, ischemic mitral regurgitation, myxomatous degeneration, degenerative (atherosclerosis) valve disease, infective endocarditis of the left heart, dilated cardiomyopathy, pulmonary embolism. At the same time, a number of studies [59] do not find a reliable connection between the severity of MV defects and the severity of TN.
Most authors [9, 27, 51, 55] consider dilatation of the fibrous ring to be the main factor in the development of regurgitation. According to G. Dreyfus et al. [25], dilatation of the annulus fibrosus is a more objective indicator of the severity of TN than the degree of TR. One cannot but agree with this, since it is known that the degree of TR, determined by echocardiography, can vary depending on the pre- and afterload and contractility of the RV. It is believed that with a dilated ring, pronounced TR will manifest itself in one way or another over time in most cases. S.G. Sukhanov et al. [5] monitored the results of combined operations—MV annuloplasty and myocardial revascularization. They consider dilatation of the fibrous ring and LV ejection fraction to be prognostic factors for the occurrence of functional TN after these operations. L.A. Bokeria et al. [1], in addition, note a correlation between the diameter of the fibrous ring and the degree of TR.
In addition to, in fact, the expansion of the fibrous ring, there is evidence of a change in the spatial three-dimensional configuration of the ring towards its “flattening” [8]. T. Ton-Nu et al. [60] studied the three-dimensional configuration of the valve in individuals without TR and with valve regurgitation. In the group without regurgitation, the valve annulus has a clearly higher position of the anteroposterior segments (relative to the mediolateral ones) and an ellipsoidal shape. As functional TN develops, the valve ring “flattens” and takes on a more rounded shape. Similar data are provided by S. Fukuda et al. [29] on a smaller number of observations.
Other factors causing regurgitation include pancreatic dilatation and subsequent displacement of the papillary muscles, which leads to an increase in the annuloapical distance, excessive chordal tension and impaired coaptation of the leaflets. This condition in the English-language literature is referred to as “tethering” or “tenting” [9, 55]. In addition, according to some data, pancreatic dilatation increases the number of complications associated with valve defects [44].
M. Antunes et al. [10] described “restriction-dilatation syndrome” as one of the mechanisms for the development and aggravation of pancreatic dysfunction, and consequently TR. This mechanism represents a “vicious circle” when LV dysfunction through increased pressure and/or volume in the pulmonary circulation leads to RV dysfunction. This in turn exacerbates LV dysfunction through interventricular communication through the interventricular septum.
Another important element in the development of TN is pulmonary hypertension. M. De Bonis et al. [23] revealed a relationship between systolic pressure in the pulmonary artery and the degree of LV dysfunction. Similar data are provided by S. Fukuda et al. [28]. In another study by R. Ghanta et al. [33] found that patients whose pulmonary artery pressure after TC repair was 52 mmHg. and higher are more susceptible to relapse of TR. J. Nath et al. [47] noted that pulmonary artery pressure was more than 40 mmHg. aggravates TR and increases mortality.
According to G. Lin et al. [39], one of the reasons for the development of TN may be the implantation of endocardial electrodes for pacemakers and cardioverter-defibrillators.
Thus, understanding the mechanisms of development of TN helps in determining the main directions for surgical correction of the defect.
For a long time, under the influence of the publication of N. Braunwald et al. [15] it was believed that correction of TR after successful correction of left heart disease was not required and that TR regressed on its own. O. Yilmaz et al. [62] in their retrospective study showed that even grade III and higher TR regressed within 3 years of follow-up after isolated MV repair. On this basis, they believe that asymptomatic TR does not require mandatory correction.
However, there are reports that contradict this belief. According to A. Calafiore et al. [16, 18], the 5-year survival rate of patients with grades II and III TR after isolated correction of mitral valve disease was 45-46%, and with grade I or without regurgitation - 74.5-88.2%. It has also been shown that, despite a decrease in the degree of regurgitation in the early postoperative period in patients with uncorrected TN, it progresses in the long-term period. H. Song et al. [57] tracked the course of uncorrected stage I and II heart failure in 638 patients operated on for left heart defects. 7.7% of patients subsequently developed grade III or IV TR. K. Matsuyama et al. [41] analyzed the long-term results of correction of defects of the left side without interventions on the TC with regurgitation within the II degree. In 16% of patients, during a follow-up period of up to 8 years, TN progressed to grade III or higher. Preoperative grade II regurgitation, atrial fibrillation, and left atrial atriomegaly greater than 60 mm were identified as factors that worsen the course of TR in the long-term period.
A. Matsunaga et al. [40] presented the results of treatment of 124 patients with ischemic mitral regurgitation. At the same time, 30% of patients had severe TR and only 43% of them underwent annuloplasty. By the end of the observation, 49% of patients had regurgitation of at least grade III; in 74% of patients without regurgitation before surgery for more than 3 years, the appearance of regurgitation on the TV was noted. In patients with TR, both before and after surgery, a high ring diameter index/body surface area was detected.
There are different opinions when determining indications for correction of functional TN. Most surgeons have no doubt about the need to correct severe regurgitation of degrees III and IV [11, 17, 23, 49]. However, the issue of correcting grade I and II regurgitation remains unresolved. G. Dreyfus et al. [25], patients with a diameter of the valve annulus less than 7 cm, measured between the anteroseptal and anteroposterior commissures, underwent correction of only the mitral defect, and those with a diameter of 7 cm or more underwent correction of the mitral defect and annuloplasty of the valve. The degree of regurgitation before surgery in both groups was on average less than I. In the long-term period, aggravation of TR by more than two degrees was observed in 48% of patients who did not undergo annuloplasty, and only in 2% of those who received annuloplasty. As a result, they concluded that focusing on the diameter of the tricuspid annulus when determining indications for TN correction gives the best result.
N. Van de Veire et al. [61] concluded that performing annuloplasty in patients with dilation of the annulus fibrosus of more than 40 mm, according to echocardiography, without significant regurgitation, prevents further progression of TN and promotes reverse remodeling of the RV. In patients with grade I and II TR and dilatation of the annulus fibrosus, who did not undergo annuloplasty, progression of TR and pancreatic dilatation were observed. Other authors [56] also suggest performing TB annuloplasty in patients with moderate regurgitation, if there is dilatation of the annulus fibrosus of more than 40 mm or pulmonary hypertension. V. Chan et al. [21] indicate that grades III and IV TR are associated with increased mortality in the postoperative period. Performing annuloplasty of the heart failure prevents the progression of failure and improves the functional class of heart failure of patients, but does not affect mid-term survival. The presence of TR in patients with grade III and higher in combination with dilatation of the fibrous ring of more than 30 mm are factors that determine further progression of TR. V.A. Ivanov [3] also considers regurgitation of degree II and higher and dilation of the annulus fibrosus more than 40 mm (according to transesophageal echocardiography) as an indication for correction of functional insufficiency of the TC.
There is a certain difference in the guidelines for the correction of TC defects proposed by the American College of Cardiology/American Heart Association (ACC/AHA) and the European Society of Cardiology (ESC).
ACC/AHA approved indications for tricuspid repair include the following:
— MV repair is indicated for severe TR in patients with MV defect requiring surgery (level of evidence B);
— Trunk replacement or repair is appropriate in patients with primary severe symptomatic TR (level of evidence C);
— TV prosthetics are advisable in cases of severe TR and altered valves in cases of ineffectiveness of plastic surgery (level of evidence C);
— Trunk annuloplasty is possible in patients with less than grade IV TR during mitral valve surgery if there is pulmonary hypertension or dilatation of the valve annulus fibrosus (level of evidence: C);
— Trunk repair or replacement is not indicated for patients with asymptomatic isolated TR and systolic pressure in the pulmonary artery less than 60 mmHg. (level of evidence C);
— prosthetics or TC repair are not indicated for patients with primary TR less than grade III (level of evidence C).
According to the guidelines for the correction of tricuspid defects adopted by the ESC, intervention on the tricuspid is indicated in the following cases:
— patients with severe TR during correction of left heart defects;
— patients with severe primary TR with clinical manifestations that are not amenable to drug therapy even in the absence of ventricular dysfunction;
- patients with severe tricuspid stenosis (with or without TR) that is not amenable to drug therapy;
- patients with severe tricuspid stenosis (with or without TR) undergoing interventions on the left side of the heart;
— patients with moderate organic TR during correction of left heart defects;
— patients with secondary moderate TR and annular dilation of more than 40 mm during correction of left heart defects;
- patients with severe symptomatic TR with corrected defects of the left side, in the absence of myocardial dysfunction, valves, RV dysfunction and pulmonary hypertension;
— patients with severe isolated low-symptomatic TR with progressive dilatation of the pancreas and an increase in its dysfunction [43].
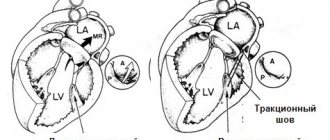
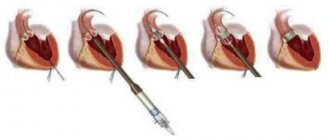
The most common methods of surgical correction of functional TN are support ring annuloplasty, proposed by A. Carpentier in 1971, and suture annuloplasty, proposed by NG De Vega in 1973, and their modifications [6, 19, 24].
I.I. Skopin et al. [4] identified 3 types of TC structure and put forward the position that the choice of the type of annuloplasty should be made taking into account the anatomical structure of the TC.
According to some data [14, 48, 49, 59], annuloplasty with a support ring provides a more durable effect in the long term and higher survival.
In particular, according to P. McCarthy et al.[42], 1 month after De Vega annuloplasty, patients with grade III and IV regurgitation accounted for 13.6%, and after 8 years - 33%. After plastic surgery with a support ring, these figures were 15.2 and 17%, respectively. Despite the low rate of reoperation (3% over 8 years of follow-up), the authors note high postoperative mortality after reoperation. Mortality was 8%, and 5- and 8-year survival rates were 65 and 50%, respectively. F. Filsoufi et al. [27] reported good short-term results after using support rings in a three-dimensional configuration and did not observe a significant increase in the degree of regurgitation.
G. Gatti et al. [31, 32] presented results using an elastic polytetrafluoroethylene strip for annuloplasty. Mortality was 5.7%, 4-year actuarial survival was 91.7%. In the mid-term period, 89.8% of patients had TR less than grade II and satisfactory functional status. A. Calafiore et al. [17] proposed a method of annuloplasty with a standard 50-mm strip without taking into account body surface area and reported satisfactory short-term results. After such plastic surgery, the diameter of the tricuspid foramen approached the N25 meter.
J. Bernal et al. [11] used De Vega suture annuloplasty and its modifications to correct functional TN. Mortality was 8.1%, survival at 6.8 years was 23.3%. The actuarial 12-year survival rate was 50.5%. Positive dynamics of echocardiographic parameters were noted in the form of a decrease in the size of the pancreas and a decrease in pressure in the pulmonary artery. During the observation period, TR was absent or grade I in 50.6% of patients, grade II in 44.8%. The degree of residual TR did not affect mortality and the rate of re-operation on the valve in the long-term period. Preoperative severe TR was associated with a higher rate of reoperation. Reoperation was required in 3.1% of patients with functional TN. The mortality rate after reoperation was 18.5%. A. Calafiore et al. [18] when performing annuloplasty according to De Vega, they reported a 5-year survival rate of 74.5% and progression of TR to grades III, IV in 5% of patients; while the mortality rate was 5.5%. S.S. Dobrotin et al. [2] also note satisfactory mid-term results after De Vega annuloplasty. A. Sarraj et al. [54] reported their use of adjustable segmental TK annuloplasty, very similar to the technique proposed by N.M. Amosov in 1972, with satisfactory mid-term results.
R. Ghanta et al. [33] did not reveal statistically significant differences in mid-term results between suture bicuspidalization and ring annuloplasty; both methods, according to the authors, give a good, lasting effect.
O. Alfieri et al. [7] in 2002 presented the “clover technique” they used to correct post-traumatic TR. Subsequently, E. Castedo et al. [20] described a similar technique in patients with recurrent TN, calling it the edge-to-edge technique. Y. Lai et al. [38] used this technique as an additional procedure in cases of inadequate correction of insufficiency with ring annuloplasty and Key repair and obtained satisfactory results. M. De Bonis et al. [23] also presented results using the “clover technique”. They used it in addition to annuloplasty for prolapse of the valves and for their tension, in 97% of cases due to displacement of the papillary muscles, in 3% in isolation. In the mid-term period, regurgitation was absent or corresponded to grade I in 87.7% of patients; there were no manifestations of TC stenosis.
G. Dreyfus et al. [26] for adequate correction of severe TR in patients with impaired coaptation due to displacement of the papillary muscles, proposed enlarging the anterior leaflet with a patch from the autopericardium, followed by implantation of a support ring. This intervention was performed in patients with a coaptation depth of more than 8 mm. During follow-up periods from 6 to 20 months, residual regurgitation was no more than grade I. F. Roshanali et al. [52] compared groups of patients with severe TR who underwent annuloplasty and annuloplasty in combination with leaflet augmentation using an autopericardial patch if the coaptation depth was greater than 8 mm or the coaptation depth area was greater than 16 mm2. After one year, residual regurgitation in the groups that underwent De Vega annuloplasty and ring annuloplasty was 28 and 14%, respectively, and in the groups with De Vega plastic surgery combined with leaflet augmentation and ring annuloplasty and leaflet augmentation - 10 and 8%, respectively.
U. Kappert et al. [35] proposed a different approach for correcting TN due to severe dilatation of the pancreas - resection of the free wall of the pancreas in combination with ring annuloplasty. After one year of observation, TR was not detected.
R. Moraca et al. [46], when analyzing the results of prosthetics and TC repair, did not reveal any differences in the postoperative and long-term periods. In conclusion, the authors indicate that in patients at risk of relapse of TR, valve replacement is preferable as a more reliable and radical intervention to reduce insufficiency. C. Park et al. [50] provide similar data; they noted only a trend toward better long-term survival in patients with valve repair. Moreover, there were no deaths in patients who underwent TC replacement in addition to correction of left heart disease. The authors also believe that in cases with severe TR and RV dysfunction, preference should be given to valve replacement. Other authors [13] identify TC replacement as a prognostic factor for increased mortality.
Despite numerous reports of good results of correction of functional TR, in some cases relapse or progression of TR is observed.
P. McCarthy et al. [42] identified a high preoperative degree of TR, LV dysfunction, the presence of an endocardial lead, atrial fibrillation, and types of annuloplasty other than ring annuloplasty as independent prognostic factors determining the progression of TR. M. De Bonis et al. [23] presented data on correction of TR in patients with dilated cardiomyopathy and mitral regurgitation. After 1.8 years of follow-up, 12% of patients had grade III or IV regurgitation, and 18% of patients with preoperative grade II or lower regurgitation subsequently progressed to two grades or higher. Severe regurgitation at the time of discharge and preoperative pancreatic dysfunction are considered as prognostic factors for the progression of TR to grades III and IV. G. Gatti et al. [32] reported that patients with preoperative regurgitation greater than grade II, RV shortening fraction less than 35%, and a permanent pacemaker tended to have recurrent TR. R. Ghanta et al. [33] found that factors such as pulmonary artery pressure after TC repair of 52 mmHg. and higher, as well as a high preoperative degree of regurgitation, determine the tendency to relapse of TR.
In a prospective study by Y. Kim et al. [36] RV dysfunction was assessed as an independent prognostic factor for the unfavorable course of TN after correction of the defect. A sensitive indicator of RV dysfunction is RV end-systolic area; Thus, with an area of less than 20 cm2, a lower incidence of cardiovascular complications in the mid-term period was noted. Similar data were presented by C. Park et al. [50].
S. Fukuda et al. [28] identified a preoperative coaptation depth (“tethering height”) of more than 0.51 cm and a “tethering area” of more than 0.8 cm2, early postoperative LV ejection fraction of less than 36.6% as echocardiographic prognostic factors relapse or persistence of TR a year or more after surgery. They also found a correlation between RV pressure and the degree of TR, and a relationship between RV pressure and LV ejection fraction at follow-up periods of more than a year. The authors obtained similar data for the early postoperative period [30].
S. Min et al. [45] in their work identified a tethering volume of more than 1.68 ml and an anteroposterior ring size of more than 36 mm as preoperative echocardiographic prognostic factors for residual TN, and pulmonary artery pressure as postoperative factors.
H. Je et al. [34] showed that age, rheumatic etiology of mitral valve disease and failure to perform the Maze procedure are predictive factors for the progression of grade II TR. Similar data were presented by H. Song et al. [57]. J. Stulak et al. [58] also compared a group of patients with successful Maze procedure and correction of mitral valve disease with a group in which the rhythm was not restored. The authors concluded that a successful Maze procedure prevents the progression of TN. In patients with sinus rhythm after surgery, the degree of TN decreased in 42% of cases, progressed in 9%, and in patients with atrial fibrillation - in 36 and 45%, respectively.
C. Yoon et al. [63] found that B-natriuretic peptide levels greater than 200 pg/ml are an unfavorable prognostic factor in patients with severe TN.
According to various data, mortality after correction of TN in combination with correction of left heart defects ranges from 0 to 18%, but most authors cite figures of 4.4-8.1%. According to J. Bernal et al. [12], the mortality rate during reoperations after previously performed TB repair was 33.8%, the rate of developmental complications was 64.9%. Actuarial survival at 10 years after reoperation was 40%, and at 26 years it was 11.8%. In other works, the authors [11, 42] cite mortality after repeated operations at 18.5–37%.
Thus, functional TN is a rather serious problem that accompanies left heart defects and aggravates their course. Severe TR should be corrected without a doubt. However, frequent unsatisfactory long-term results of uncorrected regurgitation of degrees I and II may necessitate more active surgical tactics. Other parameters need to be considered as additional or equivalent to the degree of regurgitation when determining indications for correction of the defect. It is still not completely clear which annuloplasty technique is the operation of choice, although there is a tendency towards ring annuloplasty methods. The progression of TR after correction in patients with dilated pancreas, who apparently require the use of other surgical techniques than isolated annuloplasty, also remains a problem.
Acquired heart disease - symptoms and treatment
There is no medicine that can reverse the process and restore the valve to its original state. With the help of medication, it is only possible to influence the cardiovascular system and reduce the risk of complications. In severe cases, surgical treatment methods are used.
Drug treatment
The goal of therapy is to eliminate the causes of circulatory failure, improve the functional state of the myocardium, restore normal blood circulation, microcirculation (transport of blood cells and substances to and from tissues) and prevent recurrent circulatory disorders. Treatment for chronic circulatory failure includes a complete balanced diet and drug therapy.
The main groups of drugs used for valve dysfunction:
1. ACE inhibitors (angiotensin-converting enzyme) - enalapril, lisinopril, ramipril, perindopril, fosinopril. The drugs block the conversion of the hormone angiotensin I to angiotensin II. Angiotensin II has a vasoconstrictor effect and causes a rapid increase in blood pressure. ACE inhibitors are used to treat hypertension and treat or prevent heart failure.
2. Beta blockers - carvedilol, bisoprolol, metoprolol. These drugs lower blood pressure and normalize heart rate. The action is caused by blocking beta-adrenergic receptors, which are responsible for the body's response to stress.
3. Mineralocorticoid receptor antagonists - spironolactone, eplerenone. The drugs lower blood pressure and have a diuretic effect, reducing fluid content in tissues.
There are different types of heart defects, and different combinations of drugs are used to treat each of them. Concomitant pathologies and individual characteristics of the patient are also taken into account.
For example, when congestion predominates, diuretics (furosemide, torsemide) are additionally prescribed, which reduce the volume of circulating blood, reducing congestion in the pulmonary and systemic circulation. If blood clots are present or there is a high risk of their occurrence, anticoagulants are used - drugs that reduce blood clotting (warfarin, rivaroxaban, dabigatran, apixaban).
Relatively recently, a new class of drugs was developed - angiotensin-neprilysin receptor inhibitors (sacubitril). Their main effect is to increase the amount of peptides cleaved by neprilysin. The drugs increase diuresis (urine volume), natriuresis (sodium excretion in the urine), cause relaxation of the myocardium and prevent processes of disruption of the structure and function of the heart.
If it is impossible to use beta blockers, it is recommended to replace them with If channel inhibitors (ivabradine), which also reduce the heart rate. If it is impossible to use ACE inhibitors, angiotensin receptor blockers (losartan, valsartan, candesartan, telmisartan, irbesartan, olmesartan) are used. They have properties similar to ACE inhibitors, but do not reduce the synthesis of angiotensin II, but block angiotensin receptors [6].
Surgery
If there are indications, the effectiveness of drug treatment is insufficient and there are no contraindications, surgical methods are used. Many patients are afraid of the need for heart surgery. In some cases, surgical methods do carry some risk, but there are fairly safe operations that are not open heart and without large incisions. Such operations include balloon commissurotomy for mitral valve stenosis. The method consists of widening the valve using a catheter passed through the artery.
Before surgery, the necessary laboratory and instrumental studies are carried out and the patient’s condition is stabilized with medications. When preparing for surgery, it is important to reduce shortness of breath, swelling, and normalize pulse and blood pressure.
A common surgical method is artificial or biological valve replacement. There are also valve-sparing operations, which involve plastic surgery of a damaged valve.
Surgical treatment significantly improves the quality of life, however, after surgery, medication continues, but is adjusted if necessary. In addition, after certain operations, such as artificial valve replacement, anticoagulant therapy is constantly taken. The drugs are necessary because the risk of thromboembolic complications (blockage of arteries with blood clots) increases [5].
Atresia of the tricuspid valve. Principle of Fontan operation
The word atresia means the complete absence of normal communication between the cardiac chambers or between them and the great vessels extending from them, and sometimes the absence of sections of the vessels themselves. The term atresia is applied to the tricuspid, mitral, aortic and pulmonary valve openings, the aorta and pulmonary artery.
Another term, hypoplasia, means that the message is there, but it is reduced in size compared to normal. They talk about hypoplasia of the tricuspid or mitral valves, parts of the aorta, pulmonary artery, but also about the size of the cavity of one of the ventricles (hypoplasia of the right or left ventricle).
Tricuspid valve atresia
In a normally developed heart, the tricuspid valve connects the right atrium to the right ventricle. With atresia, this valve has not developed and in its place there is a dense membrane through which blood flow cannot pass. Since this area is closed, the fetus has not developed and also lacks the entire inflow tract of the right ventricle, i.e. the first of two cones that form the ventricle. Thus, not only the valve is changed, but also the entire right ventricle - half of it is missing.
However, both the fetus and the born child can live with this, at least for some time. Blood shunting and mixing in the heart occurs through defects in the septa.
From the vena cava, blood enters the normal right atrium. But the entrance to the ventricle is closed. There is only one way left: through the atrial septal defect - into the left atrium. Blood then flows through the normally developed mitral valve into the left ventricle. But here two paths are open. One - into the aorta, the other - through the ventricular septal defect - into the remaining part of the right ventricle (the second of the cones, which is normally developed) and from here - into the pulmonary artery and lungs.
The blood flows in the left ventricle mix - venous blood from the vena cava, which reached the ventricle in such a complex way, mixes with arterial blood coming from the veins of the lungs.
The path of its further “distribution” depends on where it is easier for the main flow to go: into the aorta or through the ventricular septal defect - into the pulmonary artery and lungs. The blood is divided into two branches, and the difference in the volume of the two flows depends on the difference in the resistance of the blood vessels of the body (i.e., the systemic circulation) and the resistance created by the septal defect.
In most cases, a significantly larger volume of blood passes into the aorta and a smaller volume into the lungs. First, much of the blood remains undersaturated and the baby will be cyanotic. The degree of this cyanosis, however, is not as pronounced as with complete transposition of the great vessels - after all, mixing of arterial and venous blood still occurs in the ventricle. And, secondly, the left ventricle works in both circles, because there is almost no right one. The load that has fallen to his lot is heavy, and it can very quickly cause circulatory failure in the systemic circle - shortness of breath, enlarged liver, edema, etc.
In essence, both the quality and life expectancy of such children depend on the amount of blood flow in the lungs, i.e. determined by the size of defects in the interventricular and interatrial septa - these, in this case, are natural shunts “to the rescue”.
Over time, as the child (and heart) grows, the situation worsens, because... Less and less blood flows into the lungs. Cyanosis increases and about 90% of children will not live to see their first birthday if they are not treated surgically.
The remaining 10% have a fairly large defect in the septum, and their blood flow to the lungs is normal, and sometimes even increased. They feel well, develop relatively normally and are almost not cyanotic. Such children can live more than 10-15 years, but they will gradually develop signs of heart failure and increase cyanosis with all its complications and consequences.
What can be done and when?
In the treatment of tricuspid valve atresia, surgery, especially over the past two decades, has made significant progress and treatment tactics, the choice of surgery and its timing have now been developed sufficiently to achieve convincingly good results. But you need to understand that if the heart initially did not have one of the four valves and a large section of one of the ventricles, then they cannot be made again
. There are no such methods yet.
Therefore, the entire treatment process will come down to creating other, more optimal paths for blood flow
. In other words, the treatment will be relieving, “palliative,” and it cannot be performed at once, but must gradually prepare both the heart and lungs for new, more normal blood flow conditions than those in which they found themselves at birth.
Children with severe cyanosis during the first months should undergo surgery to create an anastomosis between the systemic and pulmonary circulation, as described for tetralogy of Fallot. This operation will significantly improve their condition at first. They will begin to develop, will look and behave according to their age, and the cyanosis will disappear.
But this is only the first, “life-saving” stage. If nothing else is done, the child will soon become very sick again and the worse his condition, the riskier the subsequent stages of treatment will be.
Between the first and second years of life, it becomes possible to perform the second stage, namely, removing the first anastomosis and creating another path for venous blood to the lungs - a direct connection of the superior vena cava with the pulmonary artery, bypassing the right half of the heart. Why don't they do this right away? The fact is that in the first months of life, the resistance of the vascular bed of the lungs may be too high. Think about it, the pressure in the veins is very low - 5-7 mmHg, and we want it to be sufficient so that without a cardiac “pump” the blood flows passively into the pulmonary artery. To do this, there must be a difference in resistance and pressure between the veins and the vessels of the lungs, otherwise blood simply will not flow into the lungs.
There are situations, and there are quite a few of them, when we perform this operation - bidirectional cavapulmonary anastomosis in the first year of life, without a previously performed systemic-pulmonary anastomosis. This clinical decision is made by your cardiologist and cardiac surgeon, thus developing the best treatment strategy for your child.
We dwell on this in such detail because... The very principle of the so-called direct cava-pulmonary anastomosis is widely used today for other defects, when the heart is so altered that the defect cannot be corrected surgically. So at this second stage, the superior vena cava connects with the pulmonary artery in such a way that blood from it flows into both lungs. In other words, blood from the upper half of the body is already going where it needs to go. But for now - only half.
Children usually tolerate surgery well. This operation will not only improve the child’s condition, but also prepare the heart and blood vessels of the lungs for the third and final stage in the treatment process - connecting the inferior vena cava with the pulmonary artery .
The best time to perform the third operation will be the age of 4-5 years, although sometimes it is done earlier than this period.
This operation is performed with artificial circulation and consists of sewing a large patch or prosthesis into the inferior vena cava at one end and into the pulmonary artery at the other. Thus, now all venous blood from the entire body goes to the lungs, and fully oxidized blood goes to the left parts of the heart, i.e. into his now only ventricle.
This operation is called the Fontan operation , named after the French surgeon who proposed it.
Today it is used not only for tricuspid valve atresia, but also for many complex congenital defects, when surgical creation of two equal ventricles is impossible. This operation revolutionized the treatment of defects that had recently been considered inoperable. Patients with such defects were simply refused admission to even the best cardiac centers. Today everything has changed, and thanks to the Fontan operation, thousands of patients can and have already been helped. The very principle of this approach—the Fontaine principle—is, however, just a palliation, albeit a well-functioning one. Fontan operation, i.e. the final stage of surgical treatment cannot be called “radical correction” (as, for example, when closing an atrial septal defect); the name “permanent palliation” is more suitable for it, i.e. This is not a correction of the defect, but the last opportunity for maximum normalization of blood circulation in this case. A person lives without a right ventricle, and blood flows passively into the lungs from the venous system. Is this person healthy? Can he endure the physical and psychological stress that awaits him in life? We will talk a little below about what is known about this today, but now let’s return to tricuspid atresia.
No matter how paradoxical it may seem, the more severe the condition of a child with this defect initially, the clearer the path to treatment. But each child is individual, and you can encounter different options and combinations of intracardiac disorders: too small a defect between the atria, too large an interventricular communication, congenital narrowing of the pulmonary artery and its branches, etc.
In each case, you need to choose the most rational and safe way to help. Sometimes it is necessary to first widen the interatrial defect in the cath lab, sometimes a cuff should be placed on the pulmonary trunk to reduce pulmonary blood flow and prevent the development of other complications. Today all these methods are well known, but treatment tactics and sequence are individual. The main thing is that the child immediately goes to a specialized institution where there is relevant experience.
Will a child be able to live a long and normal life without one of the parts of the heart - the right ventricle? It turns out that he can, for a long time, and not at all well. Today's statistics indicate that the vast majority of patients living with “Fontan circulation” belong to the so-called functional class I-II degree, i.e. practically healthy. About a quarter of them, operated on 10 or more years ago, required repeated operations to replace previously sewn-in prostheses, but today, as surgical techniques improve, we can hope that the number of such cases will decrease significantly.
The main complication in the long term after surgery is the so-called protein enteropathy, expressed in edema, accumulation of fluid in the cavities, and loss of protein. It is amenable to conservative treatment. So let's be optimistic.
It is clear that the child should be constantly (at least once every six months) observed by a pediatric cardiologist, even if there are no visible complications. The child is not disabled in any way, but he must be observed by a qualified and experienced doctor.
In any case, your child with complex congenital heart disease was given everything that modern medicine can offer, and this is as much a victory for the doctors as it is yours.
How to get treatment at the Scientific Center named after. A.N. Bakuleva?
Online consultations
Mitral stenosis (mitral valve stenosis)
From the first rheumatic attack to the appearance of complaints characteristic of mitral stenosis in countries with temperate climates, an average of 20 years passes, so complaints usually appear at 30-40 years of age. Increasingly, there are patients in whom the connection between the disease and rheumatism cannot be established. According to data obtained before the widespread use of cardiac surgery, the period from the onset of dyspnea at rest to death usually takes 2-5 years. In developing countries (South and Southeast Asia, Central America, the Middle East), the disease progresses faster and often appears by the age of 20. At the same time, in Western Europe and the USA, slowly progressive mitral stenosis in the elderly is increasingly being detected.
With mild stenosis, there are often no complaints, although physical examination reveals many symptoms of the defect. However, even with a slight increase in the transmitral pressure gradient at rest, an increase in cardiac output (during physical and emotional stress, sexual intercourse, fever, severe anemia, paroxysmal tachycardia, pregnancy, thyrotoxicosis) sharply increases PAWP, causing shortness of breath and cough. A sharp increase in blood flow against the background of severe stenosis leads to pulmonary edema. In the lying position, congestion in the lungs increases, orthopnea and nocturnal attacks of cardiac asthma occur. The smaller the area of the mitral valve opening, the worse patients tolerate physical activity and the higher the likelihood of atrial arrhythmias - extrasystole, paroxysmal tachycardia, atrial flutter, atrial fibrillation.
The high frequency of ventricular contraction during atrial fibrillation causes a sharp increase in shortness of breath. The appearance of a permanent form of atrial fibrillation is a critical moment in the course of the disease; its progression after this is noticeably faster.
Hemoptysis with mitral stenosis occurs due to rupture of the pulmonary veins when the pressure in them increases - usually with high pressure in the left atrium in the absence of a significant increase in pulmonary vascular resistance. Such hemoptysis is almost never fatal; over time, the pulmonary veins thicken and the hemoptysis goes away. It is necessary, however, to distinguish it from other types of hemoptysis, also often found with mitral stenosis and caused by pulmonary edema, pulmonary infarction or bronchitis.
As the disease progresses and pulmonary vascular resistance increases (or due to the addition of tricuspid stenosis or insufficiency), symptoms of pulmonary congestion often weaken, pulmonary edema and hemoptysis occur less frequently and are easier. However, pulmonary hypertension increases the afterload of the right ventricle, and right ventricular failure occurs: weakness, heaviness in the abdomen (due to hepatomegaly), edema, ascites, hydrothorax (usually right-sided).
Repeated pulmonary embolism, sometimes with pulmonary infarction, is one of the main causes of death in mitral stenosis.
Bronchitis, bronchopneumonia, and lobar pneumonia are common. Infectious endocardium is not typical for isolated mitral stenosis, but when stenosis is combined with mitral insufficiency it occurs quite often.
10% of patients have chest pain, usually caused by pulmonary hypertension or coronary artery disease, but often the cause is never found.
Changes in the lungs. In addition to pulmonary hypertension, sclerosis and thickening of the capillaries and alveolar septa often develop. Vital capacity, total lung capacity, maximum ventilation and the ratio of oxygen uptake to MOD decrease. The latter indicator in patients with severe mitral stenosis does not increase during exercise, as it should normally.
The increase in pulmonary capillary pressure during exercise further reduces lung compliance. Sometimes airway resistance increases. All this increases the work of breathing and causes shortness of breath. Due to sclerosis of the alveoli and decreased capillary blood flow, the diffusion capacity of the lungs may decrease, especially during exercise. Effusion into the interstitial space and alveoli also plays a role. Excess fluid is removed due to increased lymphatic drainage, which prevents alveolar pulmonary edema. Due to high pressure in the left atrium, perfusion of the lower parts of the lungs in the standing position is reduced, and the upper parts are increased.
Thromboembolism. Blood clots can occur in the left atrium, especially in its dilated appendage. This is fraught with thromboembolism of the arteries of the brain, kidneys, spleen, and limbs. The risk of thromboembolism is higher in patients with atrial fibrillation, the elderly, low cardiac output, and a history of thromboembolism. Thromboembolism is possible even with mild mitral stenosis and is its first manifestation. After a recent thromboembolism, the likelihood of detecting a thrombus in the left atrium (for example, during surgery) is lower than in the absence of thromboembolism; this suggests that thromboembolism is usually caused by fresh rather than old blood clots. Occasionally, large thrombi on a narrow base and free thrombi block the opening of the mitral valve; the symptoms in this case (fainting, chest pain, dependence of heart murmurs on body position) resemble left atrial myxoma.
Classification. The most widespread classification of mitral stenosis proposed by A.N. Bakulev and E.A. Damir. It includes 5 stages of development of the defect:
- I – stage of complete compensation of blood circulation. The patient does not make any complaints, but an objective examination reveals signs characteristic of mitral stenosis. The area of the mitral orifice is 3-4 cm2, the size of the left atrium is no more than 4 cm.
- II – stage of relative circulatory failure. The patient complains of shortness of breath that occurs during physical exertion, signs of hypertension in the pulmonary circulation are detected, venous pressure is slightly increased, but no pronounced signs of circulatory failure are detected. The area of the mitral orifice is about 2 cm2. The size of the left atrium is from 4 to 5 cm.
- III – the initial stage of severe circulatory failure. At this stage there are phenomena of stagnation in the pulmonary and systemic circulation. The heart is enlarged. Venous pressure is significantly increased. There is an enlargement of the liver. The area of the mitral orifice is 1-1.5 cm2. The size of the left atrium is 5 cm or more.
- IV – stage of pronounced circulatory failure with significant stagnation in the systemic circle. The heart is significantly enlarged in size, the liver is large and dense. High venous pressure. Sometimes slight ascites and peripheral edema. This stage also includes patients with atrial fibrillation. Therapeutic treatment gives improvement. The mitral orifice is less than 1 cm2, the size of the left atrium exceeds 5 cm.
- V – corresponds to the terminal dystrophic stage of circulatory failure according to V.Kh. Vasilenko and N.D. Strazhesko. There is a significant increase in the size of the heart, a large liver, sharply increased venous pressure, ascites, significant peripheral edema, and constant shortness of breath, even at rest. Therapeutic treatment has no effect. The area of the mitral orifice is less than 1 cm2, the size of the left atrium is more than 5 cm.