We present the next article in our series of publications devoted to various forms of IHD (coronary heart disease). Today we will talk about arrhythmias.
Heart rhythm and conduction disorders are a large group of transient or permanent disorders, mainly arising from organic lesions of the cardiovascular system. They are caused by violations of the most important functions of the myocardium: automaticity, excitability and conductivity.
Cardiac arrhythmias is a collective concept for a number of different disturbances in the frequency, rhythm and sequence of heart contractions. Diagnosis of coronary heart disease is carried out using an ECG.
The resting heart rate of most healthy people is 60–75 beats per minute.
What is atrial fibrillation?
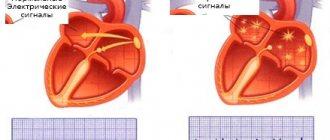
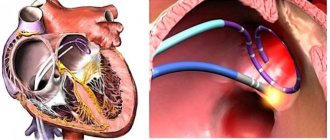
Atrial fibrillation is the most common type of arrhythmia, in which the atria contract chaotically and irregularly.
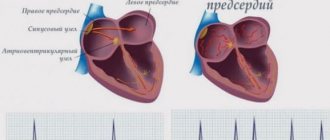
The sinus node, located in the right atrium, produces an impulse with a frequency of 60-80 per minute, which is transmitted throughout the conduction system of the heart. The organ contracts, forming a sinus rhythm. With atrial fibrillation, many foci of electrical excitation appear in them, as a result of which the atrium contracts very quickly, and at this moment the sinus node does not work. Paroxysms (episodes) of AF can be short and asymptomatic, or can last several days or months. AF itself is not dangerous, but in a third of all cases it leads to the development of ischemic stroke.
Why is atrial fibrillation dangerous?
The average Russian patient with atrial fibrillation is a 72-year-old man with coronary heart disease. AF itself is not a life-threatening condition, but can lead to the development of a severe clinical situation.
Ischemic stroke 75% of all strokes are ischemic strokes. Every third ischemic stroke occurs as a result of AF. How does this happen? With atrial fibrillation, the blood in the heart is turbulent and thrombosis occurs in the left atrium. This clot clogs the blood vessels and causes a cerebral infarction.
Myocardial infarction 6 out of 10 patients with AF have a history of coronary heart disease. If there is AF and coronary artery disease, then the risk of developing myocardial infarction increases 5 times.
Disability and death A European study based on data from 4,000 patients found that one in three people who had an ischemic stroke due to AF and did not take anticoagulants died within three months. The study showed an increase in the risk of disability by 43%, this is due to long-term rehabilitation.
| In 2020, the European Society of Cardiology developed an ABC algorithm of actions for atrial fibrillation: A - anticoagulants B - control of heart rate C - control of a person’s lifestyle |
The mechanism of pathology development
In the walls of the ventricles there are groups of cells capable of independently generating bioelectric impulses. With complete blockade of the atrioventricular node, this ability leads to the appearance of many isolated impulses circulating through the cardiomyocytes of the ventricles.
Atrioventricular block is the cause of ventricular fibrillation
Their strength is sufficient to cause weak, isolated contractions of individual groups of cells, but not enough to contract the ventricles as a whole and for a full cardiac ejection of blood.
The rate of ineffective ventricular fibrillation varies from 300 to 500 per minute, while the impulse does not weaken or interrupt, so the arrhythmia cannot stop on its own (only after cardiac arrest or artificial defibrillation).
As a result, the strength of heart contractions, ejection volume, and blood pressure rapidly drop, resulting in complete cardiac arrest.
How do I know if I have atrial fibrillation?
The most common symptoms of AF are palpitations, shortness of breath and general weakness. You should not trust subjective sensations - you need to do a cardiogram or conduct daily Holter ECG monitoring.
Holter will help more accurately determine the presence of paroxysms. If the paroxysm lasts 30 seconds or more, you can consult a doctor for anticoagulants. There are 40 thousand heartbeats per day, of which there may be 400 extrasystoles (heartbeats out of rhythm) - this is the norm.
It happens that a person does not feel paroxysms - for example, when they happen at night. This is bad because small blood clots can form that will block small vessels in the brain. Loss of blood supply to some areas will lead to their death, which significantly increases the risk of developing dementia.
In young people, extrasystoles may have extra-cardiac causes - it is necessary to check the levels of ferritin, hemoglobin, thyroid hormones, examine the gastrointestinal tract in order to exclude reflux-esophagitis, which also causes rhythm disturbances, including atrial fibrillation. People with AF need to undergo liver, kidney and general blood tests every 6 months.
| There are three forms of atrial fibrillation: — Permanent form — Persistent form (AF goes away with medical help) — Paroxysmal form (AF appears and goes away on its own) |
Characteristic symptoms
Ventricular fibrillation is a life-threatening condition with severe symptoms, equivalent to clinical death.
During arrhythmia, the function of the ventricles is impaired, blood does not enter the vascular system, its movement stops, and acute ischemia (oxygen starvation) of the brain and other organs rapidly increases. The patient is unable to move and quickly loses consciousness.
Death in 98% occurs within an hour from the appearance of the first signs of ventricular fibrillation (the time period can be much shorter).
All symptoms of fibrillation appear almost simultaneously:
- heart rhythm disturbance;
- severe headache;
- dizziness;
- heart failure;
- sudden loss of consciousness;
- interrupted breathing or complete absence of it;
- severe pallor of the skin;
- uneven cyanosis (blueness of the nasolabial triangle, tips of the ears, nose);
- absence of pulse in large arteries (carotid and femoral);
- dilated pupils of the eyes that do not respond to bright light;
- convulsions or complete relaxation;
- involuntary urination, defecation (optional).
The period of clinical death (until changes in the body become irreversible) lasts for 4–7 minutes from the moment of complete cardiac arrest, then biological death occurs (when the process of cellular decay begins).
The main drugs for preventing stroke in AF are anticoagulants
The main drugs for patients with AF that prevent stroke are anticoagulants, the effect of which reduces blood clotting. They prevent the formation of blood clots and therefore reduce the risk of stroke. Coagulation is a normal protective reaction of the body to damage to the vascular wall. With atrial fibrillation, anticoagulants are absolutely necessary for almost everyone.
The most famous anticoagulants in Russia are Eliquis, Xarelto and Pradaxa.
Also, some patients can take Warfarin (with INR monitoring). It is prescribed for low glomerular filtration rate (or creatinine clearance - an indicator of kidney function) or for prosthetic valves.
When taking Warfarin, it is necessary to monitor the INR in the blood (International Normalized Ratio) - the indicator should be from 2.0 to 3.0.
| The main drugs for patients with AF that prevent stroke are anticoagulants, the effect of which reduces blood clotting. With atrial fibrillation, anticoagulants are absolutely necessary for almost everyone. |
Diagnostics
Ventricular fibrillation is diagnosed based on external symptoms (lack of pulse, breathing, pupillary reaction to light). The electrocardiogram consistently records several stages of arrhythmia development:
- Short tachysystole or ventricular flutter (15–20 seconds).
- Convulsive stage (contraction frequency rapidly increases, rhythm is disturbed, cardiac output weakens, takes up to 1 minute).
- Actually fibrillation of the ventricles of the heart (quite large, but chaotic and frequent (300–400) flickering waves without pronounced intervals and teeth are recorded, changing height, shape, length, the stage lasts from 2 to 5 minutes).
- Atony (small, short-length and low-amplitude waves appear, lasting up to 10 minutes).
- Complete absence of heart contractions.
Since any condition with similar symptoms is a direct threat to life, resuscitation measures begin immediately, without waiting for ECG data.
Manifestation of pathology on the ECG
Anticoagulants can only be taken as prescribed by a doctor.
Standard dosage: Xarelto 20 mg / once a day Eliquis 5 mg / 2 times a day Pradaxa 150 mg / 2 times a day
Studies show that patients are more comfortable taking Xarelto - taking it once a day is easier to remember, especially for older people.
"Wafarin": take one tablet in the evening, take the INR test after 3-4 days. If the INR does not reach 2.0, then the doctor should increase the dosage. The INR should be taken every 3-4 days until the desired level is reached. Then you need to take an INR test every 3 weeks.
Important: all medications and their dosage are prescribed strictly during an in-person consultation with a doctor! This information is for informational purposes only and cannot be a guide to action. Self-medication for arrhythmia is unacceptable and can have serious health consequences.
| Reducing the dosage of anticoagulants mainly depends on only three parameters: — Creatinine clearance is less than 50. — The patient’s weight is less than 65 kg. — The patient’s age is over 85 years. In other cases, reducing the dosage risks a stroke. |
Anticoagulants thin the blood - is it dangerous?
Like any blood thinners, anticoagulants can cause bleeding. However, the risk of bleeding is 10 times lower than the risk of stroke. If a person has spontaneous bruises, nosebleeds, heavy menstruation, or blood in the urine, this is not an indication that the dosage needs to be reduced. Minor bleeding does not cause death, unlike a stroke. If you have the above symptoms of bleeding, you need to come for an examination to your attending physician - a gynecologist, urologist and, of course, a cardiologist.
| If a person takes anticoagulants, he must take a general blood test, creatinine, glomerular filtration rate (creatinine clearance), potassium, sodium, ALT, AST every 6 months. |
Ventricular fibrillation and sudden cardiac death
Previous | Contents | Ventricular fibrillation, or fibrillation, is an arrhythmic, uncoordinated and ineffective contraction of individual groups of ventricular muscle fibers with a frequency of more than 300 per minute. In this case, the ventricles do not develop pressure, and the pumping function of the heart stops. Close to ventricular fibrillation is their flutter, which is a ventricular tachyarrhythmia with a frequency of 220-300 per minute. As with fibrillation, ventricular contractions are ineffective and cardiac output is virtually absent. Ventricular flutter is an unstable rhythm, which in most cases quickly turns into fibrillation, and occasionally into sinus rhythm. Clinically equivalent to ventricular fibrillation is also frequent ventricular tachycardia with loss of consciousness (so-called pulseless ventricular tachycardia).
Ventricular fibrillation is the main cause of sudden cardiac death, occurring against the background of stable hemodynamics within 1-6 hours from the onset of symptoms of the disorder. Recently, most specialists limit this time window to 1 hour. The share of ventricular fibrillation among the immediate causes of sudden cardiac death is 75-80%.
Etiology and frequency. The etiological factors of ventricular fibrillation and associated sudden cardiac death, in order of decreasing frequency, are:
1) IHD, primarily acute violation of coronary blood circulation, acute and previous myocardial infarction (see also in the section Sudden coronary death, vol. I). According to the Framingham study, in coronary heart disease, sudden cardiac death accounts for 46% of deaths among men and 34% among women. Similar data were obtained in other studies. The highest incidence of ventricular fibrillation and sudden cardiac death is observed at the height of myocardial ischemia in the first 12 hours of acute infarction. Thrombi in the coronary arteries of the heart are found in approximately 50% of sudden out-of-hospital deaths, and new O-wave myocardial infarction is diagnosed in approximately 25% of those successfully resuscitated. Making this diagnosis is fraught with certain difficulties, since changes in repolarization and increased activity of cardiac-specific enzymes observed in some patients after resuscitation can be caused by prolonged arterial hypotension during rhythm disturbances. An increased risk of ventricular fibrillation and sudden cardiac death is also observed in patients who have had an O-wave myocardial infarction .
due to the presence of a morphological substrate for the occurrence of potentially fatal ventricular arrhythmias;
2) hypertrophic cardiomyopathy. In hypertrophic cardiomyopathy, sudden cardiac death most often occurs in young people during intense physical activity. Its risk decreases with age. In recent years, its connection with certain variants of gene mutations responsible for the occurrence of this disease has been discovered. During circulatory arrest, polymorphic ventricular tachycardia or ventricular fibrillation is usually recorded in such patients. It must be remembered that loss of consciousness and severe hemodynamic disturbances in them can also be caused by any supraventricular tachycardia with a rapid ventricular rhythm;
3) idiopathic dilated cardiomyopathy. Such patients account for about 10% of those successfully resuscitated after sudden cardiac arrest. Sudden death usually occurs against the background of severe hemodynamic disorders.
namiki in approximately half of patients with dilated cardiomyopathy. It must be remembered that in such patients, as in patients with hypertrophic cardiomyopathy, sudden death is equally often caused by ventricular fibrillation and bradyarrhythmias;
4) arrhythmogenic right ventricular cardiomyopathy. Although such patients are very susceptible to monomorphic ventricular tachycardia with frequent transformation into ventricular fibrillation, due to the rare occurrence of this disease, its proportion among the causes of sudden death from arrhythmia is very small;
5) valvular heart defects. Among them, ventricular fibrillation and sudden cardiac death most often result from aortic stenosis (congenital and acquired), which, as in the case of hypertrophic cardiomyopathy, is caused by hypertrophy of the left ventricular myocardium and the possibility of a sharp deterioration in its filling and expulsion. In patients with mitral valve prolapse, despite the significant frequency of ventricular arrhythmias, ventricular fibrillation occurs extremely rarely and is usually associated with disturbances in the electrophysiological properties of the myocardium (see below);
6) specific cardiomyopathies, primarily of an inflammatory nature, especially cardiomyopathy with sarcoidosis;
7) primary disturbances in the electrophysiological properties of the myocardium in the absence of visible structural heart diseases. This category includes prolonged O- T
(congenital and acquired, iatrogenic origin) and supraventricular tachycardia with premature ventricular excitation syndrome, pacemaker or electrical cardioversion with current pulses applied to the
T
. There are also cases of idiopathic ventricular fibrillation, which is presumably associated with dysfunction of the autonomic nervous system.
The risk of sudden death is greatest in those who have suffered sudden circulatory arrest, for whom it is 10-30% per year. In patients with idiopathic dilated cardiomyopathy, it is approximately 10% per year, in
during the first year after myocardial infarction - 5 %,
with hypertrophic cardiomyopathy and long
Q-
- 1-3%. For comparison, we can cite the following data: in the US adult population, the frequency of sudden death is 0.22% per year and in patients with mitral valve prolapse without regurgitation - 0.019% (G. Nacca-relli, 1966).
In addition to the listed cases of the development of ventricular fibrillation as a cause of sudden cardiac death as a result of primary disturbances in the electrophysiological properties of the myocardium, associated or not associated with organic heart diseases, it can be a terminal arrhythmia that occurs against the background of progressive disorders of central and peripheral hemodynamics.
Pathophysiological mechanisms. The occurrence of ventricular fibrillation is based on multiple foci of ri-n-three in the myocardium with constantly changing pathways. This is due to the heterogeneity of the electrophysiological state of the myocardium, when its individual sections are simultaneously in different time periods of de- and repolarization.
Ventricular fibrillation in more than 90% of patients is caused by ventricular tachycardia, monomorphic or polymorphic; much less often it can be induced by 1-2 early, type D, ventricular extrasystoles, causing the occurrence of unequal degrees of depolarization in different muscle fibers. Ventricular fibrillation in humans does not stop spontaneously. Only electrical defibrillation can restore sinus rhythm, the effectiveness of which depends on the nature of the underlying disease, the severity of the associated heart failure, and also on the timeliness of use. The cases of relief of ventricular fibrillation described in the literature using medications alone are rare and questionable.
Clinic. Since when ventricular fibrillation occurs, the pumping function of the heart stops, a picture of sudden circulatory arrest and clinical death is observed. Patients lose consciousness, which is often accompanied by convulsions, involuntary urination,
eating and defecation. The pupils are dilated and do not respond to light.
Diffuse cyanosis develops, there is no pulsation in the large arteries - carotid and femoral - and no breathing. If it is not possible to restore an effective heart rhythm within 4 minutes, irreversible changes occur in the central nervous system and other organs.
With ventricular flutter, MOS, consciousness and blood pressure, usually low, may persist for a short time. In most cases, however, this erratic rhythm quickly progresses to ventricular fibrillation.
On ECG
Ventricular fibrillation is manifested by chaotic flickering waves of varying amplitude and duration with non-differentiating teeth and a frequency of more than 300 per minute.
Depending on their amplitude, large-wave (see Fig. 35, e) and small-wave (see Fig. 35, e)
ventricular fibrillation can be distinguished. With the latter, the amplitude of the flicker waves is less than 0.2 mV and the probability of successful defibrillation is much lower.
Differential diagnosis. The possibility of sudden cessation of blood circulation should be borne in mind in all cases of loss of consciousness. A special chapter is devoted to this issue. Although agonal breathing may persist during the first 1–2 minutes of sudden cessation of cardiac activity, an early sign of this condition is the absence of pulsations in the large arteries and, less reliably, heart sounds. Cyanosis quickly develops and the pupils dilate. An ECG can be used to confirm the diagnosis and establish the immediate cause of sudden cardiac arrest (fibrillation, ventricular asystole, electromechanical dissociation). It must be emphasized that cardiopulmonary resuscitation should be started without waiting for ECG data, immediately after the clinical diagnosis of sudden cardiac arrest.
Large-wave ventricular fibrillation on the ECG can sometimes be difficult to distinguish from ventricular flutter and polymorphic ventricular tachycardia. Both of these forms of arrhythmias are characterized by a lower frequency of ventricular complexes, and flutter is also characterized by greater constancy of their amplitude.
Complications and outcomes of ventricular fibrillation depend on the timeliness of medical care - cardiopulmonary resuscitation (see below). The effectiveness of the latter, in turn, is determined by the nature of the organic heart disease, primarily the severity of its dysfunction, and the timeliness of the start of resuscitation measures. With the exception of relatively rare cases of early electrical defibrillation, after restoration of an effective heart rhythm, more or less pronounced complications are observed, associated both with circulatory arrest and with the resuscitation measures themselves. Possible pulmonary complications include aspiration pneumonia and lung injury from rib fractures. During cardiac arrest, total myocardial ischemia develops, and after restoration of coronary circulation, its more or less pronounced transient dysfunction occurs due to reperfusion syndrome and the so-called stunning (Stunning). In the post-resuscitation period, a wide variety of arrhythmias also often occur, caused either by the same cause as the previous ventricular fibrillation, or by disturbances in the bioelectrical and mechanical functions of the myocardium associated with circulatory arrest. Neurological complications (anoxic encephalopathy) are manifested by convulsive syndrome and coma, up to decortication. Even after a relatively long time, before. After 72 hours of unconsciousness, consciousness can be restored without residual neurological impairment. If the duration of coma exceeds 3 days, the prognosis for survival and recovery of brain function is poor.
Treatment includes emergency care - cardiopulmonary resuscitation and, if successful, measures to prevent the recurrence of ventricular fibrillation and sudden death.
Cardiopulmonary resuscitation.
Cardiopulmonary resuscitation consists of ensuring adequate ventilation of the lungs and blood circulation until the cause of the cessation of breathing and blood circulation is eliminated.
The modern concept of complex cardiopulmonary resuscitation was developed in I960 by P. Safar, W. Kouven - hoven and G . Knickerbocker. At the end of the 20s W. Kouvenhoven and his colleagues, who studied the effect of electric current on the heart in an experiment commissioned by the US Electric Company, were the first to discover that low-power current can cause ventricular fibrillation, and high-power current can eliminate it, and electrical defibrillation can be performed even without opening the chest. The first successful electrical defibrillation in the clinic was performed, however, only 20 years later by S. Beck on an open heart during cardiac surgery with variable frequency current. The modern defibrillator using pulsed current was created by V. Lown in 1960.
The effectiveness of mouth-to-mouth artificial respiration has been proven by P. Safar in 1957. In the late 60s W. Kouvenhoven and G . Knickerbocker, conducting experimental studies, discovered the possibility of increasing the duration of the time window for successful electrical defibrillation using closed cardiac massage and soon successfully used this technique in the clinic.
Previously, it was believed that when performing external cardiac massage, artificial circulation was provided by mechanical compression of the heart between the sternum and the spine. In the 70s, the heart pump theory was criticized. The basis for this was echocardiography data on the incompetence of the heart valves during resuscitation and observations on the ability of just an increase in intrathoracic pressure during coughing, without external compression of the heart, to induce artificial circulation sufficient for minimal life support. According to this thoracic pump theory, the effectiveness of external cardiac massage is based on the increase in intrathoracic pressure it creates, resulting in the collapse of the veins of the upper thoracic outlet, while the lumen of the arteries remains free. It should be noted, however, that even with optimal performance, external compression of the heart provides no more than 30% of the normal MVR value.
In accordance with the recommendations on basic life support in adults of the European Council on Resuscitation (1998), based on the corresponding recommendations of the International Consensus Committee (ILCOR), the sequence of actions when performing cardiopulmonary resuscitation at the non-specialist stage includes:
1) checking your reaction to the question: are you okay? and gentle shaking of the shoulders;
2) if there is no response with words or movements, ensure patency of the airways. To do this, the head of the patient, who should lie horizontally on his back without a pillow on a hard surface (floor, ground or shield), is carefully tilted back, placing a hand on his forehead. At the same time, the lower jaw is moved forward and upward with the other hand. As a result, the tongue moves anteriorly in the oral cavity, which prevents the root from closing the airways;
3) determining the presence or absence of breathing by assessing the respiratory movements of the chest, breathing sounds from the patient’s mouth and the sensation of his breathing on the rescuer’s cheek;
4) in the absence of breathing - carrying out two artificial breaths after a preliminary inspection of the oral cavity and (if necessary) removal of foreign bodies, dentures and vomit from it. Artificial respiration is carried out by ... blowing air from the rescuer's mouth into the patient's mouth, pinching his nose with the thumb and forefinger of the hand that finds -; on the forehead, and maintaining an elevated chin position. Insufflation is carried out relatively slowly - over 1.5-2 s, since the compliance of the lungs in this situation is significantly reduced. Slow insufflation also reduces the risk of opening the lower esophageal sphincter, filling the stomach with air and regurgitation and aspiration of its contents. The number of injections is 10-12 per minute. The recommended volume of blown air is 400-500 ml (in previous recommendations - 800-1200 ml). It has been established that, due to a significant reduction in the formation of carbon dioxide during cardiac arrest, this is sufficient for adequate ventilation.
tions. When breathing from mouth to mouth, it is necessary to monitor its effectiveness by observing the movements of the chest;
5) assessing the state of circulation by observing visible movements, including swallowing and breathing (except for rare atonal breaths), and checking the pulse in the carotid arteries. This should take no more than 10 seconds. Since it is practically impossible to determine the presence or absence of a pulse for an average person within 10 seconds, in order to move to the next stage of this population-oriented algorithm, in contrast to previous recommendations, the absence of any visible signs of life is sufficient;
6) in the absence of signs of life and blood circulation - indirect cardiac massage. It is performed by pressing the palms of two clasped hands onto the lower half of the sternum 4-5 cm deep. To ensure optimal perfusion of internal organs in this situation, the compression frequency should be 100 per minute. However, when resuscitation is carried out by one rescuer, every 15 compressions are alternated with 2 inflations, so in 1 minute only 60 compressions and 8 breaths can be performed.
Primary resuscitation continues until the arrival of qualified medical personnel and (or) the appearance of signs of life of the patient.
In recent years, there has been growing concern about the risk of transmission of infection from the victim to the rescuer during mouth-to-mouth artificial respiration. There is information about the possibility of infection with cutaneous tuberculosis, shigellosis, meningococcal meningitis, herpes simplex and salmonellosis. This risk, however, is negligible. However, reports of cases of transmission of increasingly widespread infectious diseases such as AIDS and hepatitis are completely absent. At the same time, it has been established that in case of airway patency, only correctly performed external cardiac massage ensures adequate gas exchange with maintaining arterial blood oxygen saturation by more than 90% for at least 4 minutes, which allows for
During this time, avoid mouth-to-mouth breathing. You can avoid contact with the patient’s mouth and slightly increase the effectiveness of artificial respiration when carrying out life support measures by using plastic tubes - air ducts that hold the tongue anteriorly, and a mask with an Ambu-type bag.
Cardiopulmonary resuscitation at a specialized stage begins with electrical defibrillation, which is carried out blindly, without preliminary assessment of the heart rhythm according to ECG data. The concept of the importance of electrical pulse therapy as early as possible is based on the following facts:
a) ventricular fibrillation and ventricular tachycardia, accompanied by the disappearance of the pulse, account for the vast majority - at least 80% - of cases of sudden circulatory arrest in adults;
b) ventricular fibrillation in humans cannot stop spontaneously, but can only be stopped with the help of electrical defibrillation. The latter is also the most effective method of restoring sinus or other hemodynamically effective supraventricular rhythm in ventricular tachycardia;
c) the effectiveness of defibrillation decreases rapidly over time. According to available data, the probability of successful resuscitation decreases by 7-10% with each minute that passes from the moment of clinical death (R. Cummins et al., 1991). This is due to the transition of large-wave ventricular fibrillation into small-wave ventricular fibrillation and asystole, which are associated with a significantly worse prognosis.
In this regard, every ambulance team and all departments of medical institutions must be equipped with a defibrillator, and all medical workers must be proficient in this method of resuscitation. The question is raised about the advisability of using defibrillation by all paramedical teams providing emergency medical care.
Electrical defibrillation is performed transthoracically with a sinusoidal current discharge with a
with an initial power of 200 J. Good contact of the electrodes with the skin and their correct location are important. One of them is usually placed downward from the right clavicle along the midclavicular line, and the second is above the lower ribs along the left anterior axillary line, i.e., lateral to the apex of the heart, outside the mammary gland. If defibrillation is unsuccessful, you can try changing the position of the electrodes to anteroposterior.
Before connecting the defibrillator, you can use a push technique in the precordial area, the effectiveness of which, however, has not been convincingly proven. It does not stop ventricular fibrillation, and in case of ventricular tachycardia it sometimes causes its transformation into ventricular fibrillation or asystole.
The algorithm for cardiopulmonary resuscitation at a specialized stage depends on the nature of the heart rhythm according to ECG data. In this case, depending on the presence or absence of ventricular fibrillation or ventricular tachycardia, one of two options is used.
In cases of detection of ventricular fibrillation or ventricular tachycardia, defibrillation is performed with three discharges with a power of 200, 200 and 360 J. If after this an isoline lasting more than 1 standard interval is recorded on the ECG, which may be due to electrical or mechanical stunning, it is necessary to continue cardiopulmonary resuscitation for 1 minute, then evaluate the rhythm again. In cases of persistent ventricular fibrillation or ventricular tachycardia, to ensure optimal ventilation of the lungs, tracheal intubation is performed and access is established to the central - jugular or subclavian - or peripheral vein, through which 1 mg of adrenaline hydrochloride is administered as a bolus. It has been established that this drug helps improve coronary and cerebral blood flow and survival rate during circulatory arrest in animals. The effectiveness of adrenaline hydrochloride during cardiopulmonary resuscitation is due to its ability to prevent the collapse of the carotid arteries and increase blood pressure in general both during compression of the sternum and during diastole, as well as to cause centralization.
tion of blood flow through spasm of the arteries of the abdominal organs and kidneys. The ability to further improve resuscitation outcomes in humans using higher doses of epinephrine hydrochloride than 1 mg has not yet been confirmed in placebo-controlled studies.
If ventricular fibrillation persists, defibrillation is repeated with 1-3 shocks with a power of 360 J. In cases of ineffectiveness or rapid resumption of fibrillation, the administration of adrenaline hydrochloride is repeated every 3-5 minutes in accordance with the algorithm fibrillation - defibrillation - cardiopulmonary resuscitation - adrenaline hydrochloride - rhythm assessment ECG data.
If ventricular fibrillation persists after two series of shocks and the first injection of adrenaline hydrochloride, antiarrhythmic therapy is included in the resuscitation program. However, its effectiveness has not yet been proven in controlled studies. Treatment usually begins with intravenous lidocaine as a bolus of 1–1 g5 mg/kg, which can be repeated every 3–5 minutes until a total dose of 3 mg/kg is obtained. It has been established that in humans, unlike animals, the administration of this drug before electrical defibrillation does not have a negative effect on its threshold.
An alternative to lidocaine can be the class III antiarrhythmic drug bretylium, which can increase the threshold for ventricular fibrillation and reduce the heterogeneity of the duration of the refractory period in ischemic and non-ischemic myocardium. There is evidence of its more pronounced positive effect compared to placebo on survival and the frequency of stable restoration of sinus rhythm during resuscitation in out-of-hospital conditions (D. Olson et al., 1984, etc.). In prospective randomized studies (R. Haynes et al., 1981), significant advantages of bretylium over lidocaine were not found. Bretylium is administered intravenously as a bolus at a dose of 5 mg/kg, after which transthoracic depolarization is repeated after 1-2 minutes. If there is no effect, the dose is increased to 10 mg/kg, which can be administered every 15-30 minutes, but in total - no more than 30-35 mg/kg.
Third-line drug for recurrent fibrillation
ventricles or pulseless ventricular tachycardia is procainamide, which is administered in an initial dose of 1 g at an infusion rate of 20-30 mg per minute. If the listed drugs are ineffective, you can try to administer them intravenously (3-blockers - propranolol 1 mg every 5 minutes to a total dose of 0.1 mg/kg or esmolol. These drugs are especially indicated for patients with acute myocardial infarction. There are isolated reports of the effectiveness of high doses ( 1000-1500 mg per day) of amiodarone, with the use of which less pronounced arterial hypotension is observed compared with bretylium and procainamide.
Recently, the indications for the use of buffer bases have been revised towards their limitation. It has been established that with adequate chest compressions and artificial ventilation, in the absence of significant hemodynamic disorder before cardiac arrest, pronounced acidosis does not develop in most cases. In addition, the administered sodium bicarbonate causes the formation of a large amount of carbon dioxide in the blood plasma, which diffuses into the cells faster than HC03, leading to a sharp increase in PC02 and a decrease in intracellular pH. The consequence of intracellular acidosis may be a decrease in myocardial contractility and worsening cerebral coma. Hypernatremia and hyperosmolarity also have a negative effect. Based on these premises, as well as the lack of evidence of a positive effect on survival in the experiment, the administration of sodium bicarbonate is indicated only in selected cases. These include pre-existing severe metabolic acidosis (BE less than -10) and hyperkalemia, as well as poisoning with tricyclic antidepressants and barbiturates. The initial dose is 50 mmol, or 50 ml of 8.4% solution, and repeated doses are no more than 25 mmol (administer no more than every 15 minutes). In case of out-of-hospital sudden cardiac arrest, the use of sodium bicarbonate is resorted to only with prolonged cardiopulmonary resuscitation, with the failure of defibrillation, adequate artificial ventilation, administration of adrenaline hydrochloride and antiarrhythmic drugs.
At one stage or another of resuscitation, ventricular fibrillation may develop into idioventricular rhythm and (or) asi*: stole. The tactics algorithm in this case is described in Chapter 4.
After successful resuscitation, patients usually experience hemodynamic instability, inadequate gas exchange and anoxic encephalopathy for a more or less long time, so they must be hospitalized in a block or department of intensive observation and treatment.
The central nervous system is most sensitive to ischemia and hypoxia developing during circulatory arrest. Approximately 1/3 of successfully resuscitated patients die from neurological complications, and 1/3 of survivors are left with persistent motor or sensory impairment. There are no specific treatments for encephalopathy. The symptomatic therapy carried out in such cases is aimed at correcting and preventing arterial hypotension, hypoxia, hypo- or hypercapnia, disturbances of electrolyte and carbohydrate metabolism. The effectiveness of glucocorticosteroids, often used empirically for the treatment of cerebral edema, has not been proven. It is not possible to assess the neurological prognosis in the first hours after restoration of blood circulation and breathing.
In cases of short resuscitation after a short period of ventricular fibrillation, spontaneous breathing of oxygen in high concentrations under the control of pulse oximetry data is usually sufficient to correct hypoxemia. In this case, hemoglobin oxygen saturation should be at least 95%. Inadequate spontaneous ventilation and acidosis increase the risk of recurrent cardiac arrest and contribute to secondary brain damage. Therefore, tracheal intubation and mechanical ventilation are indicated for such patients.
A number of patients after successful resuscitation experience arterial hypotension, which may be associated with the development of acute myocardial infarction or myocardial stunning as a manifestation of reperfusion. Such patients require inotropic therapy.
Common post-resuscitation complications are various heart rhythm disturbances. TO
Among the factors contributing to their occurrence during this period is hypokalemia. It occurs as a result of the transport of K+ into the cell under the influence of the action of catecholamines on β2-adrenergic receptors. In this regard, it is important to control the level of K+ in the blood plasma, which should be maintained within 4-4.5 mmol/l.
Opinions regarding the advisability of administering magnesium sulfate are contradictory. Its administration in an average dose of 2 g is certainly indicated in cases of prolonged resuscitation and persistent post-resuscitation arrhythmias.
Correction of acidosis begins with ensuring adequate ventilation of the lungs and hemodynamics, resorting to the administration of sodium bicarbonate only if these measures are insufficiently effective.
Taking into account the negative effect of post-resuscitation hyperglycemia on the neurological status, it is corrected with simple insulin according to indications.
Given the high likelihood of relapse of ventricular fibrillation and pulseless ventricular tachycardia, treatment of all successfully resuscitated patients should include measures for their secondary prevention.
They are based on a careful assessment of other possible risk factors, including data from invasive examinations - coronary angiography (for coronary artery disease) and programmed pacemaker (see Vol. I, Sudden Coronary Death). Secondary prevention includes active treatment of ischemia and heart failure, the prescription of antiarrhythmic drugs, preferably under the control of EPS data, and implantation of a cardioverter-defibrillator. The latter method, which is becoming increasingly widespread, is preferred when risk factors are ineffective in controlling risk factors using other approaches and primarily in cases where it is impossible to induce ventricular tachycardia in patients with low left ventricular ejection fraction (for more details, see vol. I, Sudden coronary death and above in the section Ventricular tachycardia).
The nearest forecast, as already happened
it is said that it depends on the timeliness of the start of resuscitation measures.
Arrest of blood circulation lasting more
than 4 minutes, as a rule, leads to irreversible changes in the central
ral nervous system. If cardiopulmonary resuscitation can be initiated within the first 3 minutes and electrical defibrillation performed within the first 6 minutes, the probability of survival reaches 70%. When defibrillation is applied too late, after more than 12 minutes, less than 20% of patients remain alive. The main cause of death in the immediate post-resuscitation period is hypoxic encephalopathy.
Long-term prognosis is determined by the state of left ventricular function, and in severe heart disease, effective prevention of fatal arrhythmia, including implantation of a cardioverter-defibrillator, often does not have a significant impact on the outcome. In patients with acute myocardial infarction, when ventricular fibrillation, as well as ventricular tachycardia, occurs in the early stages - in the first 24-48 hours - it does not significantly aggravate the prognosis, while the prognostic value of later ventricular fibrillation is very unfavorable.
Primary prevention is generally carried out in the same way as for sudden coronary death (see Vol. I).
Dangerous or useless drugs
● 50% of strokes in AF are associated with the fact that a person takes aspirin. Neither aspirin, nor Clopidogrel, nor their combination protects a person from the development of ischemic stroke. ● Many people take blood-thinning drugs when there is no reason for this, others are ready to replace anticoagulants with Cardiomagnyl, Mildronate, Riboxin - this cannot be done. ● “Preductal” can cause extrasystole and increase arrhythmia; it is used only for coronary heart disease. ● The heart cannot be “nourished” with anything; there are no “vitamins for the heart.” The heart is a reflection of your lifestyle - nutrition, movement, sleep.
Why do you need to control your lifestyle?
Because it affects the size of the left atrium. The more severe the obesity, the larger the size of the left atrium. If a person is overweight, the pressure in the heart increases, the volume of circulating blood increases, and the left atrium stretches. The larger the left atrium is from normal (400 mm), the higher the risk of developing atrial fibrillation.
Situations
With normal left atrium size, the effectiveness of radiofrequency ablation can reach 70%. The larger the size of the left atrium, the lower the effectiveness of RFA.
● If a patient is undergoing radiofrequency ablation, it is important to remember: the more abnormal the atrial function is, the higher the risk of recurrent atrial fibrillation. ● When a person’s heart rhythm is lost, during an ultrasound they pay attention to the size of the left atrium: if it is slightly dilated, it makes sense to try to restore this rhythm using medications or electrical pulse therapy. But if the left atrium is significantly dilated, there is no particular point in restoring the rhythm - sooner or later it will break down again.
What to do?
● Control your weight. ● Avoid alcohol - alcohol increases the risk of bleeding and aggravates the course of the arrhythmia or increases the frequency of breakdowns of this arrhythmia. ● Stop smoking. ● Stop taking dietary supplements - most of them can enhance the effect of anticoagulants. ● Increase physical activity. ● Monitor the condition of the thyroid gland by an endocrinologist.
Treatment
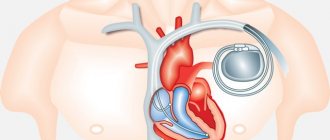
It is impossible to cure fibrillation; this form of arrhythmia is a deadly complication that usually occurs unexpectedly. In some cardiovascular diseases, it can be predicted and prevented by installing a pacemaker or cardioverter-defibrillator.
Treatment of fibrillation consists of first aid and cardiac resuscitation, in 20% the victim’s life can be saved.
First aid
If cardiac arrest as a result of ventricular fibrillation did not occur in a hospital, first aid must be provided before the arrival of a professional medical team. There is very little time allotted for it - the heart must be started within 7 minutes, then the victim’s chances rapidly drop.
First stage of emergency care
Call out to the person, give him a boost, hit him hard on the cheek, and perhaps the person will come to his senses.
Place your hand on your chest; its movement indicates the presence of breathing.
Place your ear on your chest in the sternum area (a palm below the subclavian fossa), so you can hear the sound of your heartbeat or feel how your chest rises in time with your breathing.
With your fingers (middle and index) pressed together, try to feel the pulse in any accessible large blood vessel (carotid, femoral artery).
Absence of pulse, breathing, or chest movements is a signal for first aid.
Second stage of emergency care
Lay the victim face up on a flat surface.
Tilt his head back, try to determine with your fingers what is interfering with breathing, clear the airways of foreign objects, vomit, and move the receding tongue to the side.
Ventilate the lungs: hold the victim’s nose with one hand and forcefully blow air “mouth to mouth.” At the same time, evaluate how much the chest rises (artificial respiration prevents the lungs from collapsing and stimulates the movement of the chest).
Kneel to the side of the victim, fold your hands on top of each other (crosswise), begin to rhythmically press on the lower third of the sternum with crossed palms on outstretched arms.
For every 30 rhythmic chest compressions, take 2 deep mouth-to-mouth breaths.
After several cycles of direct massage and ventilation, assess the condition of the victim (perhaps he has a reaction, pulse, breathing).
Direct cardiac massage is performed intensively, but without sudden movements, so as not to break the victim’s ribs. Do not try to start the heart with an elbow strike to the sternum - only very qualified specialists can do this.
First aid is provided before the arrival of the medical team, who must be called before resuscitation begins. The time during which it makes sense to provide first aid is 30 minutes, after which biological death occurs.
Professional Cardiac Resuscitation Techniques
After the arrival of doctors, measures to restore heart function and hemodynamics continue in the ambulance and in the intensive care unit of the hospital.
Apply:
- Electrical defibrillation of the heart (with the help of electrical impulses of different frequencies and strengths, disturbances in the conductivity and excitability of the ventricular myocardium are eliminated and rhythm is restored). If there are no serious organic changes in the myocardium, in the first minutes a defibrillator restores heart function in 95%; against the background of serious pathologies (cardiosclerosis, aneurysm), stimulation is only 30% effective.
- An artificial lung ventilator (the lungs are ventilated manually, using an Ambu bag, or connected to an automatic device, supplying the respiratory mixture through a tube or mask).
The introduction of drugs corrects disturbances in electrolyte metabolism, eliminates the consequences of accumulation of metabolic products (acidosis), maintains heart rhythm, and has a positive effect on the conductivity and excitability of the myocardium.
| Groups and drugs | What are they intended for? |
| Adrenergic agonists (adrenaline) | Increase the tone and resistance of myocardial cells, stimulate their synchronous contraction, improve hemodynamic parameters (coronary and cerebral blood flow) |
| Antiarrhythmics (lidocaine, ornid, novocainamide) | Improve conductivity and reduce excitability of cardiomyocytes, suppress circulating excitation impulses in the myocardium |
| Regulators of acid-base and electrolyte balance (sodium bicarbonate, sodium lactate) | Used to neutralize metabolic products and restore acid-base balance during acidosis |
After an attack of ventricular fibrillation, patients spend some time in intensive care units, during which time the attending cardiologist decides how to improve the prognosis (options being considered are implantation of a cardioverter-defibrillator or a pacemaker).
Complications of the post-resuscitation period
Resuscitation measures (direct massage, defibrillation) manage to save the lives of 20% of patients.
Typical complications of the post-resuscitation period:
- chest injuries and rib fractures (due to intense direct massage);
- hemothorax and pneumothorax (accumulation of blood or air in the pleural cavity of the lungs);
- aspiration pneumonia (due to the contents of the stomach, nasopharynx and oral cavity entering the respiratory tract and lungs);
- disturbances in the functioning of the heart (myocardial dysfunction);
- arrhythmia;
- thromboembolism (blockage of the pulmonary artery with a blood clot);
- disturbances in the functioning of the brain (against the background of hemodynamic disturbances and oxygen starvation).
The result of restoration of heart function and hemodynamics after a long time (10–12 minutes after the onset of clinical death) can be irreversible changes in brain tissue caused by oxygen deficiency, coma, and complete loss of mental and physical disability. Only 5% of cardiac arrest survivors do not have significant brain problems.