Description of the pathology
Changes in the myocardium of the left ventricle can provoke various diseases or metabolic disorders in the heart muscle.
Moderate cardiac dysfunction can be diffuse or focal. The first type is characterized by failure of the myocytes of the left ventricle, as a result of which they contract incorrectly. That is, the electrical impulse is carried out incorrectly through these cells. The second type is focal changes. In this case, scars form on the wall of the left ventricle. They consist of connective tissue that is not capable of conducting electrical impulses.
Moderate metabolic disorders can return to normal on their own, but if such disruptions occur frequently, the myocardium cannot recover.
Thus, changes can transform into irreversible ones. As the situation worsens, they can provoke cardiac pathologies.
When there is a discrepancy between energy consumption and its entry into the myocardium, the consequence will be degenerative changes. But even dystrophy does not always manifest itself, and if there are symptoms, it is often increased fatigue, which is not always paid attention to.
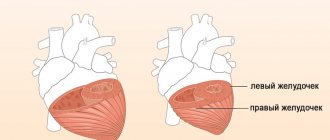
Left ventricular hypertrophy is a condition that the body activates to compensate for the blood flow process. This happens especially often if there is mitral valve insufficiency. Hypertrophy affects the condition of the walls of the left ventricle; they lose elasticity. This also applies to the septum between the ventricles.
With hypertrophy, thickening of the walls also occurs. It is not always uniform; it can occur according to a focal principle, that is, only in a certain area of a given cavity. And myocardial dystrophy leads to the fact that the wall of the left ventricle becomes significantly thinner, and the chamber cavity stretches.
Myocardial ischemia and impaired left ventricular diastolic function
In recent years, the attention of many researchers has been attracted by the possibility of studying myocardial function in the diastole phase, i.e. diastolic function of the left ventricular myocardium.
Interest in this problem is based on the fact that a number of studies have demonstrated the leading role of impaired diastolic function of the left ventricle in the development of heart failure in many diseases. It is also known that some rhythm disturbances are accompanied by symptoms of diastolic dysfunction. All of the above makes the problem of studying the process of relaxation of the left ventricle very relevant.
The data accumulated to date indicate that the diastolic filling of the left ventricle is determined by many factors, among which the greatest importance is given to the active relaxation of the left ventricular myocardium in the early phase of diastole, the elastic properties of the myocardium itself, in particular, the degree of its rigidity, the pressure that is created in the left the atrium at the time of its systole, the state of the mitral valve and associated subvalvular structures [1-4]. In various heart diseases, pathological changes in the left ventricular myocardium itself can lead to disruption of the diastolic function of the left ventricle.
It is customary to distinguish the following periods of diastole:
the period of early diastolic filling of the left ventricle, which consists of a phase of fast and slow filling, and the period of late diastolic filling of the left ventricle, coinciding with the systole of the left atrium [8,13]. The volume of blood flow through the mitral valve and its velocity during early diastolic filling is determined by the active energy-dependent relaxation of the left ventricular myocardium, chamber stiffness [2,3] and the level of pressure in the left atrium at the beginning of left ventricular diastole [4,5]. A number of studies have shown that relaxation of the left ventricle in early diastole is an active energy-dependent process controlled by such basic mechanisms as the load of contraction, relaxation, and heterogeneity of load distribution [2]. The period of early diastolic filling of the left ventricle is influenced by the diastolic deformation of the ventricular cavity, as well as intraventricular pressure at the moment of opening of the mitral valve [1,2]. The combination of the effects of these factors creates the so-called suction function of the left ventricle, which determines the movement of part of the blood volume from the cavity of the left atrium to the cavity of the left ventricle. At the end of rapid filling, the pressure difference between the left chambers decreases, and a slow filling phase begins, during which the gradient between the atrium and the ventricle is small and the blood flow from the atrium to the ventricle is small. By the time of left atrium systole, this gradient begins to increase again, which is manifested in the re-acceleration of blood flow through the mitral valve [5].
During atrial systole, the volume of transmitral blood flow entering the left ventricular cavity depends on the pressure in the left atrium during systole, on the rigidity of the walls of the left ventricle, and the end-diastolic pressure in the ventricular cavity. An additional factor influencing the filling process should also be considered blood viscosity [1,2]. Normally, the volume and velocity of blood flow through the mitral valve during early diastole significantly exceed these values during atrial systole.
Methodological issues for determining diastolic function
In recent years, with the introduction of Doppler cardiography into widespread practice, it has become possible to measure transmitral blood flow velocities in various periods of diastole non-invasively.
It should be noted that a Doppler study of transmitral blood flow can reliably verify only the phase of early fast diastolic filling and the phase of atrial systole, since the L wave, reflecting slow diastolic filling, can be detected on a Dopplerogram only in 25% of cases and, moreover, is very variable in magnitude and duration [1].
In the absence of disturbances in the diastolic function of the left ventricle in healthy young and middle-aged individuals, the peak velocity E (Emax) and the area under the curve E (velocity integral E, denoted Ei) exceed the value of the peak and integral velocities A
(Amax and Ai, respectively) [1.6-8]. According to various authors, the ratio of the velocities of the periods of early and late diastolic filling of the left ventricle ranges from 1.0 to 2.2 for velocity integrals and from 0.9 to 1.7 for peak velocities. The time of isometric relaxation of the left ventricular myocardium, measured by simultaneous recording of mitral and aortic flows, also largely depends on age, most often it is 74 ± 26 ms [1,2].
A number of studies have also shown the relationship between the increase in the contribution of the atrial component of diastolic filling of the left ventricle and the age of the subjects, which is expressed by a decrease in the ratio of the rates of the early and late diastolic filling periods due to an increase in the rates of the atrial systole period and a decrease in the rates of the early diastolic filling period. It should also be noted that the data on phase analysis of diastole in the literature are incomplete and heterogeneous in terminological definition, which requires further study of this issue.
Based on the above, we can conclude that normally the diastolic function of the left ventricle is determined by the following most significant points:
diastolic deformation of the left ventricle, pressure in its cavity at the time of opening of the mitral valve, rigidity of the walls of the left ventricle, preservation of the structures of the mitral complex and the rheological properties of the blood itself.
Impaired diastolic function in myocardial ischemia
In the presence of chronic myocardial ischemia, the rigidity or rigidity of its walls increases [4,6,9]. In particular, a number of researchers have convincingly shown the existence of a close correlation between the diastolic properties of the heart and the maximum oxygen consumption of the myocardium at rest and during exercise.
At the current level of development of this issue, the pathogenetic mechanism of impaired diastolic relaxation of the left ventricle is as follows:
insufficient oxygen supply to the myocardium leads to a deficiency of high-energy compounds, which in turn leads to a slowdown in the process of early diastolic relaxation of the left ventricle.
These changes affect the process of filling the ventricular chamber in early diastole: due to a slower than usual decrease in pressure in the left ventricular chamber, the moment when the pressure levels between the ventricle and atrium are comparable is reached later. This leads to an increase in the duration of the period of isometric relaxation of the left ventricular myocardium. Once the mitral valve opens, the pressure gradient between the ventricle and atrium is less than normal and, therefore, early diastolic filling flow is reduced. A kind of compensation is provided during atrial systole, when the volume of blood required for adequate filling of the left ventricle enters during active contraction of the atrium chamber. Thus, the atrial contribution to the formation of the stroke volume of the chamber increases [1–4, 6, 8, 10]. The above hemodynamic changes are attributed to the early type of ventricular diastole disorder, in which there is no significant increase in pressure in the chamber of the left atrium, and, accordingly, changes in the hemodynamics of the pulmonary circulation and signs of congestive heart failure are not observed [2,11].
The explanation of the pathogenetic aspects of the influence of ischemia in patients with impaired diastolic function of the restrictive type looks much more complicated. For the formation of this type of diastole disorder, the following main points are necessary: high end-diastolic pressure in the cavity of the left ventricle, formed by the significant rigidity of its myocardium [2,11,12], high pressure in the cavity of the left atrium [4,11,13], ensuring adequate filling of the ventricle in early diastole, decreased systolic function of the left atrium. Most authors in this regard point to the rather rare occurrence of a restrictive type of diastole impairment in patients with coronary artery disease [4, 6], since high myocardial stiffness is more often associated with its organic damage, for example, with restrictive cardiomyopathy, infiltrative cardiopathy [7,8]. Patients with coronary heart disease are characterized by the presence of focal myocardial pathology and the formation of high myocardial stiffness
due to prolonged, chronic ischemia and the development of fibrosis.
Thus, today it is quite obvious that myocardial ischemia has a negative effect on the process of diastolic filling of the left ventricle. Therefore, it is advisable to also touch upon the issues of diagnosing impaired diastolic function in the category of patients under consideration.
Diagnostics
Doppler cardiography has become increasingly important in recent years.
[8,11,12]. It is generally accepted today to distinguish 2 types of impaired diastolic function of the left ventricle according to Doppler cardiography data [8].
1st type
, in which, as a result of a violation of the early phase of ventricular diastole, the speed and volume of blood flow through the mitral orifice in the early phase of diastole (Epic) decrease and the volume and speed of blood flow increase during atrial systole (Apic), while an increase in the time of isometric relaxation of the left ventricular myocardium is noted ( VIRM) and prolongation of the deceleration time (SD) of flow E.
Type 2, designated pseudonormal
, or restrictive, which assumes the presence of significant rigidity of the ventricular myocardium, which leads to an increase in diastolic pressure in the ventricular chamber, and then in the atrium, and the pressure in the atrium chamber can significantly exceed the pressure in the ventricular cavity by the time the latter begins diastole, which ensures the presence of significant pressure gradient between chambers at the beginning of diastole; at the same time, the nature of the transmitral blood flow changes: Epik increases and Apik decreases, and the previously indicated time intervals (VIRM and VZ) are shortened.
A number of authors suggest dividing disorders of left ventricular diastolic function into 3 types: early, pseudonormal and restrictive
. Thus, E. Braunwald [2] proposes to differentiate the pseudonormal type of disorder from the normal and restrictive type based on the duration of the slowdown of the E peak of early filling, which, as is known, is shortened in pseudonormal and restrictive types of diastole disorder. The validity of this approach is questionable in light of the presence in the literature of data on a significant influence on the duration of diastole time intervals of heart rate at the time of the study.
Other authors point out the possibility of differentiating between the pseudonormal type of disorder and the norm by assessing flows in the pulmonary veins. With the pseudonormal type, there is an increase in pressure in the left atrium, which affects the filling nature of the left atrium [11].
The role and place of color Doppler M-modal echocardiography in the differential diagnosis between the above types of left ventricular filling is not entirely clear today. A number of authors believe that this technique helps to distinguish the pseudonormal type of filling from the restrictive and normal ones [1,2], while at the same time the question remains open about the degree and nature of the influence of factors such as heart rate, blood viscosity on the accuracy of measurements in this mode , the state of the myocardium of the left atrium, etc. It seems that color Doppler mapping in this situation does not have any fundamental advantages over a conventional Dopplerogram, because with M-modal scanning of a color Doppler image, the time intervals described above are also measured, which means that the influence remains all previously mentioned limiting factors.
It is important to study segmental diastolic function
using the Doppler tissue imaging method with M-modal scanning [8,11]. The use of this method makes it possible to assess not only the general state of diastolic function, but also the nature of relaxation of individual segments, which is especially important when assessing the effect of myocardial ischemia on these parameters at rest and during stress tests.
Clinical significance of left ventricular diastolic dysfunction and the possibility of drug intervention
IHD is one of the most common causes of left ventricular diastolic dysfunction [1,4,6] due to impaired early diastolic relaxation against the background of acute or chronic ischemia, increased myocardial stiffness at the site of the post-infarction scar and the formation of connective tissue against the background of chronic ischemia. In addition, an increase in the stiffness of hypertrophied intact myocardium in patients with coronary artery disease may be associated with ischemia due to coronary insufficiency
due to stenosis of the artery supplying blood to this area of the myocardium, and as a result of relative coronary insufficiency, which often occurs with hypertrophy. It is also known that diastolic dysfunction can occur without impairment of left ventricular systolic function [2,14]. But impaired diastolic function, even in isolated form, leads to a significant deterioration in central hemodynamics and may contribute to the onset or progression of pre-existing systolic heart failure [11,14].
The prognosis for patients with coronary heart disease who have diastolic dysfunction is more unfavorable [1,2,14], which makes the problem of its drug correction urgent.
Few studies have been devoted to the issues of drug therapy for impaired diastolic function in patients with coronary artery disease. In addition, to date there is no large study on this issue. In recent years, the scientific literature has published mainly experimental work on animals devoted to studying the effect of antianginal drugs of various groups
, as well as
ACE inhibitors (enalapril - SOLVD - investigators)
on the process of diastolic relaxation of the myocardium [15,16,17,18].
According to the results of these studies, the greatest effectiveness was noted with the use of calcium antagonists, b-blockers, and ACE inhibitors
.
For example, E.Omerovic et al. (1999) demonstrated the positive effect of the selective b1-blocker metoprolol
on the state of systolic and diastolic function of the left ventricle during myocardial infarction.
There are also separate clinical works devoted to this issue. A. Tsoukas et al. (1999), studying the effect of combination therapy with diuretics and ACE inhibitors
on the state of central hemodynamics in patients with a restrictive type of transmitral blood flow and reduced left ventricular ejection fraction (<40%), noted a positive effect of this combination of drugs in 25% of patients.
Elimination of diastolic dysfunction in the presence of myocardial ischemia is largely determined by the adequacy of individually selected antianginal therapy or surgical revascularization of the myocardium [7,14,16].
For this purpose,
calcium antagonists (in particular amlodipine), b-blockers, and nitrates are most often used.
The data of C. Stanescu et al. are also interesting. (published in the proceedings of the 21st Congress of the European Association of Cardiology in 1999) on the frequency of prescription of various groups of drugs in patients with heart failure of various etiologies (coronary artery disease - 35%, hypertension - 24%, valvular heart disease - 8%, cardiomyopathies - 3 %, other reasons - 17%). According to these authors, of 1360 patients hospitalized for heart failure, diastolic heart failure was diagnosed in 38% of cases. After an echocardiographic study, the frequency of prescription of various drugs in these patients was as follows: diuretics - 57%, calcium antagonists - 44%, b-blockers - 31%, ACE inhibitors - 25%, cardiac glycosides - 16%. While before echocardiographic examination and determination of the presence of diastolic form of heart failure, the frequency of prescription of the above drugs in these patients was as follows: diuretics - 53%, calcium antagonists - 16%, b-blockers - 10%, ACE inhibitors - 28%, cardiac glycosides - 44%. Thus, after the echocardiographic study, calcium antagonists were prescribed 3 times more often, and cardiac glycosides - less often than before the study.
In conclusion, it is advisable to note that the problem of correcting diastolic dysfunction in coronary patients is far from being resolved.
Some issues regarding the diagnosis of diastolic dysfunction remain controversial, and there is no consensus regarding drug therapy. It seems that many aspects of this problem will be resolved when the results of large studies appear on the effect of therapy on the state of diastolic function in coronary patients. Literature
1. Barats S.S., Zakroeva A.G. Diastolic cardiac dysfunction in terms of transmitral blood flow and flow in the pulmonary veins: controversial issues of pathogenesis, terminology and classification. Cardiology 1998; 5: 69-76.
2. E. Braunwald ed., Heart disease, 5th Ed., WB Saunders company 1997.
3. Caash WH, Apstein CS, Levine HJ et al. Diastolic properties of the left ventricle. In.- The LV-basic and clinical aspects. Ed. HJLevine. Boston. 1985; 143.
4. Choong CY Left ventricle: diastolic function — its principles and evaluation.-Principles and practice of echocardiography. Ed. A. Weiman. Philadelphia. Lea and Febiger. 1994; 1721-9.
5. Bonow PO, Frederick 1.M., Bacliarach SJ et al. Atrial systole and left ventricular filling in Hypertrophic cardiomyopathy: effect of verapamil. Amer J Cardiology 1983; 51:1386.
6. Baschinsky S.E., Osipov M.A. Diagnostic value of studying left ventricular diastolic function during stress Doppler echocardiography in patients with coronary heart disease. Cardiology 1991; 9: 28-31.
7. Bessen M., Gardin JN. Evaluation of left ventricular diastolic function. Cardiol.Clinics 1990; 18: 315-32.
8. Feigenbaum H. Echocardiography.- 5th Edition.- Lea & Ebiger.-Philadelphia. 1994; 166-72,189-91.
9. Zhelnov V.V., Pavlova I.F., Simonov V.I., Batishchev A.A. Diastolic function of the left ventricle in patients with coronary heart disease. Cardiology 1993; 5:12-4.
10. Dobrotvorskaya T.E., Suprun E.K., Shukov A.A. The effect of enalapril on systolic and diastolic function of the left ventricle in congestive heart failure. Cardiology 1994; 12: 106-12.
11. Christopher P., Appleton MD Doppler assessment of left venricular diastolic function: the refinements continue. JACC 1993; 21(7): 1697–700.
12. Cecconi M., Manfrin M., Zanoli R. et al. Doppler echocardiographic evaluation of left ventricular end-diastolic pressure in patients with coronary artery disease. J Am Soc Echocardiol 1996; 110: 241–50.
13. Castello D, Vaughn M, Dressler FA et al. Relation between pulmonary venous flow and pulmonary weige pressure: influence of cardiac output. Amer Heart J 1995; 130: P.127-31.
14. Vasan RS, Benjamin EJ, Levy D. Congestive heart failure with normal left ventricular systolic function. Arch Intern Med. 1996: 156: 146-57.
15. Barbier R., Tamborini G., Alioto G., Pepi M. Acute filling pattern changes of the failing left ventricle after captopril as related to ventricular structure. Cardiology 1996; 87: 153–60.
16. Goldstein S. Beta-blockers: insights into the mechanism of action in patients with left ventricular dysfunction. J Heart Failure. 1996: 13: 115.
17. Poultur H., Rousseau M.F., van Eyll C., et. al. Effects with long-term enalapril therapy on left ventricular diastolic properties in patients with depressed ejection fraction. SOLVD Investigators. Circulation 1993 Aug 88: 2 481-91
18. Sasaki M., Oki T., Inchi A., Tabata T., et. al. Relationship between the angiotensin converting enzyme gene polymorphism and the effects of enalapril on left ventricular hypertrophy and impaired diastolic filling in essential hypertension: M-mode and pulsed Doppler echocardiographic studies. J Hypertens 1996 Dec 14:12 1403-8
Enalapril –
Ednit (trade name)
(Gedeon Richter)
Amlodipine –
Amlovas (trade name)
(Unique Pharmaceutical Laboratories)
| Applications to the article |
| Diastolic filling of the left ventricle is determined by many factors, among which the greatest importance is given to the active relaxation of the myocardium of the left ventricle in the early phase of diastole, the elastic properties of the myocardium itself, in particular, the degree of its rigidity, the pressure that is created in the left atrium at the time of its systole, the state of the mitral valve and associated subvalvular structures |
| IHD is one of the most common causes of left ventricular diastolic dysfunction due to disruption of early diastolic relaxation against the background of acute or chronic ischemia, increased myocardial stiffness at the site of the post-infarction scar and the formation of connective tissue against the background of chronic ischemia |
| In patients with coronary heart disease who have diastolic dysfunction, the prognosis is more unfavorable, which makes the problem of its drug correction relevant. |
Causes
Myocardial changes occur for many reasons, and it is very important to diagnose them correctly. Some of them are diseases that can even be life threatening.
The causes and results of myocardial disorders are:
- atrial fibrillation;
- steanosis of the heart valve (aorta);
- muscular dystrophy.
Pathological changes in the myocardium of the left ventricle can occur due to inflammatory diseases. This is myocarditis, which provokes both diffuse and focal disorders. And it, in turn, is caused by such pathologies as rheumatism, influenza, measles, rubella. Various autoimmune diseases also provoke changes in the myocardium.
It is very important for the body that metabolic processes function normally; otherwise, dystrophic changes occur, as a result of which the myocytes change. Metabolic disorders mean that the heart muscle does not receive enough nutrients and oxygen. This condition is also called cardiac dystrophy.
Cadydystrophy can occur due to:
- Kidney and liver failure.
- Diabetes mellitus.
- Disorders of the thyroid gland, namely its hyperfunction.
- Anemia.
- Infectious diseases of both acute and chronic nature, the most popular are influenza and tuberculosis.
- Intoxication of the body - alcohol, drugs, poisoning with drugs and other chemicals.
Additionally, the causes of cardiac dystrophy can be excessive physical exertion, emotional shock, and stressful situations. All these factors lead to chronic fatigue. Metabolic disorders are also caused by fasting or poor nutrition.
Children may also experience changes in the myocardium of the left ventricle, and the cause of this condition is cardiac dystrophy. Factors that provoke its manifestation in a child can be mental overload and decreased motor activity.
Metabolic disorders in the myocardium can occur due to a failure of the repolarization process. At the same time, the processes of potassium and sodium exchange at the intracellular level are disrupted. Metabolic disorders also arise due to the following factors:
- hypothermia;
- increased stress, both emotional and physical;
- obesity;
- chronic diseases.
In addition, changes in the LV myocardium occur as a result of the progression of atherosclerosis, ischemia, hypertension, and arrhythmia. These are serious diseases that provoke myocardial hypertrophy.
Symptoms
Quite often, these changes are asymptomatic for several years, or appear slightly.
One of the most common signs of pathological changes in the heart muscle is angina pectoris. Because when the wall of the left ventricle thickens, compression of the vessels that feed the muscle occurs.
Atrial fibrillation and ventricular fibrillation can be the causes of the development of myocardial changes, as well as their consequence.
Another symptom of myocardial changes is “heart stopping.” In this case, the person feels that the heart does not beat for several seconds. As a result, he may lose consciousness.
Additionally, the following symptoms may occur:
- persistent increase in blood pressure, frequent changes;
- headache;
- pain in the heart area;
- weakness, fatigue;
- sleep disorders.
Transmural myocardial infarction
Transmural myocardial infarction means necrosis of all three layers of the heart muscle - the outer (epicardium), middle (myocardium) and inner (endocardium). Therefore, a transmural infarction is called penetrating (translated as “through-the-wall”). On an electrocardiogram with a transmural infarction, a Q-shaped wave is clearly visible. Another name for this type of myocardial infarction is associated with this - Q-infarction.
Transmural infarction is dangerous, first of all, because of its relapses. The small focal form of tissue necrosis, despite its penetrating nature, does not lead to serious complications. However, a repeated heart attack causes extensive symptoms that threaten not only the health, but also the life of a person. Large-focal infarction, in which a significant area of the heart is affected, poses a great danger even at the first attack.
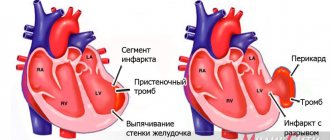
The main cause of transmural myocardial infarction is atherosclerosis of the coronary arteries. With this disease, plaques form in the walls of blood vessels, which narrow the lumen of the blood vessels, impeding blood flow and blood flow to the heart. The situation is aggravated by physical exertion and nervous stress, during which the pulse quickens and blood flow accelerates and intensifies. As a result, blood turbulence occurs at the site of atherosclerotic plaques, red blood cells stick together and blood clots form. These blood clots represent the main danger of developing a transmural infarction. Rupture of an atherosclerotic plaque or detachment of a blood clot leads to blockage of the vessel supplying the heart. Left without blood supply and oxygen supply, muscle tissue cells die - a focus of necrosis is formed.
This process is accompanied by pain in the heart area, which is the stronger the pain at the affected area. Pain during transmural myocardial infarction has a wave-like character - sometimes rolling, then receding, it can last tens of minutes, several hours and even several days. Pain in the heart during transmural infarction is acute, squeezing, radiating under the left shoulder blade, into the left arm, jaw, teeth , ear, accompanied by restlessness, anxiety, excitement, fear of death.
A feature of extensive transmural myocardial infarction is the addition of motor disorders (paralysis of the limbs), dysfunction of internal organs, difficulty speaking and other symptoms associated with impaired blood supply to the body. Other serious complications of transmural infarction are pulmonary edema and cardiac asthma.
Diffuse changes
What are “diffuse-type changes in the left ventricular myocardium”? This type is the most common. In this case, not only the left ventricle is affected, but also the entire myocardium, since diffuse changes are characterized by uniform damage.
Diffuse disorders appear both in moderate pathological processes and in acute situations, such as myocardial infarction. In the latter case, there are changes in the structure of tissues and disruption of metabolic processes. Diffuse changes are an accumulation of myocytes in the left ventricle, which, under the influence of certain factors, have changed and do not conduct impulses.
With diffuse disorders of the left ventricular myocardium, swelling of the legs, tachycardia, and even fluid accumulation in the lungs are added to the general symptoms.
Diffuse changes in the myocardium of the left ventricle can provoke a deterioration in the circulatory process, myocardial hypoxia and the appearance of necrotic foci. The most dangerous consequence of these disorders is myocardial infarction.
The real basis of myths about myocardial changes: focal and diffuse
ECG reports drawn up by functional diagnostic specialists will certainly contain “focal” or “diffuse” changes. But, strangely enough, these terms and concepts cannot be found in international and national recommendations. And in the manuals written by the masters, there is a passing conversation about focal changes in M. S. Kushakovsky and about “changes” (in context - diffuse) in M. I. Kechker. It's a rather strange situation, isn't it?
In the conclusion I wrote “focal changes in the myocardium of a cicatricial nature,” after which the head of the department asked me not to use this phrase, because, having read about focality, clinicians immediately assume a heart attack. Indeed, the most dangerous manifestation of acute focal changes is the formation of pathological Q or QS, while the severity of the pathological process is judged by changes (elevation or depression) of the ST segment. Pathological Q and QS are observed as a manifestation of cicatricial post-infarction changes, as well as with certain turns and rotations of the heart.
In all cases where it is possible to determine the electrophysiological nature of the pattern and/or topic of the process, we can talk about focal changes in the myocardium. It turns out that this definition includes extrasystole and parasystole, escaped ectopic contractions, replacement rhythms and paroxysmal tachycardias, conduction disorders in the His-Purkinje system. However, an indication of a specific electrophysiological mechanism of rhythm disturbance and/or conduction is quite sufficient in conclusion and without mentioning the focal nature of the process.
With regard to changes in the ST segment, i.e. its elevation or depression, the diagnostic search is more complex and deeper. Such changes may be caused by the state of coronary blood flow, the inflammatory process in the myocardium and pericardium, or the manifestation of benign or malignant early ventricular repolarization syndrome.
“There are many diseases, but there is only one T wave” - this catchphrase from doctors of functional diagnostics reflects the depth and complexity of diagnosing changes in the T wave of the electrocardiogram - oxygen starvation of the myocardium, electrolyte imbalance, inadequacy of hormonal and humoral regulatory factors.
The specificity of changes in the ST segment and T wave is very low, but these changes need to be analyzed, interpreted and then described in the final report. There will always be terra incognita in medicine, and the conclusion “diffuse changes in the myocardium” has its right to exist. And there is no doubt that such a conclusion from a functional diagnostics doctor will necessarily require a fellow clinician to continue the diagnostic search.
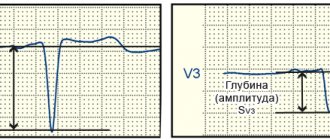
I note that among diffuse changes there are mild, moderate and pronounced. The severity of the changes is determined by the number of ECG leads in which changes in the T wave are recorded: one to two, three to four or five to six leads. Diffuse changes consist in the registration of several patterns - a decrease in the amplitude of the T wave down to isoelectric T, inversion of the T wave into negative T, biphasic (plus-minus or minus-plus) morphology of the T wave.
Nonspecific abnormalities
These violations are recorded on the ECG. The diagnosis sounds like “moderate nonspecific changes in the myocardium.” They have a direct connection with repolarization processes. This pathological condition affects the process of recovery of myocytes after an impulse has passed through them.
As a rule, such disorders are not dangerous, and, if necessary measures are taken, are completely reversible, since they are provoked by various past diseases, hormonal imbalances, and impaired metabolic processes.
Complications can include angina, heart failure, and even myocardial infarction.
Changes in the left ventricular myocardium may not be dangerous to human health. Quite often they are diagnosed during routine examinations, that is, by accident. This means that there are almost no characteristic symptoms. But you should not underestimate this condition - if you do not take the necessary measures, the condition may worsen. Usually, with moderate changes, doctors recommend changing your diet, giving up bad habits, and improving your psycho-emotional state.