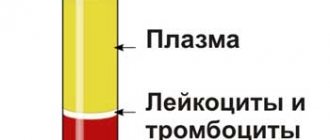
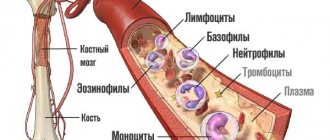
Every practicing doctor knows how difficult it is to find the cause of a disease. Often you have to spend a lot of time, effort and money on this. Currently, new testing methods are emerging to help identify the cause of the disease. One such method is hemoscanning.
–
dark-field microscopy of native blood.
The method was developed by Dr. Kurt Grange
(Master of Nutrition Science, Doctor of Philosophy in Nutrition Science and Doctor of Naturopathy from Clayton University). What is special about this method? First of all, blood microscopy is performed at a magnification of 1800–2000 times in the presence of the patient. The blood is not dried or tinted, due to this, blood cells, plasma and its other components continue to live for some time (under certain conditions, up to 5–7 days). Examination of blood using dark-field microscopy allows one to evaluate the quantitative and qualitative composition of blood, the functional activity of its components, to identify the presence of bacteria, fungi, viruses, parasites, protozoa, as well as inclusions in the form of crystals of cholesterol, bilirubin, uric acid, sugar, etc. Based on the totality of all By identifying signs in the blood, it is possible to determine with fairly high certainty whether the patient has certain diseases or a predisposition to them.
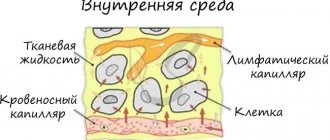
Hemoscanning of blood: how it is done
The microscope is connected to a video camera, which displays the image on the monitor and also makes it possible to take photographs and videos of interesting objects. It is known that blood performs very important functions in the body. First of all, blood is a transport system that connects the work of all organs and systems. Blood interacts with every cell of the body, delivering them oxygen, bioactive, nutrients, and removes waste products, toxins and waste products that cells release during their functioning. Blood transports immune cells to areas of inflammation or the introduction of foreign agents (infections, allergens), and delivers platelets to areas of bleeding. The condition and composition of the blood is reflected in all pathological processes that occur in the body - inflammatory, infectious, metabolic, immune, allergic, etc. In case of digestive disorders, the function of the excretory organs (kidneys, intestines, skin and lungs), with dysbacteriosis and parasitosis, the blood becomes acidified, overloaded with toxic substances and free radicals.
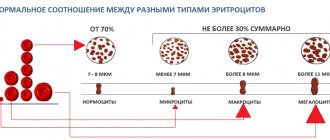
Leukocyte activity, eosinophil and basophil content
The state of the human immune system can be judged by the number, size, and activity of leukocytes
, quality composition. The presence of inactive, small cells, prone to grouping into groups of two or three, a decrease in the total number of leukocytes indicates a decrease in immunity, the presence of an inflammatory process (acute or chronic) in the body. An increase in blood pH inhibits the phagocytic activity of leukocytes and reduces the immunological response.
With an increase in the number of eosinophils and basophils
one can judge the predisposition to allergic reactions and the presence of parasitic infestation in the body.
Blood diseases affecting red blood cells
Blood diseases that affect red blood cells include:
Anemia: People with anemia have low red blood cell counts. Mild anemia often causes no symptoms. More severe anemia may cause fatigue, pale skin, and shortness of breath with exertion.
Iron deficiency anemia: The body needs iron to make red blood cells. Low iron intake and blood loss due to menstruation are the most common causes of iron deficiency anemia. It can also be caused by blood loss from the gastrointestinal tract due to ulcers or cancer. Treatment includes iron tablets or, less commonly, blood transfusions.
Anemia of Chronic Disease: People with chronic kidney disease or other chronic diseases are prone to developing anemia. Anemia of chronic disease usually does not require treatment. Injections of the synthetic hormone epoetin alpha (Epogen or Procrit) to stimulate blood cell production or blood transfusions may be necessary in some people with this form of anemia.
Pernicious anemia (B12 deficiency): a condition that prevents the body from absorbing enough B12 in the diet. This may be caused by a weakened stomach lining or an autoimmune disease. In addition to anemia, nerve damage (neuropathy) may eventually occur. High doses of B12 prevent long-term problems.
Aplastic anemia: In people with aplastic anemia, the bone marrow does not produce enough blood cells, including red blood cells. It can be caused by a number of conditions, including hepatitis, Epstein-Barr or HIV medication side effect, chemotherapy drugs, pregnancy. Treatment for aplastic anemia may require medications, blood transfusions, and even a bone marrow transplant.
Autoimmune hemolytic anemia: In people with this disease, an overactive immune system destroys the body's own red blood cells, causing anemia. Medicines that suppress the immune system, such as prednisone, may be needed to stop this process.
Thalassemia: This is a genetic form of anemia that mainly affects people of Mediterranean origin. Most people have no symptoms and do not need treatment. Others may require regular blood transfusions to relieve symptoms of anemia.
Sickle cell anemia : a genetic disease that mainly affects people whose families come from Africa, South or Central America, the Caribbean islands, India, Saudi Arabia and Mediterranean countries including Turkey, Greece and Italy. In sickle cell anemia, the red blood cells are sticky and hard. They can block blood flow. Severe pain and organ damage may occur.
Polycythemia vera: The body produces too many blood cells, for an unknown reason. Excess red blood cells usually do not cause any problems, but can cause blood clots in some people.
Malaria: A mosquito bite carries the parasite into a person's bloodstream, where it infects red blood cells. Periodically, red blood cells rupture, causing fever, chills and organ damage. This blood infection is most common in parts of Africa, but can also be found in other tropical and subtropical areas around the world; those traveling to affected areas should take preventive measures.
What can the platelet count tell you?
Platelets
are cells of the blood coagulation system. Changes in the number, shape, and aggregation of platelets are signs of a violation of homeostasis: with blood loss, deregulation of the coagulation system, dehydration, pH shift to an acidic environment, acute infections, anemia, metabolic disorders, intravascular coagulation syndrome. An increase in the number of platelets and their aggregation is caused by provoking and contributing factors: hypokinesia, obesity, excessive physical activity, cooling, intravenous manipulation, taking hormonal contraceptives.
Plasma analysis
The liquid part of blood is plasma
, gives us a fairly large amount of information about the state of the internal environment of the body. The presence of various inclusions in it: cholesterol crystals, uric and orthophosphoric acid salts, bacteria, larvae, fungi and their spores, the rapid appearance of fibrin threads is an indication of a predisposition to the development of a particular disease. Cholesterol crystals form when lipid metabolism is disrupted. Failure of the digestive process, liver and pancreas function leads to hyperlipidemia, which over time leads to a pathological condition - hypercholesterolemia. Cholesterol crystallizes, adsorbs waste products and bacterial waste products on its surface, becomes fixed in the vessels and becomes the basis of atherosclerotic lesions. This can cause the development of cardiovascular pathology.
Helpful information
T. Shuzel
A simple blood smear test provides accurate and quick information. Additional quantitative counting of blood cells is provided using a hematological automatic counter.
Blood smears are used to count and evaluate different morphological forms of blood cells. It should be borne in mind that complete information cannot be obtained only using automatic meters.
This article describes methods for preparing and analyzing blood smears, as well as obtaining additional information through the use of automatic research methods.
TECHNIQUES FOR PERFORMING BLOOD Smears
There are many indications for blood tests, and they are aimed not only at diagnosing anemia. Most systemic diseases also lead to changes in the hemogram, the analysis of which is the basis for diagnosis.
1. Taking material for research
Most often, a blood smear is performed by applying a drop of venous blood taken from a tube preserved with ethylenediaminetetraacetate (EDTA) onto a glass slide, which preserves the morphology of the cells. The percentage of blood/anticoagulant must be observed: excess EDTA causes pseudothrombopenia artifact and changes cell morphology (serrated edge of red blood cells).
The material is applied to a glass slide immediately after collection, because cell damage occurs during storage (vacuolation of leukocytes, nuclear pyknosis1).
1 Pyknosis (gr. puknosis, condensation) [English. pyknosis]. Transformation of the nucleus in a cell with chromatin condensation. The nucleus becomes homogeneous and has a uniform color. The manifestation of this phenomenon is associated with cell death.
2. Method of applying the material
- A drop of blood is applied to the surface of a glass slide, positioned horizontally, using a pipette close to its edge (photo 1).
Photo 1. Performing a blood smear: Place one drop of blood on the end of a slide. Another glass, with which you need to make a smear, is placed on the slide in front of the drop of blood at an angle of approximately 30-45° (photo 2). By sliding it along the slide, it is brought closer to the drop of blood until the latter is distributed along the moving edge (photo 3). After this, the glass is moved with a one-way movement along the surface of the glass slide towards the opposite edge. The slide is marked before or after application of the material, which avoids possible errors.
| Photo 2. Performing a blood smear: a glass for performing a blood smear is placed on a glass slide with a drop of blood at an angle of 30-45°. | Photo 3. Performing a blood smear: the slide is moved close to the drop of blood until it begins to distribute along the entire front of its edge due to capillarity. |
- The purpose of applying the material to a glass slide is to obtain a homogeneous smear with a thinning end (tails, also called pigtails). In this thin part of the smear, blood cells are arranged in one layer, which allows you to examine their morphology (photo 4). Figure 1 shows how a blood smear should be performed.
| Photo 4. Performing a blood smear: the blood smear has a homogeneous structure with a cellular thinned monolayer located in the part of the slide opposite to the drop of blood applied to it. | Fig.1. Picture of a smear after applying the material to a glass slide |
- If cell counting cannot be performed immediately on site, the resulting smears, delivered with the rest of the material to the laboratory, are not stained.
- Before staining, smears must be dried in fresh air, avoiding high temperatures, because this can lead to artifacts due to deformation of the surface of red blood cells.
These precautions are extremely important because when animals with erythrocyte invasion are infected with rickettsia (Hemobartonella felis) and piroplasmas (Babesia canis), artifacts arising from staining smears can make identification of the pathogens difficult. Using the May-Grunwald-Giemsa dye (Pappenheim method), a very high-quality microscopic picture of the smear is obtained with detail of the nuclear and cytoplasmic apparatus of the cell (Appendix 1). It should be noted that this painting method takes a long time (more than 20 minutes). In addition, rapid rinsing may damage the test material. But quick staining of smears (Diff-Quik, RAL) in emergency cases allows you to obtain the necessary analysis results in a short period of time. The main disadvantage of using quick sets (Kits) is that they do not allow sufficient detailing of the nuances inside the cell itself (the genesis of chromatin artifacts, qualitative assessment of the cytoplasmic state).
Appendix 1. Painting scheme according to May-Grunwald-Giemsa
Classic paint scheme
— Place the slide with the material applied on it into the May-Grunwald stain and leave for 4 minutes. — Place the slide with the material applied to it in May-Grunwald paint, diluted by 50% with water, and leave for 2 minutes. — Rinse the slide with distilled water. — Place the slide in a container with a 5% solution of Giemsa paint diluted with tap water and leave for 15 minutes. — Rinse the slide and dry.
Simplified diagram
— MayGrunwald-Giemsa stain (2-3 ml) is poured onto the smear, while the slide should be placed horizontally in the supporting device. Coloring is carried out within 5 minutes. — Carefully drain off the dye and reapply the Giemsa dye solution diluted 1:10 with water extemporaneously for 10 minutes. — Rinse the slide and dry.
ARTIFACTS
- Artifacts that can occur due to very rapid or insufficient drying, as well as due to high temperature or excess EDTA, have the following manifestations:
- jagged forms of erythrocytes or echinocytosis (must be differentiated from acanthocytosis2); - refraction of light under a microscope in bodies located on red blood cells (must be differentiated from piroplasmas or babesias) (photo 5).
2 Acanthocytosis (Gr. akanta, thorn, needle; kutos, cell) [English. akanthocytosis]. Deformation of red blood cells that resembles a hedgehog's spine.
| Photo 5. Artifact from improper drying of red blood cells: light-refracting bodies located on the surface of red blood cells. Activated lymphocyte. |
Artifacts resulting from painting due to the use of an old dye solution or improper painting conditions (insufficient soaking time):
— weak coloring of cells, mainly the nuclear apparatus; — dye depot (photo 6); — pseudo-inclusions (superposition of two elements).
| Photo 6. Coloring artifact: dye depot. |
READING METHOD
Blood smear analysis is always carried out in the same way, following the appropriate steps.
The collection of information begins from the moment the material is applied to a glass slide, before the study of blood cells under immersion.
1. Macroscopic examination
- Interpretation of the blood smear begins by examining it with the naked eye immediately after applying the blood to the slide. At this stage, the quality of the resulting blood smear is assessed (the thinness of the end of the smear and homogeneity, the presence of agglutination of a drop of blood).
- The presence of macroscopic agglutination before drying and staining is characterized by heterogeneous distribution of blood on the slide in the form of “dot clots”, which indicates autoagglutination or the presence of erythrocyte columns (photo 7).
| Photo 7. Macroscopic agglutination. Attention should be paid to the lack of homogeneity of the blood. This agglutination is caused by autoagglutination or persistence of erythrocyte (coin) columns. |
- In dogs, autoagglutination occurs due to sensitization of red blood cells by antibodies. This is serious evidence of hemolytic processes of an immune nature, while the presence of the formation of coin (erythrocyte) columns mainly occurs in the case of dysproteinemia. To distinguish between these two phenomena, a drop of blood is mixed with saline solution. This causes the agglutination to disappear if a coin column is formed. The persistence of this blood condition indicates autoagglutination.
- Macroscopic agglutination in a cat, manifested by a accumulation of red blood cells, is nonspecific and cannot be interpreted.
Then the slide with the blood smear applied on it is dried, stained and examined under a microscope.
2. Examination of a smear under low magnification
The first stage of microscopic examination of the material is carried out at low magnification (×10 or ×20 objective). This allows you to assess the quality of the smear and obtain the first information.
In the area of the thinnest part of the smear, a large accumulation of leukocytes is noted, which makes it possible to determine their number already at the first assessment (photo 8).
| Photo 8. Examination of the pigtail of the blood smear at low magnification. Red blood cell counting is possible. This image shows pronounced leukocytosis with a predominance of neutrophils. | Photo 9. Examination of the smear at low magnification: an accumulation of platelets is observed. |
Detection of platelet accumulations (Figure 9) indicates an artifact that may lead to a false conclusion of thrombocytopenia.
- At the level of the monocellular layer, a complete visual examination of the slide at low magnification (×40) allows one to calculate the leukocyte formula.
- It is recommended to search for nucleated red blood cells. If we find them in large quantities, then this indicates a violation (we may be talking about the circulation of microfilariae, or we are dealing with abnormal cells). The researcher always focuses his attention on the suspected presence of macroscopic agglutination of red blood cells (photo 10) or the formation of coin columns (photo 11).
- The various cells are then examined under high magnification.
| Photo 10. Examination of the cellular monolayer (×40): microscopic autoagglutination. | Photo 11. Study of the cellular monolayer (×40): erythrocyte columns. |
ASSESSMENT OF THE CONDITION OF RED CYTES
- The condition of red blood cells is assessed under immersion (×100) in the middle part of the smear: in the monocellular layer, where they are well separated and not deformed.
- Morphological assessment reveals symptoms of medullary regeneration, as well as changes in size, shape and the presence of inclusions.
- Normal red blood cells in domestic carnivores are round, anucleate cells in the shape of a biconcave disk (discocyte), the diameter of which is approximately 7 μm in a dog and 6 μm in a cat. In the monocellular zone they are represented by a monocellular layer, have a round shape with a pale glow in the center (practically absent in cats), and are colored yellow-orange (moderate eosinophilia). In this case, physiologically moderate anisocytosis3 can be observed (photo 12).
| Photo 12. Red blood cells and normal platelets in a dog. |
3 Anisocytosis (gr. an, negative prefix; isos, equal; kutos, cell) [English. anisocytosis]. The pathological state of red blood cells, when they vary greatly, while normally they should be the same. This term is also acceptable in relation to variations in leukocyte diameter.
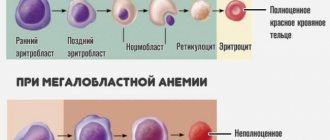
1. Signs of regeneration
- The information obtained from the blood test does not indicate the cause of the anemia or its regenerative nature. But it allows you to assess the severity of disorders by counting the number of red blood cells, determining the concentration of hemoglobin and hematocrit.
Examination of blood smears of an animal suffering from anemia, in combination with hematological analysis performed using automated technology, allows us to assess the degree of regeneration and, in some cases, identify the cause of anemia.
- Pathognomonic symptoms of regeneration in blood smears are as follows: polychromatophilia; anisocytosis; Havel-Joly bodies; erythroblasts (red blood cells containing a nucleus) associated with reticulocytosis observed with special staining.
- Polychromatophilia (photo 13) is determined by the presence in the blood of large-diameter red blood cells of a violet color with elements of basophilia, which contrast with the orange color of normal red blood cells. These polychromatophilic cells correspond to immature forms of red blood cells that are widely distributed in the bone marrow and are seen in degenerative bone marrow changes. In the cytoplasm they contain remnants of ribosomal RNA, which gives a purple color. This corresponds to reticulocytes with intravital coloring (shimmering cresyl blue) in dogs and cats.
| Photo 13. Examination of the monocellular layer in a smear with high magnification (×100). It should be noted the presence of polychromatophilic erythrocytes, anisocytosis of erythrocytes and the presence of spherocytes. The erythroblast is observed at the lower left. | Photo 14. Examination of the monocellular layer at high magnification (×100). You should pay attention to erythrocyte anisocytosis and small azurophilic inclusions of a round shape, which are contained in some erythrocytes: Havel-Joly bodies. |
- Anisocytosis (photo 14) - variations in the size of red blood cells. The number of large red blood cells in the blood increases. Polychromatophily is associated with large size and corresponds to anisocytosis. The presence of red blood cells with more intense color and larger red blood cell size is a strong argument in favor of regenerative anemia.
- Erythroblasts (nucleated red blood cells, immature forms), usually found in the bone marrow, tend to sometimes appear in small numbers in the circulating blood of a healthy body.
They are characterized by their large size, have a grayish-violet cytoplasm, and a voluminous, round, intensely colored nucleus. Their number increases with regenerative anemia, but the presence of these cells alone does not allow making a diagnosis of the suspected disease. Erythroblasts can be observed in large numbers in the circulating blood during lead poisoning, severe septicemia, and various blood diseases (myeloproliferation syndrome, etc.).
- To confirm regenerative anemia, it is necessary to count reticulocytes circulating in the blood after staining with blue brilliant cresyl (Appendix 2). When carrying out this staining, mature forms of erythrocytes with blue intense staining of the cytoplasmic reticulum are distinguished. In a dog, all cells with this color are counted, whereas in a cat, only reticular erythroblasts are counted (recognized due to the pronounced accumulation of cytoplasmic granulation).
Appendix 2. Methods for determining the number of reticulocytes
Method Execution
- Mix 500 µl of blood with 500 µl of blue brilliant cresyl solution (1 g of brilliant blue cresyl powder and 0.4 g of citrate, diluted in 100 ml of isotonic solution). - Leave for 10-20 minutes at room temperature. — Apply this mixture to a glass slide, as is done for obtaining a blood smear. — Count the number of observed reticulocytes at high magnification (approximately to visualize 100 red blood cells in the field of view of the microscope under immersion) and carry out this procedure in 10 objective fields. The resulting amount is expressed as the percentage of circulating reticulocytes. The relative estimate is transformed into an absolute value by multiplying this number by the total number of red blood cells.
Interpretation
Some authors talk about bone marrow regeneration at a quantitative rate of 80,000-30,000 reticulocytes per mm3 in dogs and cats. Others talk about 0 reticulocytes in mm3. If the assessment is questionable, or one indicating the lower limit of the norm, a repeat count is carried out after two or three days. Reticulocyte kinetics facilitates more accurate interpretation.
Havel-Joly bodies (photo 14) are single inclusions of small size, have an azurophilic and round shape, and are present in young red blood cells that are in the maturation phase. They belong to the nuclear material phase of erythroblasts and are eliminated upon maturation. A small percentage (less than 1%) may be present in cats under normal physiological conditions. Their number increases with regenerative anemia, splenectomy, and in animals treated with glucocorticosteroids and chemotherapy drugs.
2. Size anomalies
- Spherocytosis is the most common abnormality of red blood cell size. It indicates an immunological effect on red blood cells during the development of hemolytic anemia. The presence of spherocytes is explained by partial lysis of erythrocytes coated with antibodies by macrophages of the liver and spleen. Spherocytes are recognized by their small size, have a homogeneous cytoplasm and intense coloring, they do not have a pale spot in the center. These cells are more difficult to identify in a cat: healthy red blood cells in this species do not have a pale color in the center.
In immunoglobulin-mediated hemolytic anemia, smears indicate a pronounced concentration of spherocytes, which is associated with signs of bone marrow regeneration (the presence of polychromatophils, Havel-Joly bodies and anisocytosis).
- Symptoms of medullary regeneration with the presence of large (macrocytes) or small (microcytes) red blood cells can be observed in the circulating blood. These cells have the tinctorial and morphological properties of normal red blood cells. It is much more difficult to detect an abnormality: it is necessary to estimate the mean volume of red blood cells (VGM) using an automatic counter.
Causes of decreased VGM may include portosystemic shunting or regenerative anemia caused by iron deficiency. An abnormal size is an early sign of sideropenia (low iron). Hypochromasia (reduced coloration) manifests itself much later and occurs several months after the onset of the disease.
- Some individuals belonging to certain breeds of dogs (for example, the poodle) have a VGM that exceeds the norm. Such macrocytes are sometimes detected in blood smears.
3. Shape anomalies
- Dog and cat red blood cells circulating in the blood are usually round in shape. An increased content of red blood cells of various forms is called poikilocytosis (photo 15). This nonspecific term refers to variations in the shape of red blood cells in relation to the physiological norm, without indicating the nature or severity of the abnormalities. This anomaly is considered an artifact or is associated with a variety of systemic diseases.
| Photo 15. Examination of the monocellular layer at high magnification (×100). Note the varying shapes of red blood cells (erythrocyte poikilocytosis) and large platelets (macroplatelet). |
- Many terms, some of which are intended to describe the cytological picture, are used to describe the form of red blood cell abnormalities: codocytes, dacryocytes, keratocytes, stomatocytes, and so on. The presence of individual forms is a pathognomonic sign of certain disorders and correlates with the clinical picture of the disease. With the development of poikilocytosis, one should mainly look for severe disorders associated with the inflammatory process or pathology of the hepatobiliary system.
- With metabolic disorders, red blood cells can be deformed due to an abnormality in their membrane. Acanthocytes are deformed red blood cells whose elongated shape resembles a finger. They are formed due to membrane abnormalities caused by liver disorders (bypass surgery, liver neoplasms, lipoproteinopathy, hypercholesterolemia).
- Mechanical fragmentation of erythrocytes during hemocirculation disorders leads to the formation of schizocytes: these are fragments of erythrocytes of various sizes that may have an irregular shape (dots, sickle, etc.). They occur due to blood turbulence, for example, with valve stenosis. Schizocytes are characteristic of caval syndrome. This is a pronounced hemolytic crisis in individuals that are carriers of various parasites, for example adult forms of Dirofilaria immitis. They persist in the caudal vena cava and cause secondary rheological blood disorders. Also, mechanical fragmentation of erythrocytes can be caused by immunological or physical aggressions (DIC, hemangiosarcoma, severe inflammation, etc.).
4. Color abnormalities
The color intensity of red blood cells is proportional to the concentration of hemoglobin they contain.
- Hypochromia (photo 16) is determined by the presence of pallor in the central part of the red blood cell due to a pronounced decrease in hemoglobin content. Often detected in sideropenic anemia. Hemoglobin is concentrated in a thin peripheral ring in red blood cells called annulocites.
| Photo 16. Examination of the monocellular layer at high magnification (×100). It should be noted the presence of annullocytes: red blood cells have a pronounced clearing in the center due to the reduced hemoglobin content. |
Most of these anemias are associated with iron deficiency due to chronic loss in the digestive system (ulcers, collapsing tumors, etc.).
- On the contrary, a certain degree of hyperchromia (optical) can be detected in hemolytic anemia by the presence of spherocytes, uniform erythrocytes with intense coloring.
5. Presence of inclusions
Typically, red blood cells have a uniform (homogeneous) color without any inclusions. A large number of erythrocyte inclusions have been identified in dogs and cats, some of which are present normally.
- Heinz bodies appear as fawn-colored protuberances on the membrane or inside red blood cells, caused by the oxidation of hemoglobin molecules. They are much easier to identify using special stains (methylene blue, cresyl blue brilliant), but they can be observed with regular stains due to their large size.
- The presence of Heinz bodies is usually observed in felines in small quantities (less than 3%). This is due to the high content of sulfhydryl groups on the hemoglobin molecule and explains the increased sensitivity to oxidation processes. If there are a large number of Heinz bodies in a cat, poisoning with toxic doses of drugs that cause oxidation (paracetamol, consumption of onions), as well as diseases associated with metabolic processes (diabetes, hyperthyroidism), or oncological nature (malignant lymphoma) are assumed primarily. With severe intoxication, oxidized hemoglobin can condense into some red blood cells. The remaining cytoplasm in the optical field appears empty and forms an eccentric cell. Its morphology is similar to Heinz bodies.
- Blood smear examination can identify some pathogens in dogs and cats. The most common are hemobartenellosis (Hemobartonella felis) and piroplasmosis (Babesia canis).
- Hemobartenella have the form of small cocci or rods localized on the surface of the red blood cell membrane, which are sometimes difficult to differentiate from artifacts that arise when preparing a blood smear.
- Pyroplasmas are characterized by pear-shaped basophilic inclusions in the cytoplasm of red blood cells. The double form is classic for the disease, but intra- or extracellular persistence of the four parasites can also be observed in smears.
- Inclusions of viral particles during plague can also be found in red blood cells. They are located in the cytoplasm, have a round shape, blue and pale pink color. They are mainly observed during the acute course of an infectious disease, when the symptoms are of a weakly specific nature. Despite their rare occurrence, these inclusions may be the only indicator when diagnosing a viral disease.
PLATELET STUDY
- Blood platelets, or platelets, have a round or oval shape, a poorly differentiated contour and pink granularity. Their size is smaller than that of red blood cells.
- If accumulations are detected in the blood smear, this indicates artifactual thrombocytopenia during puncture (activation of primary hemostasis, platelet aggregation in the test tube, which excludes the possibility of counting) or excess EDTA.
- The quantitative content of platelets is determined under an immersion lens (Appendix 1). This research method is used in cats. Some machines often make errors due to poor volume differentiation between red blood cells and platelets, which causes inaccurate counting.
- The presence of megathrombocytes (size equal to, sometimes exceeding, the volume of a red blood cell) is often associated with early, as well as secondary regeneration of platelets during their destruction or peripheral disposal. Much less frequently, enlarged platelets can be a consequence of bone marrow dysplasia, where the megakaryocyte cell line is affected (in an infectious disease caused by the feline leukemia virus).
- The presence of microplatelets in blood smears indicates their destruction mediated by an immune reaction (thrombocytopenic purpura of an immune nature). These small platelets are fragments coated with antibodies. They have a meaning similar to spherocytes, which are identified in anemia of an immune nature.
- Inclusions in platelets are rare, but they are detected during infection with Ehrlichia platys (causes cyclic thrombocytopenia).
Appendix 3. Platelet count formula (immersion lens)
Dog: platelets/µl. The number of platelets in the field of view of the microscope is determined (×100, ×15,000).
Cat: platelets/µl. The number of platelets in the field of view of the microscope with magnification (×100, ×20,000) is determined.
A healthy animal has from 8 to 25 platelets, observed at medium magnification in the field of view of the microscope. This count makes it possible to identify thrombocytopenia (less than 3 platelets per field of view) and severe thrombocytosis with high accuracy.
RESEARCH OF LEUKOCYTES
- Leukocyte control is carried out at the first stage of the study using automated counting. This study is carried out in pigtails of a smear at low magnification.
- The density of leukocytes at the end of the smear and in its central part allows you to determine their number, while a differentiated count of 100 or 200 leukocytes allows you to more accurately determine the leukocyte formula of the blood than can currently be done using most machines used for this purpose. Qualitative assessment in visual examination requires the qualifications of the researcher. The number of formed elements and the leukocyte formula calculated in blood smears must correspond to the results of an automatic study.
- Counting the formula or examining the lines of developing leukocytes makes it possible to recognize abnormal or blast cells in the circulating blood in malignant hemopathy. Currently used Coulter-type machines do not allow differentiating normal from abnormal leukocytes. These cells are counted as normal white blood cells and their presence may not be apparent to the clinician (Figure 17).
| Figure 17: Abnormal or blast cells that may not be identified by automated imaging. |
- Next, morphological analysis is performed at a higher magnification (×40 or ×100) in the area of the smear with a monocellular layer where the morphology of the cells is clearly visible. Neutrophil granulocytes (polynuclear neutrophils, PNN) are determined. Lymphocytes and monocytes are present in sufficiently large quantities in the circulating blood, which allows for morphological analysis. In domestic carnivores, the blood formula has a neutrophilic profile. In young individuals, lymphocytes may predominate (observed up to the age of one year).
- The most common modifications are associated with changes in the morphology of polynuclear neutrophils during systemic inflammatory processes or cellular pathology (in the case of sepsis, endotoxemia or severe necrosis). In this case, we are talking about toxic polynuclear neutrophilia. The first stage indicates moderate toxicity and is determined by the detection of Doyle bodies: grayish, irregular inclusions in the cytoplasm that are caused by aggregation of the cytoplasmic reticulum. These difficult-to-detect cytoplasmic inclusions are the earliest indicator. Diffuse cytoplasmic basophilia occurs in more severe cases. With increased toxicity, two listed signs are observed: microvacuolization of the cytoplasm, which has a foam pattern (“soap bubbles”) (photo 18). In critical cases, cellular gigantism is noted. Complete lysis of the nuclear apparatus completes the picture.
| Photo 18. Leukocyte assessment. A polynuclear neutrophil with cytoplasmic microvacuoles in the form of “soap bubbles” is presented: we are talking about a toxic “polynuclear cell”. | Photo 19. Leukocyte assessment. Hypersegmented polynuclears (more than 5 lobes in polynuclears). Examination of these cells mainly indicates the physiological nature of the cell changes (cell aging). |
- Nuclear hypersegmentation (right-sided deviation of the nucleus or Arnett curve) without signs of toxicity is often a morphological change in the neutrophil population (Figure 19). This condition is determined in the population of neutrophil polynuclear cells by pronounced segmentation of the nucleus (more than 5 lobes), which mainly indicates aging of blood cells. Increased contact with EDTA (long-term preservation of blood before performing a smear), corticosteroid therapy with a decrease in tissue circulation, chronic inflammation are the causes of a non-physiological increase in the hypersegmentation of polynuclear neutrophils.
- The stage of incomplete maturation, or “band form” (photo 20), is characterized by incomplete segmentation of nuclei shaped like a horse’s shoe or a groundnut (peanut). The presence of this imperfect stage of cells in large numbers is associated with neutrophilia (deviation of the nucleus or Arnett curve to the left). Indicates an increase in granulopoiesis, often due to an increase in phagocytosis in peripheral tissue, for example, during acute purulent inflammation.
| Photo 20. Assessment of leukocytes. Young neutrophilic polynuclear cell, or “band form”: its nucleus is in a state of hyposegmentation and has the shape of a peanut. Erythrocyte anisocytosis may also be noted. |
- Other groups of leukocytes may also change, which has the following explanation: lymphocytes do not show signs of toxicity, but react when stimulated by an antigen of an infectious agent, as well as during an immune or neoplastic process. These reactive lymphocytes, or “immunocytes,” are characterized by increased size and marked basophilia. Other modifications, such as azurophilic cytoplasmic inclusions, can also be found in lymphocytes called “granular lymphocytes” (Figure 21). They are observed in the circulating blood during nonspecific stimulation of the immune system (viral diseases, recent vaccination), as well as in the case of neoplasms and some chronic disorders (erlichiosis). These activated cells are NK (natural killer) cells.
| Photo 21. Leukocyte assessment. It should be noted the presence of a lymphocyte in a granular state, which contains azurophilic inclusions in the cytoplasm. |
- Leukocyte inclusions are extremely rare. They belong to the morulae of canine ehrlichiosis (Ehrlichia canis) in monocytes and bodies of Lentz in distemper, which are included in lymphocytes, monocytes and neutrophil granulocytes.
- The presence of erythrophagocytes (monocytes that phagocytose red blood cells) can be observed in hemolysis of an immune extracellular nature, for example in babesiosis.
A clinician who has automated blood testing equipment that provides results during the consultation period has great advantages. However, additional blood smear studies are recommended to evaluate and explain systemic abnormalities.
Appendix 4. Basic provisions
Drying of blood smears is carried out in fresh air, without a heat source, which can cause the presence of artifacts that interfere with the assessment of the morphological status of blood cells.
Macroscopic agglutination is physiological in the cat. In the dog, it may correspond to both the formation of coin columns and microscopic agglutination, often occurring in hemolytic anemia.
It is necessary to clarify the presence of thrombocytopenia, determined using an automated study, through careful examination of smears to identify accumulations of platelets responsible for pseudothrombocytopenia.
The presence of polychromatophils, the presence of erythrocyte anisocytosis and Havel-Joly bodies on the blood smear are symptoms of regenerative anemia.
In the case of the presence of an increased content of activated lymphocytes, it is necessary to conduct a systematic search for the persistence of chronic inflammation.
SVM 5/2004
Presence of uric acid crystals in the blood
Presence of uric acid crystals
in the blood allows you to assess the level of protein metabolism in the body, the functioning of the kidneys and liver. Uric acid is a byproduct of protein digestion. As uric acid passes through the liver, it is converted into urea, after which the kidneys remove it from the body. Violation of protein metabolism leads to excessive formation of uric acid, its salts (urates), the formation of stones in the urinary tract, deposits in the joint capsules and articular surfaces.
Conditions for sample collection and storage
A clinical blood test is performed from venous blood stabilized with potassium salt EDTA, unless otherwise specified in the instructions for the analyzer. Blood collection is performed on an empty stomach. The blood sample should be mixed immediately after collection 9 times by gentle inversion, foam formation and sudden shaking should be avoided. Before testing, the blood sample can be stored at room temperature (23–24 °C) for 24 hours in a rack, in an upright position, away from light.
When using a capillary blood sample for clinical analysis, it is necessary to obtain free-flowing drops of capillary blood from the prewarmed puncture area. Collecting capillary blood without squeezing a finger ensures the safety of cells. Applying pressure to the puncture area and collecting a sample from a cooled limb will distort the hemogram results. Capillary blood samples must be stabilized with potassium EDTA, so K3EDTA-treated capillaries should be used for sample collection. Samples can be stored at room temperature (23–24 °C) for 24 hours on a rack, in an upright position, away from light.
What do orthophosphoric acid salts indicate?
Phosphoric acid salts
in the blood indicate a pronounced disturbance of phosphorus-calcium metabolism, calcium deficiency in the body, which again is a prerequisite for the development of various diseases. Phosphoric acid is formed during the disposal of proteins, heavy physical activity, consumption of products with thickeners, flavors, etc. Phosphoric acid is a “marker” of calcium loss. It saturates itself with calcium ions, and when the body removes it, it throws out the calcium accumulated in it along with it.