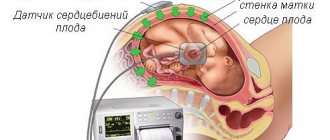
The main heart defects in the fetus are formed in the 1st trimester of pregnancy at a period of 12-14 weeks. This may be a reaction to external factors or genetic problems. By this time, the formation of the fetal heart muscle occurs, so the expectant mother needs to undergo an ultrasound examination to identify pathologies of the organ.
In intrauterine development, heart defects arise as a reaction of the body to impaired placental circulation or exposure to carcinogenic substances (formaldehyde, nicotine, toxic substances).
When and why genetic pathologies of the fetus occur: risks by age
Anomalies in fetal development occur already at the moment of fertilization of an egg by a sperm. For example, a pathology such as triploidy (the presence of three chromosomes in a row of a chain, and not two, as expected), occurs when two sperm penetrate the egg, each of which leaves one chromosome. Naturally, with such a set, a living organism cannot survive, so at a certain stage a miscarriage or frozen pregnancy occurs.
Spontaneous miscarriages occur in 50% of abnormal fertilizations. This is how nature protects humanity from complete degeneration.
In general, chromosomal pathologies are divided into 4 groups:
- Gametopathy.
The pathology is present even before conception in the sperm or egg itself, i.e. This is a genetic disease - a congenital pathology. - Blastopathy
. Anomalies occur in the first week of zygote development. - Embryopathy
. The embryo receives damage from 14 to 75 days after conception. - Fetopathy
. It consists in the formation of pathology of fetal development starting from the 75th day after fertilization.
No one is immune from the birth of a baby with genetic disorders. If previously the risk group included mothers over 35 years of age, diabetics, women with chronic diseases (kidney failure, thyroid problems), today sick children are born to young mothers aged 20 to 30 years.
These statistics lead to gloomy thoughts. Thus, the risk of having a baby with chromosomal abnormalities in 20-year-old women is 1:1667, and in 35-year-old women it is already 1:192. But in reality, this means that in 99.5% of cases, a child of a thirty-five-year-old mother will be born healthy.
HGLOS - Hypoplastic left heart syndrome
Until recently, this defect was fatal in absolutely all cases. The child died during the first week of life, and it was impossible to provide him with any surgical assistance.
Today the situation is different and, if everything is done on time and correctly, then there is a good chance of saving it. Precisely to save, because Immediately after birth, the time of his life counts not months and days, but hours and even minutes.
The word hypoplasia means “reduction in size.” With this defect, the left ventricle, i.e. the main one, the main one, was almost completely undeveloped. But this is not enough. Neither the entrance to it, i.e. mitral valve, nor outlet, i.e. section of the ascending aorta along with its valve. Instead of the aorta, there is a small vessel with a diameter of 1-3 millimeters, through which blood flows into the coronary arteries, but there is no section of the ascending aorta.
The huge right ventricle, receiving blood from the right atrium and left atrium through a defect in the interatrial septum, pumps all the blood into the pulmonary artery and lungs. Some of it enters the systemic circle through the open ductus arteriosus, but only a small part. Life, therefore, depends on the presence of two communications - interatrial and interarterial, which, firstly, are insufficient, and secondly, immediately after birth they can close, as is normal. Otherwise, as a hemodynamic catastrophe
it cannot be called, and without the most urgent measures the child will die.
The diagnosis can be made in utero, and this is an extremely important moment when both parents and doctors must weigh all available options for providing immediate assistance. What is important here is not only preparation for immediate action, but also absolute determination to go through with the child the entire difficult step-by-step path of his further treatment, which will take the first few years of his life.
Each of these stages may be the last for a child, since they are all associated with enormous risk. But always, even if this is little consolation, we must remember that both you and the doctors did everything possible today to save him.
Stages of treatment
The first operation is performed in the first days of life. Approaches to the first stage of surgical correction can be different and depend on the clinic, the skill of the surgeon, and the readiness of the cardiac surgery hospital to care for and manage this extremely complex category of patients. This can also be an operation with artificial circulation, sometimes quite long, because it is necessary to perform a complete reconstruction of the sharply narrowed ascending aorta. This is achieved by sewing in an elastic biological prosthesis of such a shape that, as a result, the pulmonary artery and aorta become the only vessel extending from the right (single) ventricle. To ensure sufficient mixing of blood, a large atrial septal defect is created, and blood flow to the lungs is directed through an arteriopulmonary anastomosis. As can be seen from this description, this is a very large and complex operation that can only be performed in centers with extensive experience in heart surgery on newborns.
But even in the largest and world-famous US institutions, the mortality rate after this stage reaches 20-40 percent.
We present these figures to emphasize that for hypoplastic left heart syndrome, surgery is the only possible, but far from safe, path. But, since we are talking about saving lives, even the attempts themselves are always justified.
In recent years, several clinics around the world have introduced a method, the survival rate of which is close to 90% - the so-called hybrid operation of patent ductus arteriosus stenting and bilateral separate narrowing of the pulmonary arteries .
If everything went well, then the second operation is recommended to be done at 4-10 months. This involves an anastomosis between the superior vena cava and the pulmonary artery, or “half” of the Fontan procedure. And finally, at the age of one and a half to two years, the child undergoes the third stage - the final Fontan palliation, after which the blood circulation is completely separated.
In clinics with extensive experience in such operations, the second and third stages are much less risky than the first, the most difficult. The child will have to live with one ventricle, and everything said above about the defects for which the Fontan operation is the final stage of surgical treatment applies in full to this defect.
Just 10-15 years ago, children with hypoplastic left heart syndrome were not subject to surgery at all. But the syndrome is one of the five most common congenital heart defects
.
Today, helping these children is possible, although not always. But if you and your cardiologists know this, then you need to act. And let the “little orchestra of hope led by love” never leave you on this difficult path. How to get treatment at the Scientific Center named after.
A.N. Bakuleva? Online consultations
What genetic diseases of the fetus can be seen on ultrasound, when to undergo it
It cannot be said that an ultrasound shows 100% of all abnormalities, but with a high degree of probability a woman will know about the health status of her unborn baby. During the entire pregnancy, a woman undergoes at least three ultrasound examinations: in the 1st, 2nd and 3rd semesters. They are called screening ultrasounds.
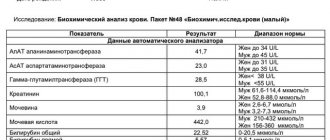
In the 1st semester, from 10 to 14 weeks (until the 10th week, ultrasound is not informative), the pregnant woman undergoes a study called screening. It consists of a biochemical blood test and ultrasound examination of the embryo. The result of screening is the identification of the following pathologies:
- Down syndrome
- Patau syndrome
- Edwards syndrome
- Shereshevsky-Turner syndrome
- Carnelia de Lange syndrome
- Smith-Lemli-Opitz syndrome
- Prader-Willi syndrome
- Angelman's syndrome
- Langer-Gideon syndrome
- Miller-Dicker syndrome
- DiGeorge anomaly
- Williams syndrome
- Wilms tumor
- triploidy (when there are not 46 chromosomes in each pair, but 69, i.e. three, not two)
- neural tube defect
At 20-24 weeks another ultrasound is done. Among the genetic diseases of the fetus visible on ultrasound examination in the 2nd semester, the following can be noted:
- anencephaly (absence of the brain, diagnostic accuracy 100%)
- pathology of the abdominal wall (86%)
- pathology of limb development (90%)
- spinal cord herniation (87%)
- developmental pathology or absence of kidneys (85%)
- the presence of a hole in the diaphragm, which separates the abdominal cavity and chest (85%)
- hydrocephalus or dropsy of the brain (100%)
- heart abnormalities (48%)
In the 3rd semester, Doppler ultrasound is performed - an ultrasound examination to determine the vascular system of the fetus, placenta and mother. Starting at 23 weeks of pregnancy, the umbilical artery, uterine artery and middle cerebral artery are checked. Systolic (when the heart muscle contracts) and diastolic (when the heart muscle relaxes) blood flow is examined. A baby with chromosomal disorders has atypical blood flow.
Also in the 3rd semester, fetal fetometry is required - measurement of size in order to identify developmental anomalies.
If an ultrasound examination reveals markers of fetal chromosomal pathology
- Dear patients, first of all, we ask you to remember the most important thing: if markers (signs) of chromosomal pathology of the fetus were found during an ultrasound examination, this does not mean that the fetus has a chromosomal pathology, and the pregnancy must be terminated.
- All women who have been found to have ultrasound markers of chromosomal pathology of the fetus are offered invasive prenatal diagnostics - today, depending on the stage of pregnancy, we offer three types of invasive diagnostics: chorionic villus aspiration (performed at 11-14 weeks), amniocentesis (16-18 weeks), cordocentesis (19-20 weeks). More information about invasive diagnostic methods can be found at this link.
The most common ultrasound markers of chromosomal abnormalities are:
Increase in TVP.
This parameter is assessed at the first screening ultrasound (11-14 weeks)
TVP (thickness of the collar space) may be greater than normal for several reasons.
Why may the fetus exhibit an increase in TVP?
Parents are extremely excited and want to immediately get answers to all the questions they have - what is involved, what to do, and many others. Questions that cannot be answered immediately. After all, there are many reasons for the increase in TVP. This finding can occur in completely healthy fetuses; this is not a developmental defect, it is only a signal for a more in-depth examination, because such a feature can occur in fetuses with chromosomal abnormalities, heart abnormalities, or other congenital or hereditary diseases. When increasing the maximum TVP threshold, it is IMPORTANT that the doctor evaluates all other ultrasound markers (signs) and also conducts a detailed assessment of the fetal anatomy. Perhaps the reason for the increase in TVP lies in a violation of fetal development (for example, abnormalities in the structure of the heart).
What to do if an increase in TVP is detected in the fetus?
If your fetus is diagnosed with an enlarged TVP, you will definitely be referred for a consultation with a geneticist, who, after collecting an anamnesis, assessing all the risks, will give recommendations on additional research methods (invasive diagnostics). Next, an expert ultrasound of the fetus will be required at 20 weeks for a detailed assessment of the anatomy. If all these studies do not reveal any deviations, then the chances of giving birth to a healthy child are high even with a significant amount of TVP.
Hypoplasia/aplasia of the nasal bones.
Hypoplasia of the nasal bones is a reduction in the size of the nasal bone depending on the CTE of your baby.
Aplasia of the nasal bones is the lack of visualization of your baby's nasal bone.
The lack of visibility of the bony part of the nasal dorsum in the fetus or its underdevelopment (not bright enough) at the first screening is associated with delayed calcium deposition. This situation may be somewhat more common in fetuses with Down syndrome, but it is important that:
- the absence of nasal bones on ultrasound is not a developmental anomaly in itself; can occur in completely healthy fetuses (in 3% of cases);
- to assess the degree of individual risk, it is necessary to evaluate the remaining ultrasound markers (thickness of the fetal nuchal translucency, blood flow indicators on the heart valve, blood flow indicators in the ductus venosus, fetal heart rate) and biochemical analysis of maternal serum (PAPP-A, hCG);
- If the result of a combined screening (evaluation of ultrasound and blood test data in a special program) shows a LOW risk of chromosomal pathology, there is no need to worry. Be sure to undergo a follow-up ultrasound at 19-20 weeks of pregnancy, where a thorough assessment of the fetal anatomy will be carried out and certain ultrasound markers of the second trimester of pregnancy will be examined.
- What to do if the combined screening result is HIGH? – There’s no need to worry. You will definitely be referred for a consultation with a geneticist, who, having collected an anamnesis, assessed all the risks, will give recommendations on additional research methods (invasive diagnostics).
Hyperechoic intestine.
This is a term that refers to increased echogenicity (brightness) of the intestine on an ultrasound image. The finding of hyperechoic bowel NOT a malformation of the bowel, but simply reflects the nature of its ultrasound image. It must be remembered that the echogenicity of the normal intestine is higher than the echogenicity of its neighboring organs (liver, kidneys, lungs), but such intestine is not considered hyperechoic. Only intestines whose echogenicity is comparable to the echogenicity of fetal bones are called hyperechoic.
Why can the fetal intestine be hyperechoic?
Sometimes hyperechoic intestine is detected in completely normal fetuses, and this sign may disappear with dynamic ultrasound. Increased echogenicity of the intestine may be a manifestation of chromosomal diseases of the fetus, in particular Down syndrome. Therefore, when hyperechoic bowel is detected, a careful assessment of fetal anatomy is performed. However, if a hyperechoic intestine is detected, we can only talk about an increased risk of Down syndrome, since such changes can also occur in completely healthy fetuses. Sometimes hyperechoic bowel may be a sign of intrauterine fetal infection. Hyperechoic bowel is often found in fetuses with intrauterine growth restriction. However, this will necessarily reveal a lag in the size of the fetus from the gestational age, oligohydramnios, and impaired blood flow in the vessels of the fetus and uterus. If none of the above is detected, then the diagnosis of fetal growth restriction is excluded.
What to do if hyperechoic intestine is detected in the fetus?
You should contact a genetic specialist who will once again evaluate the results of biochemical screening and give the necessary recommendations for further management of pregnancy.
Hyperechoic focus in the ventricle of the heart.
This is a term that refers to the increased echogenicity (brightness) of a small area of the heart muscle on an ultrasound image. Identification of a hyperechoic focus in the heart NOT a cardiac malformation, but simply reflects the nature of its ultrasound image. A hyperechoic focus occurs at the site of increased deposition of calcium salts on one of the heart muscles, which does not interfere with the normal functioning of the fetal heart and does not require any treatment.
Why can a fetus have a hyperechoic focus in the heart?
Sometimes a hyperechoic focus in the heart is detected in completely normal fetuses, and this sign may disappear with dynamic ultrasound. The presence of a hyperechoic focus in the fetal heart may be a manifestation of fetal chromosomal diseases, in particular Down syndrome. In this regard, when a hyperechoic focus is detected, a careful assessment of the fetal anatomy is carried out. However, this marker refers to the “small” markers of Down syndrome, therefore, identifying only a hyperechoic focus in the heart does not increase the risk of having Down syndrome and is not an indication for other diagnostic procedures.
What to do if a hyperechoic focus is detected in the fetal heart?
If the fetus has ONLY a hyperechoic focus in the heart, then no additional examinations are required; the risk of Down's disease does not increase. At a planned ultrasound at 32-34 weeks, the fetal heart will be examined again. In most cases, the hyperechoic focus in the heart disappears by this stage of pregnancy, but even if it continues to remain in the heart, this does not in any way affect the health of the fetus and the management of pregnancy.
The only artery of the umbilical cord.
A normal umbilical cord consists of three vessels - two arteries and one vein. Sometimes, instead of two arteries, only one artery and one vein are formed in the umbilical cord, thus, only two vessels are identified in the umbilical cord. This condition is considered a malformation of the umbilical cord, but this defect does not have any effect on the postpartum condition of the child and its further development.
Why can a single umbilical cord artery be identified in a fetus?
Sometimes a single umbilical cord artery is identified in completely normal fetuses; After the birth of a child, this fact does not have any impact on its further development. Sometimes a single umbilical cord artery is combined with defects of the fetal cardiovascular system, therefore, when a single umbilical cord artery is identified, a detailed examination of the anatomy of the fetus and, in particular, the cardiovascular system is carried out. In the absence of other malformations, a single umbilical cord artery is able to provide adequate blood flow to the fetus. Somewhat more often, a single umbilical cord artery is detected in fetuses with Down syndrome and other chromosomal diseases. However, this marker is a “minor” marker of Down syndrome, so identifying only a single umbilical cord artery does not increase the risk of Down syndrome and is not an indication for other diagnostic procedures. A single umbilical cord artery sometimes leads to intrauterine growth retardation. In this regard, if a single umbilical cord artery is detected, an additional ultrasound is recommended at 26-28 weeks of pregnancy, and a planned one at 32-34 weeks. If a lag in the size of the fetus from the gestational age or disruption of blood flow in the vessels of the fetus and uterus is not detected, then the diagnosis of fetal growth retardation is excluded.
What to do if a single umbilical cord artery is identified in the fetus?
Identification of only a single umbilical cord artery does not increase the risk of Down syndrome and is not an indication for genetic counseling or other diagnostic procedures. A control ultrasound is required at 26-28 and 32 weeks of pregnancy to assess the rate of fetal growth and evaluate its functional state.
Choroid plexus cysts (CPC).
The choroid plexus is one of the first structures to appear in the fetal brain. It is a complex structure, and the presence of both choroid plexuses confirms that both halves develop in the brain. The choroid plexus produces fluid that nourishes the brain and spinal cord. Sometimes the fluid forms collections inside the choroid plexus, which appear as a “cyst” on ultrasound. Choroid plexus cysts can sometimes be found on ultrasound between 18 and 22 weeks of pregnancy. The presence of cysts does not affect the development and function of the brain. Most cysts disappear spontaneously by 24-28 weeks of pregnancy.
Are choroid plexus cysts common?
In 1-2% of all normal pregnancies, the fetuses have CSS, in 50% of cases bilateral choroid plexus cysts are found, in 90% of cases the cysts spontaneously disappear by the 26th week of pregnancy, the number, size, and shape of cysts can vary, cysts are also found in healthy children and adults. Somewhat more often, choroid plexus cysts are detected in fetuses with chromosomal diseases, in particular with Edwards syndrome (trisomy 18, extra chromosome 18). However, with this disease, the fetus will always have multiple malformations, so identifying only choroid plexus cysts does not increase the risk of having trisomy 18 and is not an indication for other diagnostic procedures. In Down syndrome, choroid plexus cysts are usually not detected. The risk of Edwards syndrome when CSS is detected does not depend on the size of the cysts and their unilateral or bilateral location. Most cysts resolve by 24-28 weeks, so a control ultrasound is performed at 28 weeks. However, if choroid plexus cysts do not disappear by 28-30 weeks, this does not affect the further development of the child.
Enlargement of the renal pelvis (pyelectasia).
The renal pelvis is the cavity where urine from the kidneys collects. From the pelvis, urine moves to the ureters, through which it enters the bladder.
Pyeelectasia is an enlargement of the renal pelvis. Pyeelectasis is 3-5 times more common in boys than in girls. Both unilateral and bilateral pyeloectasia occur. Mild forms of pyelectasis often go away on their own, while severe forms sometimes require surgical treatment.
The cause of dilation of the renal pelvis in the fetus.
If there is an obstacle in the way of the natural outflow of urine, urine will accumulate above this obstacle, which will lead to expansion of the renal pelvis. Pyeelectasis in the fetus is diagnosed by routine ultrasound examination at 18-22 weeks of pregnancy.
Is pyeelectasis dangerous?
Moderate expansion of the renal pelvis, as a rule, does not affect the health of the unborn child. In most cases, during pregnancy, spontaneous disappearance of moderate pyelectasis is observed. Severe pyelectasis (more than 10 mm) indicates a significant difficulty in the outflow of urine from the kidney. Difficulty in the outflow of urine from the kidney may increase, causing compression, atrophy of the kidney tissue and decreased kidney function.
In addition, a violation of the outflow of urine is often accompanied by the addition of pyelonephritis, an inflammation of the kidney that worsens its condition. Slightly more often, dilation of the renal pelvis is detected in fetuses with Down syndrome. However, this marker refers to the “minor” markers of Down syndrome, therefore, detecting only dilation of the renal pelvis does not increase the risk of having Down syndrome and is not an indication for other diagnostic procedures. The only thing you need to do before giving birth is to undergo a control ultrasound at 32 weeks and once again assess the size of the renal pelvis.
Is it necessary to examine the baby after birth?
In many children, moderate pyelectasis disappears spontaneously as a result of the maturation of the urinary system after the birth of the child. For moderate pyelectasis, it may be sufficient to conduct regular ultrasound examinations every three months after the birth of the child. If a urinary infection occurs, antibiotics may be necessary. As the degree of pyelectasis increases, a more detailed urological examination is necessary.
In cases of severe pyelectasis, if the dilation of the pelvis progresses and a decrease in kidney function occurs, surgical treatment is indicated. Surgery can remove the obstruction to the flow of urine. Some surgical interventions can be successfully performed using endoscopic methods - without open surgery, using miniature instruments inserted through the urethra. In any case, the issue of surgical treatment is decided after the birth of the child and its complete examination.
What to do if ultrasound markers of chromosomal pathology are detected in the fetus?
You should contact a genetic specialist who will once again evaluate the results of ultrasound and biochemical screening, calculate the risk individually for your case and give the necessary recommendations for further management of pregnancy.
Types of ultrasound examinations
Ultrasound diagnostics represents a wide range of studies. There are several types of ultrasound that accurately determine intrauterine malformations of the baby.
Standard ultrasound
. It is usually combined with a biochemical blood test. It is carried out no earlier than 10 weeks of pregnancy. First of all, the thickness of the fetal collar zone is detected, which should not exceed 3 mm, as well as visualization of the nasal bone. In a baby with Down syndrome, the nuchal region is thicker than normal, and the nasal bones are not developed. The following factors also influence the increase in thickness:
- heart disease
- stagnation of blood in the neck veins
- lymphatic drainage disorder
- anemia
- intrauterine infections
Doppler - uh
This is an unusual ultrasound test that evaluates fetal blood flow. The difference between the sent and reflected signal indicates the norm or pathology of the fetus-placenta-mother chain.
- 3D ultrasound allows you to see a color image of the baby, see the limbs, the absence of fused fingers, underdeveloped feet, etc. The accuracy of diagnosing the nuchal translucency increases by 30%. The doctor can tell for sure whether there are pathologies in the development of the neural tube.
- 4D ultrasound does not differ in operating principle from simpler options, but has many advantages. The doctor sees a three-dimensional image of the heart and a view of the fetus from different angles. It is 4D diagnostics that finally dots all the i’s, whether there are chromosomal abnormalities or not. It can be stated with 100% accuracy whether there are malformations of the nervous system, skeletal dysplasia, cleft lip or cleft palate.
Congenital heart defects
The five most common defects are tetralogy of Fallot, ventricular septal defect, transposition of the great vessels, coarctation of the aorta, and left chamber hypoplasia.
The optimal period for ultrasound diagnosis of the fetal heart is considered to be 24-26 weeks of pregnancy. It is at this time that the anatomical structures of the heart are visualized to the maximum, and at earlier stages only obvious and global heart defects can be seen.
The most informative is an ultrasound examination of a 4-chamber section of the heart. After which, in case of any deviation from the norm, the woman is sent for a more detailed examination of the fetus using Doppler echocardiography. Karyotyping is also performed because in 30% of cases the abnormalities are the result of chromosomal abnormalities.
What does an ultrasound of general fetal pathologies look like: photos and interpretation of ultrasound results
Genetic pathologies can be both specific (Down syndrome, Wilms tumor) and general, when the internal organ develops incorrectly. To identify common abnormalities, anatomical examination of the fetus is available. It is carried out in the 2nd semester starting from the 20th week of pregnancy. During this period, you can see the baby’s face and determine its gender.
With an anatomical ultrasound, all organs of the fetus are displayed on the screen in a section, and in the image the bones will appear white, and the soft tissues will appear in various shades of gray. The specialist can clearly see the structure of the brain; he is also able to see abnormalities in development. A cleft in the upper palate, called a cleft lip, becomes noticeable.
Longitudinal and transverse projections of the spine confirm or refute the correct location of the bones; one can verify the integrity of the abdominal wall. The absence of heart pathologies is confirmed by the identical sizes of the atria and ventricles. The normal functioning of the stomach is indicated by its fullness with amniotic fluid. The kidneys should be in their place, and urine from them should flow freely into the bladder. The doctor clearly sees the fetal limbs, except for the toes.
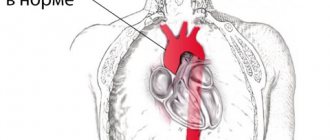
Abnormalities of fetal heart position
Among the anomalies of the location of the heart, ectopia of the heart (placement outside the chest) is distinguished. Such pathologies include dextrocardia (displacement of the heart to the right side relative to the normal position) and mesocardia (the location of the heart is not on the left side of the sternum, but along the midline of the body).
Ectopy of the heart in the fetus occurs 14-18 days after conception, the mesaderm begins to develop incorrectly, which causes improper fusion of the abdominal wall. The fetus either has no diaphragm at all or no diaphragmatic segment of the pericardium.
Due to the hole in the wall between the right and left ventricles, intracardiac murmurs are heard. Also, in the fetus, ectopia of the heart is often accompanied by other anomalies - hydrocephalus, encephalocele, etc.
It must be said that there is a high probability of misdiagnosis based on the position of the heart. With a breech presentation of the fetus, the heart is visualized on the right side on ultrasound, although in fact it is located in the right place.
In 71% of cases, ectopia of the heart is caused by pleural effusion, cystic adenomatous malformation of the lung, or diaphragmatic hernia.
There are four types of ectopia:
- abdominal (the heart is located in the peritoneum);
- thoracic (the heart comes out through defects in the sternum);
- thoracoabdominal (Cantrell's pentad is a complex pathology with a complex of deviations from the norm);
- cervical (the heart moves to the heart area).
Thoracic ectopia occurs in 55-60% of cases, thoracoabdominal - in 38%, cervical - in almost 3%. Survival rate is about 10%. In most cases, with ectopia, the baby is either stillborn or dies immediately after birth.
The pathology is accompanied by displacement of other internal organs, which are not protected from mechanical damage and are more susceptible to infections and viruses than usual.
Genetic pathologies of the fetus: what they look like on ultrasound and prognosis of the pathology
| Pathology | How and when is it detected? | What is the essence of pathology | Character traits | Mental and intellectual development |
Down syndrome | A chorionic villus biopsy is performed, enlarged nuchal translucency in the fetus, underdevelopment of the nasal bones, enlarged bladder, fetal tachycardia | Chromosomes of the 21st pair, instead of the required 2, are represented by 3 in the chain | Slanting Mongoloid eye shape, regardless of the child’s race, undeveloped bridge of the nose, shallow-set eyes, semicircular flat ear, shortened skull, flat back of the head, shortened nose | Delayed intellectual development, small vocabulary, lack of abstract thinking, lack of concentration, hyperactivity |
| FORECAST | They live up to 60 years of age; in rare cases, subject to constant activities with the child, his socialization is possible. This child needs constant supervision | |||
Patau syndrome | Small head at 12 weeks on ultrasound, asymmetrical hemispheres, extra fingers | Trisomy is present on chromosome 13 | Children are born with microcephaly (underdevelopment of the brain), low forehead, slanted palpebral fissures, cleft lip and palate, corneal clouding, heart defects, enlarged kidneys, abnormal genitals | Profound mental retardation, lack of thinking and speech |
| FORECAST | 95% of children with Patau syndrome die before the age of one year, the rest rarely live to 3-5 years | |||
Edwards syndrome | Chorionic villus biopsy, intrauterine blood sampling from the umbilical cord, microcephaly is visible on ultrasound | There is trisomy on chromosome 18 | Mostly girls (3/4) are born, and the male fetus dies in the womb. Low sloping forehead, small mouth, underdeveloped eyeball, cleft upper lip and palate, narrow ear canal, congenital dislocations, clubfoot, severe abnormalities of the heart and gastrointestinal tract, underdeveloped brain | Children suffer from oligophrenia (organic brain damage), mental retardation, imbecility (moderate mental retardation), idiocy (lack of speech and mental activity) |
| FORECAST | During the first year of life, 90% of sick children die, less than 1% die before the age of 10 years. | |||
Shereshevsky-Turner syndrome | X-ray of fetal bone structures, MRI of the myocardium | An abnormality occurring on the X chromosome | It occurs more often in girls. Shortened neck with folds, swollen hands and feet, hearing loss. Drooping lower lip, low hairline, underdeveloped lower jaw. Height in adulthood does not exceed 145 cm. Joint dysplasia. Abnormal development of teeth. Sexual infantilism (no follicles in the ovaries), underdevelopment of the mammary glands | Speech and attention suffer. Intellectual abilities are not impaired |
| FORECAST | Treatment is carried out with anabolic steroids; girls from 14 years of age are prescribed female hormonal drugs. In some cases, it is possible to overcome the disease, and the woman can become pregnant using IVF. Most patients remain infertile | |||
Polysomy on the X chromosome | Screening at 12 weeks of pregnancy, chorionic villus biopsy, amniotic fluid analysis. The enlargement of the collar area is alarming | Instead of two X chromosomes, there are three or more | It occurs in girls and rarely in boys. Characterized by sexual infantilism (secondary sexual characteristics do not develop), high growth, curvature of the spine, skin hyperpigmentation | Antisocial behavior, aggression, mental retardation in men. |
| FORECAST | With regular classes with teachers and involvement in work activities, the child’s socialization is possible | |||
Polysomy on the Y chromosome | Instead of XY chromosomes there is an extra Y chromosome | Occurs in boys. They grow tall from 186 cm, heavy massive lower jaw, convex brow ridges, narrow shoulders, wide pelvis, stoop, belly fat | Mental retardation, aggression, emotional instability | |
| FORECAST | You need to work with the child, direct him to peaceful activities, involve him in sports | |||
Carnelia de Lange syndrome | When analyzing the blood of a pregnant woman, plasma protein-A (PAPP-A), which is usually high, was not detected in the serum | mutations in the NIPBL or SMC1A gene | Thin fused eyebrows, shortened skull, high palate, abnormally erupted teeth, underdeveloped limbs, marbled skin, congenital malformations of internal organs, growth retardation | Profound mental retardation, |
| FORECAST | Average life expectancy 12-13 years | |||
Smith-Lemli-Opitz syndrome | Ultrasound shows abnormalities of the skull in the fetus, the rib bones are not visible | mutation in the DHCR7 gene, responsible for the production of cholesterol | Narrow forehead, drooping eyelids, squint, skull deformation, short nose, low-set ears, underdeveloped jaws, genital abnormalities, fusion of fingers | Increased excitability, aggression, decreased muscle tone, sleep disturbances, mental retardation, autism |
| FORECAST | Therapy using dietary cholesterol | |||
Prader-Willi syndrome | There is low fetal mobility, abnormal position, | Chromosome 15 is missing the paternal part of the chromosome | Obesity with short stature, poor coordination, weak muscle tone, squint, thick saliva, bad teeth, infertility | Mental retardation, speech delay, lack of communication skills, poor fine motor skills. Half of the patients have an average level of intelligence and can read |
| FORECAST | With constant practice, a child can learn to read, count, and remember people. Overeating should be fought | |||
Angelman syndrome | Starting from the 12th week, there is a delay in fetal development in height and weight | The UBE3A gene is absent or mutated on chromosome 15 | Frequent unreasonable laughter, small tremors, many unnecessary movements, wide mouth, tongue hanging out, walking with absolutely straight legs | “Happy puppet syndrome”: the child laughs often and for no reason. Mental retardation, hyperactivity, impaired motor coordination, chaotic hand waving |
| FORECAST | Antiepileptic therapy is carried out, muscle hypotonicity is reduced by massage, in the best case, the child will learn non-verbal communication and self-care skills | |||
Langer-Gideon syndrome | Maxillofacial anomaly is visible on 4D ultrasound | trichorinophalangeal syndrome, which consists of a violation of the 8th chromosome | Long pear-shaped nose, underdeveloped lower jaw, very protruding ears, uneven limbs, curvature of the spine | Mental retardation, varying degrees of mental retardation, lack of speech |
| FORECAST | Poorly amenable to correction, low life expectancy | |||
Miller-Dicker syndrome | Ultrasound shows an abnormal structure of the skull, facial disproportions | Pathology in the 17th chromosome, causing smoothing of the cerebral convolutions. Caused by fetal intoxication with aldehydes due to maternal alcohol abuse | Dysmorphia (alcohol syndrome), heart defects, kidney defects, seizures | Lissencephaly (smooth gyri of the cerebral hemispheres), underdevelopment of the brain, mental retardation |
| FORECAST | Survival up to 2 years. Children can only learn to smile and make eye contact. | |||
DiGeorge Anomaly | In some cases, ultrasound reveals various defects of the baby’s organs, especially the heart (tetralogy of Fallot) | Disease of the immune system, a violation of a region of the 22nd chromosome | Hypoplasia of the thymus (underdevelopment of the organ responsible for the production of immune cells), deformation of the face and skull, heart disease. There are no parathyroid glands responsible for the metabolism of calcium and phosphorus | Atrophy of the cerebral cortex and cerebellum, mental retardation, difficulties with motor skills and speech |
| FORECAST | Treatment with immunostimulants, thymus transplantation, calcium-replenishing therapy. Children rarely live past the age of 10 and die from the consequences of immunodeficiency | |||
Williams syndrome | Ultrasound shows imbalances in skeletal development and joint elasticity | Genetic disease caused by a missing link on chromosome 7 | The synthesis of elastin protein is impaired; children have a typical “Elf face”: swollen eyelids, low-set eyes, sharp chin, short nose, wide forehead | Increased sensitivity to sound, impulsivity, obsessive sociability, emotional instability, anxiety, expressive speech |
| FORECAST | Speech is well developed, even better than that of healthy peers. Pronounced musical abilities (absolute pitch, musical memory). Difficulty solving math problems | |||
Beckwith-Wiedemann syndrome | Ultrasound reveals abnormally disproportionate limbs, excess body weight, and kidney pathology | Genetic disease caused by a missing link on chromosome 11 | Rapid growth at an early age, abnormally large internal organs, a tendency to cancer. The child has an umbilical hernia, an abnormally large tongue, and microcephaly (underdevelopment of the brain). | Emotional and mental development in some cases does not lag behind the norm. Severe mental retardation sometimes occurs |
| FORECAST | Life expectancy is the same as that of ordinary people, but there is a tendency to cancerous tumors | |||
Treacher Collins syndrome | Ultrasound shows pronounced asymmetry of facial features | Genetic mutation on chromosome 5 that causes abnormal bone structures | The child has practically no face, a pronounced physical deformity | Absolutely normal psycho-emotional development |
FORECAST | Surgical interventions are performed to eliminate deformities | |||
What is the Astraia program
Astraia is a professional program that calculates the likelihood of chromosomal abnormalities in a fetus. The program was developed by the Fetal Medicine Foundation (FMF) in London and was successfully tested on a huge amount of clinical material in many countries around the world. It is constantly being improved under the leadership of a leading specialist in the field of prenatal diagnostics, Professor Kypros Nicolaides, in accordance with the latest world advances in the field of fetal medicine.
The specialist conducting early prenatal screening must have an international FMF certificate, which gives the right to perform this diagnosis and work with the Astraia program. The certificate is confirmed annually after a statistical audit of the work done during the year and passing the certification exam. This ensures high diagnostic accuracy of the obtained risks.
Conducting early prenatal screening using this program is regulated by Order of the Ministry of Health of the Russian Federation dated November 1, 2012 No. 572n “On approval of the procedure for providing medical care in the field of obstetrics and gynecology (except for the use of assisted reproductive technologies).”
Early prenatal screening allows you to calculate the following risks:
- Down syndrome (trisomy 21) in the fetus;
- Edward syndrome (trisomy 18) in the fetus;
- Patau syndrome (trisomy 13) in the fetus;
- the risk of developing early (before 34 weeks) and late (after 37 weeks) preeclampsia (preeclampsia) in the pregnant woman herself.
Causes of fetal pathologies: what affects the birth of children with genetic abnormalities
Factors contributing to the birth of children with genetic abnormalities include:
- Genetic predisposition . Genes are information inherited from both parents. Indicators such as height, eye and hair color are determined. In the same way, various deviations are laid down if both or one of the parents has a damaged gene. This is why close relatives are prohibited from marrying. After all, then the likelihood of bearing a fetus with a genetic pathology increases. With a partner who has the opposite genetic makeup, you are more likely to give birth to a healthy baby.
- Age of parents
. The risk group includes mothers over 35 years of age and fathers over 40 years of age. With age, immunity decreases, chronic diseases arise, and a woman’s immune system simply “will not notice” genetically damaged sperm. Conception will occur, and if a young woman’s body itself rejects the defective fetus, the pregnancy of an older mother will proceed more calmly. - Mom's bad habits
. Almost 90% of pathological pregnancies occur with oligohydramnios. In a smoking woman, the fetus suffers from hypoxia; the breakdown products of aldehydes (alcohols) in the early stages of pregnancy lead to mutations and abnormalities. In 46% of cases, alcoholics have children born with genetic pathologies. Alcohol also “breaks” genetic chains in fathers who like to drink. - Infections
. Diseases such as influenza, rubella, and chickenpox are especially dangerous. The fetus is most vulnerable until the 18th week, until the amniotic sac is formed. In some cases, a woman is offered an abortion. - Taking medications
. Even regular chamomile tea is toxic for a pregnant woman. Any medication taken should be accompanied by consultation with a doctor. - Emotional turmoil
. They cause the death of nerve cells, which invariably affects the development of the fetus. - Bad ecology and climate change
. If you become pregnant while on vacation in Thailand, there is a chance that along with your pregnancy you will bring a dangerous infection, which will begin to develop slowly in your native land, affecting the health of the baby.
How to prevent fetal defects and where to get a fetal ultrasound in St. Petersburg
You can prevent most problems with pregnancy and fetal pathologies by planning your pregnancy in advance. When undergoing examination during planning, both partners undergo tests that clearly show the likelihood of genetic abnormalities. A range of tests are also carried out for infections that can cause deformities in the baby (TORCH complex) and other studies.
We invite you to undergo an ultrasound scan for fetal pathology in St. Petersburg at the Diana Clinic. We have installed the latest ultrasound machine with Doppler. The examination is carried out in 3-D and 4-D formats. A disc with the recording is given to you.