Truncus arteriosus (TCA) refers to complex congenital malformations of the heart and blood vessels, in which only a single common blood vessel, not divided into the pulmonary artery and aorta, departs from the heart, carrying blood throughout the pulmonary and systemic circulation, and leading to deep hemodynamic disorders. This abnormal vessel for an already born child during intrauterine development is called the common arterial trunk.
Such a complex heart and vascular defect occurs in 2-3% of patients with congenital defects, and it is always combined with an anomaly such as a ventricular septal defect. OSA is often combined with other malformations of the blood vessels and heart: patent atrioventricular canal, abnormal drainage of the pulmonary veins, coarctation of the aorta, one ventricle, mitral valve atresia, etc. In addition, such anomalies may also be accompanied by noncardiac malformations of the skeleton, genitourinary or digestive system.
Clinical manifestations of OSA are expressed in two syndromes: congestive heart failure and arterial hypoxemia. With this malformation of the heart, symptoms of profound hemodynamic disturbances begin to appear immediately after birth, and if there is no narrowing of the mouth of the pulmonary artery, then the condition of the newborn is assessed as critical from the first minutes of his life. About 75% of children with OSA die before the first year of life, and 65% of them die before 6 months. A fatal outcome with the common truncus arteriosus is caused by overcrowding of the pulmonary vessels with blood and severe heart failure.
Treatment for OSA can only be surgical and should be carried out in the first months of the child’s life. Often, for patients who are 2-3 years old, it is already too late to operate and they, as a rule, can only live up to 10-15 years. In the absence of timely cardiac surgery, less than 10% of patients survive to 20-30 years.
In this article we will introduce you to the presumed causes, types, symptoms, mechanisms of hemodynamic disorders, methods of identifying and treating the common arterial trunk. This information will help you understand the essence of the disease, and you can ask your doctor any questions you have.
Causes
Bad habits of the expectant mother significantly increase the risk of developing heart defects in the fetus.
Like all congenital heart defects, the occurrence of OSA can be provoked by negative factors affecting the body of the pregnant woman and the fetus. Their effects are especially dangerous during the 3-9th week of gestation, since it is during this period that ebryonogenesis of the heart of the unborn child occurs.
Such unfavorable factors include:
- genetic disorders;
- infectious agents (Coxsackie B viruses, herpes, rubella, influenza, enterovirus, etc.);
- bad habits of the expectant mother (smoking, taking drugs and alcohol);
- unfavorable ecology (electromagnetic radiation, radiation, toxic emissions, etc.);
- occupational hazards;
- taking certain medications;
- diseases of the pregnant woman (especially diabetes mellitus, autoimmune reactions).
Possibilities of fetal echocardiography in the first trimester of pregnancy (11-14 weeks)
Ultrasound machine HM70A
Expert class at an affordable price.
Monocrystal sensors, full-screen display mode, elastography, 3D/4D in a laptop case. Flexible transformation into a stationary scanner with a cart.
Introduction
The relevance of the topic of prenatal diagnosis of congenital heart defects (CHD) is clear to all doctors who are involved in prenatal diagnosis, neonatology, pediatrics, cardiology, and genetics. CHDs are one of the leading causes of perinatal mortality and are registered with a frequency of 4-13 per 1000 live births [1]. Due to the fact that preventive measures to prevent congenital heart disease are not sufficiently successful, their prenatal ultrasound diagnosis seems relevant and necessary.
Numerous studies by foreign and domestic colleagues have repeatedly formulated and studied various risk groups for the occurrence of congenital heart disease. This was done to potentially narrow the group of pregnant women eligible for echocardiography at a tertiary center. Among these risk groups were:
- Families with a child with congenital heart disease.
- Families with congenital heart disease in one or both spouses.
- Women suffering from diabetes, systemic connective tissue diseases, hypothyroidism.
- Pregnant women with teratogenic exposure in early pregnancy (herpes before the 6-7th week) [2].
However, in parallel, other scientists rejected these risk groups, because most congenital heart diseases occurred in fetuses and children whose mothers were not included in any of the proposed risk groups. The only reasonable criteria for the so-called selective selection were recognized as pregnant women who were at risk after screening in the first trimester and pregnant women with suspected congenital heart disease during ultrasound examination of the fetus [3].
It is undeniable that the optimal period of pregnancy for studying the fetal heart is 20-22 weeks, however, most lethal and clinically significant heart defects can be diagnosed at the end of the first trimester of pregnancy. Let us quote the words of the head of the Fetal Medicine Foundation, Kipras Nicolaides, expressed on the pages of the FMF website (www.fetalmedicine.com): “An ultrasound specialist should assure most parents from the 12th week of pregnancy that their child does not have major congenital heart defects. In the case of large congenital heart defects, early detection can lead to a correct diagnosis or at least raise suspicion for ultrasound monitoring.”
The main goal of prenatal diagnosis has been formulated by prenatal diagnostic specialists around the world - to provide women with the maximum possible information about the defect as early as possible. We must give the woman and the family as a whole the right to decide on prolonging pregnancy with gross malformations in the fetus [4].
Every year, an increasing number of publications are devoted to the diagnosis of congenital heart disease in the early stages - in the first trimester of pregnancy [5-8]. Almost none of the issues of the ISUOG journal (Ultrasound In Obstetrics and Gynecology, or the “white” journal, as experts call it) ignores the topic of early diagnosis of congenital malformations.
The most famous website in the world of prenatal diagnostics, www.thefetus.net (Philippe Jeanty, USA), has already published more than 30 cases of congenital heart disease in the first trimester of pregnancy. However, in Russian periodicals there are only a few works on this topic. All of them belong to the “pen” of prenatal diagnostic specialists of the Russian Association of Ultrasound Diagnostics in Perinatology and Gynecology, although for many specialists, both before and now, examination of the fetal heart at 11-14 weeks consists only of ascertaining the number of heartbeats.
The purpose of echocardiography in the first trimester of pregnancy is to identify fatal and clinically significant congenital heart disease. This study does not aim to identify stenoses and hypoplasias of the outflow tracts, diagnose minor septal defects, pathologies of the aortic arch and ductus arteriosus. Many of these defects are not only technically impossible to suspect in the first trimester, they manifest themselves after the 30th week of pregnancy, i.e. their diagnosis is the prerogative of research in the third trimester.
The accuracy of prenatal diagnosis of congenital heart disease at all stages of pregnancy varies widely. The reasons for this may be different experience of specialists, obesity of the pregnant woman, frequency of ultrasound transducers used and class of ultrasound machine, previous abdominal surgeries, gestational age, amount of amniotic fluid and fetal position. However, we note that many of these factors lose their relevance precisely when transvaginal echocardiography is performed in the first trimester of pregnancy. Timely diagnosis of congenital heart disease makes it possible to identify high-risk fetuses based on genetic syndromes, which is important during prenatal counseling and has a significant impact on obstetric tactics.
results
From 2006 to 2011, 125 congenital heart diseases were detected prenatally in the first trimester of pregnancy. Of these, 68 (55%) CHDs were combined with various chromosomal anomalies (CA) of the fetus, 30 (24%) were part of various multiple congenital malformations (MCDM), 27 (21%) CHDs were isolated.
Echocardiography examined a four-chamber section of the fetal heart (Fig. 1) and a section through three vessels (Fig. 2). Ultrasound was performed with a transabdominal sensor; only when necessary (difficult visualization) was an intracavitary sensor used. A four-chamber section of the fetal heart during ultrasound scanning with a transabdominal sensor was visualized in 85% of cases, a section through the vessels - in 73%, when using a transvaginal sensor, these figures increased significantly to 100 and 91%, respectively. Optimization of prenatal diagnosis of congenital heart disease can be achieved through strict adherence to basic methodological rules. When assessing a four-chamber section of the fetus, it is necessary to evaluate the normal location of the fetal heart, excluding its ectopia (Fig. 3), the position of the axis of the fetal heart, which does not present any difficulties, the normal proportions and sizes of the chambers of the heart, the movement of the atrioventricular valve leaflets should be free, the septal leaflet of the tricuspid the valve should be located closer to the apex of the heart (Fig. 4). When assessing a section through three vessels, it is necessary to evaluate the relative position of the vessels and their diameter.
Rice. 1.
Pregnancy 12 weeks. Four-chamber section of the fetal heart. The chambers of the heart are clearly visible.
Rice. 2.
Pregnancy 12 weeks. Section through three vessels. The aorta and pulmonary trunk are visualized. The vessels are located in one line and have normal sizes.
Rice. 3.
Pregnancy 8 weeks. Ectopia of the heart. The heart is located outside the chest cavity.
The case was published on the website www.thefetus.net.
Rice. 4.
Pregnancy 13 weeks. Four-chamber section of the fetal heart. The chambers of the heart are clearly visible. Position of atrioventricular valves.
Advanced echocardiography involves the use of additional modes and sections - a section through the aortic arch (Fig. 5), a section through the outflow tract of the left ventricle (Fig. 6), a CD mode (Fig. 7), pulse Doppler, STIC technology (Fig. 8-11 ). This examination is carried out when abnormal screening projections of the fetal heart are detected, markers of CA [widening of the thickness of the nuchal space - TVP, hypoplasia/absence of the nasal bone (Fig. 12, 13), regurgitation in the ductus venosus (Fig. 14), tricuspid regurgitation (Fig. 15 )] and/or congenital malformations of the fetus.
Rice. 5.
Pregnancy 13 weeks. Section through the aortic arch. Three brachiocephalic vessels extending from the arch are clearly visible.
Rice. 6.
Pregnancy 12 weeks. Tetralogy of Fallot. Color flow mode. Section through the left ventricular outflow tract. The “equestrian aorta” is visible, sitting above the VSD. The case was published on the website www.thefetus.net.
Rice. 7.
Pregnancy 13 weeks. Double exit of vessels from the right ventricle. Color flow mode. Parallel exit of vessels from the right ventricle.
Rice. 8.
Pregnancy 12 weeks. Atrioventricular communication (AVC) in Down syndrome. STIC mode.
Rice. 9.
Pregnancy 12 weeks. Tetralogy of Fallot. STIC mode. The “horsewoman” aorta sits above the VSD. The case was published on the website www.thefetus.net.
Rice. 10.
Pregnancy 13 weeks. Transposition of the great vessels. STIC mode. A parallel course of efferent vessels is visible, the upper of which leaves the left ventricle and divides into a bifurcation (pulmonary artery).
Rice. eleven.
Pregnancy 12 weeks. Common arterial trunk. STIC mode. A single efferent vessel of two ventricles is visible.
Rice. 12.
Pregnancy 11.4 weeks. Multiple markers of CA. Patau syndrome (trisomy 13). Increased TVP, abnormal profile with hypoplasia of the nasal bone, protuberance on the upper jaw (a sign of a cleft face), polydactyly. The fetus was diagnosed with hypoplasia of the left heart.
Rice. 13.
Pregnancy 12 weeks. Multiple markers of CA. Down syndrome (trisomy 21). Increased TVP, abnormal profile with nasal bone hypoplasia. AVC was detected in the fetus.
Rice. 14.
Pregnancy 12 weeks. Reverse blood flow in the ductus venosus in a fetus with heterotaxy.
Rice. 15.
Pregnancy 12 weeks. Tricuspid regurgitation in a fetus with a common truncus arteriosus.
The nosology of the congenital heart diseases we identified was as follows:
- hypoplastic left heart syndrome (HLHS) - 29 cases (Fig. 16);
- atrioventricular communication (AVC) - 23 (Fig. 17, 18);
- ventricular septal defect (VSD) - 19 (Fig. 19);
- pathology of the great vessels - 19 (of which transposition - 3, double origin of vessels from the right ventricle - 2, tetralogy of Fallot - 5, common truncus arteriosus - 9);
- pathology of the right heart (pathology of the tricuspid valve) - 3;
- heterotaxy syndrome - 6;
- single ventricle - 4;
- ectopia of the heart - 7;
- combined forms of congenital heart disease occurred in 15 cases.
Rice. 16.
Pregnancy 13 weeks. Hypoplastic left heart syndrome in a fetus with Turner syndrome (45X). Single flow through the tricuspid valve. Shunting of blood into the hypoplastic left ventricle through the VSD.
Rice. 17.
Pregnancy 11.4 weeks. Four-chamber section of the heart. Single atrioventricular valve. There is no “cross” of the normal relationship between the atrioventricular valves and the heart septa.
Rice. 18.
Pregnancy 11.4 weeks. Four-chamber section of the heart. Color flow mode. Single atrioventricular valve.
Rice. 19.
Pregnancy 12 weeks. Color flow mode. Extensive VSD in a fetus with Edwards syndrome (trisomy 18).
When karyotyping fetuses with a prenatal diagnosis of congenital heart disease at 11-14 weeks, 68 chromosomal abnormalities were diagnosed:
- trisomy 21 (Down syndrome) was detected in 23 (34%) cases,
- trisomy 18 (Edwards syndrome) - in 19 (28%);
- trisomy 13 (Patau syndrome) - in 7 (10%);
- monosomy X (Turner syndrome) - in 6 (9%);
- triploidy - in 8 (12%);
- other chromosomal imbalances - in 5 (7%).
It should be especially noted that in 8 cases of detected CA, the indication for karyotyping was the identification of congenital heart disease. These fetuses had normal values for both TVP and nasal bone length.
In case of CA, the identified congenital heart disease according to nosology had the following features: in the majority of fetuses with Down syndrome, AVK and VSD were diagnosed; with Patau syndrome - GLOS and VSD; with Edwards syndrome - VSD, tetralogy of Fallot and OSA; with Turner syndrome - GLOS and aortic pathology - coarctation of the aorta in a typical place (Fig. 20).
Rice. 20.
Pregnancy 12 weeks. Section through the aortic arch. Color flow mode. Narrowing of the aorta in a “typical” location in a fetus with Turner syndrome (45X).
It is necessary to separately consider the issue of verification of ultrasound diagnosis. All pregnancies with isolated congenital heart disease in the first trimester were prolonged until the second trimester, when 100% morphological verification of the diagnosis is possible. In modern conditions, verification of diagnoses after termination of pregnancy in the first trimester is a rather significant problem. However, with specialized training of specialist morphologists, verification of congenital heart disease is also possible during termination of pregnancy in the first trimester (Fig. 21, 22). This undoubtedly depends on the quality of the material obtained, the qualifications of the morphologist and the special equipment required in some cases, as well as on the general methodological approaches to anatomical and morphological diagnosis, regardless of gestational age.
Rice. 21.
Pregnancy 13 weeks. Increased TVP in a fetus with hypoplasia of the left heart.
Rice. 22.
The same fruit. Morphological examination - hypoplasia of the ascending aorta and cystic cavities (marked by arrows).
Conclusion
From the above, the following conclusions can be drawn.
The fetal heart should be assessed in all pregnant women during a screening examination in the first trimester (11-14 weeks). Since the modern concept of the development of prenatal diagnostics within the framework of the “pilot” project of the Ministry of Health of the Russian Federation implies a screening examination in the first trimester by an expert doctor, it is he who should evaluate the fetal heart and suspect congenital heart disease at the end of the first trimester of pregnancy.
To exclude lethal and clinically significant cardiac pathology in the first trimester, it is necessary to evaluate the four-chamber projection of the fetal heart and the section through three vessels.
Extended echocardiography should be performed if abnormal screening projections of the fetal heart, markers of CA and/or fetal congenital malformation are detected.
If congenital heart disease is detected in the first trimester, fetal karyotyping is indicated.
Literature
- Office for National Statistics. Mortality statistics. Childhood, Infancy and Perinatal. Series DH3. Stationary Office: London, 2007; 40.
- Novikova I.V., Pribushenya O.V., Rumyantseva N.V. Formation of risk groups for prenatal diagnosis of congenital heart defects. Instructions for use. Minsk, 2004.
- Carvalho JS, Moscoso G, Tekay A et al. Clinical impact of first and early second trimester fetal echocardiography on high risk pregnancies. Heart. 2004; 90: 921-926.
- Becker R., Wegner R.-D. Detailed screening for fetal anomalies and cardiac defects at the 11-13 week scan. Ultrasound Obstet Gynecol 2006; 27; 613-618.
- Allan LD, Sharland GK, Milburn A. et al. Prospective diagnosis of 1,006 consecutive cases of congenital heart disease in the fetus. J Am Coll Cardiol 1994; 23: 1452-1458.
- Huggon IC, Ghi T, Cook AC et al. Fetal cardiac abnor-malities identified prior to 14 weeks' gestation. Ultrasound. Obstet. Gynecol. 2011; 20: 22-29.
- Persico N., Moratalla J., Lombardi CM et al. Fetal echocardiography at 11-13 weeks by transabdominal high-frequency ultrasound Ultrasound Obstet. Gynecol. 2011; 37: 296-301
- Allan LD Echocardiographic detection of congenitalheart disease in the fetus: present and future. Br. Heart. J. 1995; 74: 103-106.
Ultrasound machine HM70A
Expert class at an affordable price.
Monocrystal sensors, full-screen display mode, elastography, 3D/4D in a laptop case. Flexible transformation into a stationary scanner with a cart.
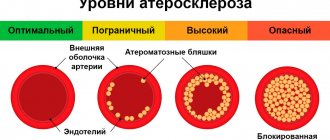
Varieties
Depending on the location of the OSA relative to the ventricles of the heart, there are three options:
- mainly over the right ventricle – in almost 42% of patients;
- equally over both the right and left ventricles - in almost 42% of patients;
- mainly over the left ventricle - in approximately 16% of patients.
Depending on the origin of the pulmonary arteries, cardiologists distinguish four main types of OSA:
- I - the trunk of the pulmonary artery branches off from the single arterial trunk and is divided into the left and right pulmonary artery;
- II – both pulmonary arteries branch from the surface of the posterior wall of a single arterial trunk;
- III – both pulmonary arteries branch from the surface of the lateral walls of a single arterial trunk;
- IV – there are no pulmonary arteries, and blood is delivered to the lungs by bronchial arteries branching from the aorta.
Type IV OSA is now considered by cardiologists as one of the severe forms of tetralogy of Fallot.
Hemodynamic disorders
OSA develops in the fetus at 5-6 weeks of pregnancy. Under the influence of unfavorable factors at this stage, the division of a single trunk into the main vessels - the pulmonary artery and the aorta - does not occur. There is no normal partition between them, and they continue to communicate with each other.
The common trunk delivers blood to both ventricles of the heart, and this process leads to mixing of venous blood with arterial blood. In this case, the pressure remains the same in the truncus arteriosus, cardiac chambers and pulmonary arteries.
In OSA, there is always a defect in the septum between the ventricles. In addition, if the development of other partitions of the heart chambers is delayed, the heart may have only 2 or 3 sections. The valve located in the truncus arteriosus may have 1, 2, 3 or 4 leaflets.
Equal pressure in the heart chambers, pulmonary artery and aorta causes overfilling of the vessels in the lungs and a rapid increase in heart failure, which at a certain point provokes death. In surviving children, such profound hemodynamic disturbances cause the development of severe pulmonary hypertension.
In the presence of narrowing of the pulmonary artery, overload of the pulmonary vessels does not occur, but a pressure gradient is created in the “aorta - trunk of the pulmonary artery” variant. This change in hemodynamics leads to the development of right and left ventricular failure.
Depending on the form of OSA, hemodynamic disturbances can occur in three ways:
- normal or slightly increased blood flow in the lungs with a mildly expressed blood discharge - manifested by the occurrence of cyanosis during exercise and is not accompanied by heart failure;
- decreased pulmonary blood flow due to stenosis of the pulmonary artery orifice - manifested by constant cyanosis due to impaired oxygen enrichment of the blood;
- increased pulmonary blood flow and increased pressure in the pulmonary vessels - expressed in pulmonary hypertension and cardiac failure, which are not corrected by the therapy.
Common truncus arteriosus
The name of this rare heart defect defines its essence. Instead of two main vessels—the aorta and the pulmonary artery—from the heart, one large vessel departs, carrying blood into the systemic circulation, into the lungs and coronary vessels. This vessel - the arterial trunk - did not divide, as it should, at 4-5 weeks of intrauterine life of the fetus, into the aorta and pulmonary artery, but “sitting” astride the two ventricles, carries mixed blood into both circles of blood circulation (the ventricles communicate with each other through huge ventricular septal defect). The pulmonary arteries to both lungs can depart from the trunk in one common vessel (and then divide into branches) or separately, when both the right and left depart directly from the trunk.
It is clear that with this defect the entire circulatory system is deeply disturbed. The flow of venous and arterial blood mixes in the ventricles. This oxygen-undersaturated mixture enters the systemic circle and the lungs under the same pressure, and the heart works with enormous load. Manifestations of the defect become obvious already in the first days after birth: shortness of breath, fatigue, sweating, cyanosis, rapid pulse, enlarged liver - in a word, all signs of severe heart failure. These phenomena may decrease in the first months of life, but in the future they will only increase. In addition, the response of the pulmonary vessels to increased blood flow leads to their changes, which will very soon become irreversible. According to statistics, 65% of children with a common arterial trunk die during the first 6 months of life, and 75% do not live to see their first birthday. For patients, even those who have reached only two or three years of age, it is usually too late to operate, although they can live up to 10-15 years.
Surgical treatment is quite possible, and its results are quite good. The most important condition for success will be the timely admission of the child to a specialized cardiology center and the performance of the operation in the first months of life. Delay in these cases is like death.
If for some reason it is impossible to perform a radical operation, then a palliative option exists and has proven itself: applying a cuff to both pulmonary arteries arising from the common trunk. This operation (see the chapter on ventricular septal defects) will protect the pulmonary vessels from increased blood flow, but it must be done very early - in the first months of life.
Radical correction of the common arterial trunk is a major intervention, performed, of course, under artificial circulation. The arteries leading to the lungs are cut off from the common trunk, thus turning the trunk only into the ascending aorta. Then the cavity of the right ventricle is incised, and the septal defect is closed with a patch. The normal path for left ventricular blood has now been restored. The right ventricle is then connected to the pulmonary arteries using a conduit. A conduit is a synthetic tube of one or another diameter and length, in the middle of which a biological or (less often) mechanical prosthesis of the valve itself is sewn. We discussed the various designs of artificial valves and their disadvantages and advantages above (see the chapter on Ebstein's anomaly). Let's just say that as it grows, the entire sewn-in conduit can grow with its own tissue and be destroyed, and the valve can gradually become overgrown and poorly cope with its original function. In addition, the size of the conduit that can be sewn into a six-month-old child is clearly insufficient to function normally for many years: after all, synthetic tubes and artificial valves do not grow. And, no matter how large the conduit is, after a few years it will become relatively narrow. In this case, over time the question of replacing the conduit will arise, i.e. about a repeat operation, but this can happen after many years of the child’s normal life. However, the need for constant and regular cardiac monitoring should be obvious to you.
It is possible that by the time you read this, they will have already created prostheses coated on the inside with their own cells, taken in advance from the child’s tissues. For now, this is only experimental work and it will take time for it to become a clinical reality. But with today’s dizzying pace of scientific development, this is quite possible in the near future.
How to get treatment at the Scientific Center named after. A.N. Bakuleva?
Online consultations
Symptoms
Clinical symptoms of this congenital heart and vascular defect are expressed in arterial hypoxemia, manifested by cyanosis, and heart failure. The severity of these phenomena depends on the type of OSA.
With such a congenital heart defect, the following symptoms may be present:
- shortness of breath that occurs at rest;
- excessive sweating;
- decreased endurance;
- cyanosis (minimal to significant);
- rapid pulse;
- enlarged liver and spleen;
- retardation in physical development;
- “heart hump” (not always present);
- deformation of fingers in the form of “drum sticks” and nails in the form of “watch glasses”.
In type I OSA, cyanosis is expressed in a mild form, and in types II-III it manifests itself to a greater extent, but signs of heart failure are not always detected.
When examining a patient, a doctor may detect the following symptoms of OSA:
- increase in pulse pressure;
- increased heart rate;
- single and loud II tone and ejection click;
- holosystolic murmur on the left edge of the sternum with an intensity of 2-4/6;
- decreasing diastolic murmur in the third intercostal space to the left of the sternum (with valvular insufficiency of the arterial trunk);
- murmur heard at the apex on the mitral valve in mid-diastole (not always).
Diagnostics
Suspicion of this pathology will arise during pregnancy, or rather, at a later stage when a woman undergoes an ultrasound.
Suspicion of the presence of OSA in the fetus may arise during an ultrasound at 24-25 weeks of pregnancy. Often, women who discover such a pathology in their unborn child are advised to terminate the pregnancy, since even timely operations to correct OSA are not always successful. In addition to this developmental anomaly, approximately half of the fetuses have extracardiac defects (anomalies of the bone skeleton, digestive or genitourinary system, DiGeorge syndrome).
Identifying the above-described symptoms and physical examination of the child helps to suspect the presence of OSA in a newborn. To clarify the diagnosis, the following examination methods are prescribed:
- X-ray of the chest - the spherical shape of the heart is determined, enlarged shadows of the branches of the pulmonary artery and great vessels, hypertrophy and an increase in the volume of the ventricles;
- ECG - deviation of the EOS to the right and overload of the ventricles of the heart are detected;
- phonocardiography - deviations in tones and heart murmurs are determined (loud II tone, systolic, and sometimes diastolic, murmur);
- Echo-CG – the connection of the pulmonary arteries with OSA is revealed;
- cardiac catheterization - with such a congenital defect, the catheter easily penetrates the OSA, the same pressure is determined in the ventricles, aorta and pulmonary artery, and when the pulmonary artery is narrowed, a pressure gradient is present;
- angiocardiography - radiography with contrast reveals specific anomalies of vascular development, an increase in the volume of the ventricles and right atrium, blurred and unusual structure of the roots of the lungs and increased or unified pattern of the lungs;
- aortography is an X-ray study with contrast that determines the level of origin of the pulmonary artery and evaluates the condition of the valve leaflets.
The most informative procedure in examining newborns is angiocardiography, which allows one to assess the type of OSA. Data from all studies enable the doctor to draw up the correct treatment plan and determine the extent of the necessary surgical correction.
Endovascular closure of patent ductus arteriosus using an occluder
Introduction
Patent ductus arteriosus (PDA) is a congenital heart defect (CHD) characterized by the presence of abnormal vascular communication between the aorta and the pulmonary artery
In the prenatal period, everyone has a PDA; this is a normal component of the fetal circulation. After the first breath of the born child, the pulmonary vessels open, the pressure in the right ventricle drops, the PDA gradually ceases to function and closes (obliterates). Obliteration of the duct occurs at different times. In 1/3 of children it closes by two weeks, in the rest - within eight weeks of life.
Hemodynamic disorders are associated with abnormal discharge of blood from the aorta to the pulmonary artery, since the pressure in the aorta is much higher than in the pulmonary artery.
The volume of blood discharged depends on the size of the duct. As a result of circulatory disorders, a smaller volume of blood enters the systemic circulation than it should, which affects vital organs (brain, kidneys), and skeletal muscles. Passing through the vessels of the lungs, this blood returns to the left atrium, the left ventricle, which, experiencing excessive load, increase in size (hypertrophy), then, under the influence of an ever-increasing volume of blood supersaturated with oxygen, changes occur in the vessels of the lungs and pulmonary hypertension occurs.
Manifestations and natural course of the defect
Children are born with normal body weight and length. Further manifestations of the disease are related to the size of the duct. The shorter and wider the PDA, the greater the volume of blood discharged through it and the more pronounced the clinical picture of the disease. With narrow and long PDAs, sick children are no different from healthy ones. The only sign indicating the presence of congenital heart disease is a murmur heard by the pediatrician over the heart area. With wide and narrow PDAs, already in the first months and even days of the child’s life, all the symptoms (manifestations) of the defect can be detected. Such children experience constant pallor; during physical activity (straining, sucking, screaming), transient cyanosis (blue skin tint) is noted, mainly on the legs. Children are lagging behind in physical development. They have a tendency to recurrent bronchitis and pneumonia.
The most difficult periods during the course of the defect are the adaptation phase during the newborn period and the phase of terminal pulmonary hypertension in older children. During these periods, children die from heart failure, cerebrovascular accidents (stroke), pneumonia, and infective endocarditis. The average life expectancy for a PDA without surgical treatment is 25 years, although many patients with a narrow and long PDA live to old age. If an adult patient with a PDA develops signs of circulatory decompensation, surgery to close the duct is also recommended.
Treatment
There are two methods of treating PDA: conservative, or medicinal, and surgical. Drug treatment of PDA is used only in the maternity hospital for newborns during the first two weeks of life; later it becomes ineffective. This method is not always effective and has many contraindications, so the main treatment is mechanical closure of the duct.
Previously, the most common intervention was ligation of the duct after thoracotomy. Nowadays, the operation of PDA ligation is performed very rarely. Endovascular occlusion of the PDA has taken on a leading role. The essence of the intervention is to occlude (close) the duct with specially made spirals and occluders. The technique has almost no complications; it is performed on young children under general anesthesia, and on teenagers and adults under local anesthesia. Access is carried out by puncture, through the femoral artery. The effectiveness of the operation is almost one hundred percent; recanalization of the PDA is occasionally observed, which is subsequently eliminated in the same way. For wide and short PDAs, when endovascular occlusion of the PDA with coils is technically impossible, the PDA is closed using specially designed occluders, which are also delivered through the femoral artery.
Description of a clinical case.
Patient S, 67 years old, was admitted to the inpatient emergency department on September 18, 2017 with a diagnosis of congenital heart disease: patent ductus arteriosus (diameter 4 mm, length 13 mm). Upon admission, he complains of weakness and shortness of breath when walking. He has a history of hypertension with a maximum increase in blood pressure up to 200/100 mmHg, adapted to 140/80 mmHg. Takes Concor, amlodipine, Torvacard. Over the course of a year, he has noticed the appearance of shortness of breath when walking. During examination, according to ECHO data, congenital heart disease was detected: patent ductus arteriosus, signs of moderate pulmonary hypertension. According to MSCT data, the diameter of the ductus arteriosus was 4 mm.
Considering the deterioration of health and the appearance of pulmonary hypertension, a decision was made to perform transcatheter closure of the PDA.
Description of the operation (09/22/2017)
The right common femoral artery and common femoral vein were punctured under local anesthesia. A diagnostic catheter was inserted into the thoracic aorta. Angiography was performed and a patent ductus arteriosus connecting the aorta and pulmonary artery was visualized. Next, through the introducer in the right OBV, a catheter is inserted through the guidewire through the ductus arteriosus into the descending aorta. The catheter was replaced with an occluder delivery device. An Amplatzer Duct Occluder was implanted with the distal occluder disk located on the side of the aorta, and the proximal one on the side of the pulmonary artery. On control angiography, the position of the occluder in the ductus arteriosus is optimal, and a reduction in blood flow through the ductus arteriosus is noted. A good angiographic result was obtained.
The next day the patient was discharged from the hospital in satisfactory condition.
Treatment
OSA can only be eliminated by surgery. Conservative treatment for this cardiac malformation is ineffective and is prescribed to maintain the patient’s condition until cardiac surgery.
Conservative therapy
The purpose of such treatment is aimed at the following aspects:
- decreased physical activity;
- ensuring thermal comfort;
- decrease in circulating blood volume;
- elimination of symptoms of heart failure.
The patient may be prescribed the following medications:
- diuretics;
- Digoxin;
- ACE inhibitors.