The Innovative Vascular Center has all the necessary technologies for the treatment of acute and chronic occlusion of the inferior vena cava. Our specialists have successful experience in solving this complex problem.
Acute thrombosis of the inferior vena cava requires treatment in a specialized vascular surgery hospital. The goal of treatment is to restore the patency of the IVC. This problem is successfully solved using endovascular surgery methods. There are modern thrombolytic drugs and endovascular probes for removing thrombotic masses.
Thrombolysis
Dissolution of venous blood clots with special drugs - thrombolytics. These include streptokinase, urokinase and actilise. Only direct administration of a thrombolytic through a catheter into a thrombus with regular monitoring of the patency of the affected segment is effective. Thrombolysis options may include the use of a special Angiojet device. The thrombolytic solution is supplied through a special probe under high pressure, and then the blood clots are sucked out by a special suction. Another similar device used in our clinic is the Aspirex probe. This is a special spiral suction that gently removes thrombotic masses. The use of Aspirex in the inferior vena cava is limited due to its large diameter, so catheter thrombolysis is the most acceptable method. Thrombolysis is possible only in the first 10 days from the onset of the disease, while the blood clots have not yet healed.
May–Turner syndrome and varicose veins of the pelvic organs in men
A.A. Kapto Federal State Autonomous Educational Institution of Higher Education "Russian Peoples' Friendship University"; Russia, 117198 Moscow, st. Miklouho-Maklaya, 6
Introduction
Compression of the left common iliac vein and fibrous adhesions in it were first described by the German pathologist R. Virchow in 1851 [1]. He noted that deep ileofemoral thrombosis was 5 times more common in the left leg than in the right. According to researchers [2–5], compression of the left common iliac vein is a widespread pathology that occurs in 22–50% of the population (without division by gender) (Table 1).
Table 1. Frequency of detection of fibrous adhesions in the lumen of the left common iliac vein during autopsies (without division by gender)
| Author | Year of publication | Number of autopsies, abs. | Frequency of detection of adhesions, % |
| JP McMurrich [2] | 1908 | 57 | 30 |
| WE Ehrich, EB Krumbhaar [3] | 1943 | 412 | 23,8 |
| R. May, J. Thurner [4] | 1957 | 430 | 22 |
| N. Usui et al. [5] | 1978 | 90 | 50 |
In the English-language literature, compression syndrome of the left common iliac vein is more often called May-Thurner syndrome. Asymptomatic compression of the left common iliac vein in the adult population, according to HC Baron et al., occurs in 16–20% of cases [6]. The natural course of May–Turner syndrome is a prerequisite for the development of ileofemoral thrombosis and postthrombotic syndrome. The detailed clinical picture of ileofemoral thrombosis in patients with compression syndrome of the left common iliac vein is also called Cockett syndrome [7, 8].
Until recently, the relevance of the problem of May–Turner syndrome was associated only with its thrombotic complications. With non-thrombotic compression of the left common iliac vein, a clinical picture develops due to congestion of both the internal and external iliac veins. The plethora of the internal iliac vein causes the plethora of the veins of the pelvic organs due to the development of collateral circulation. Congestion of the external iliac vein leads to the appearance of retrograde blood flow in the left cremasteric vein flowing into it and the formation of an ileospermatic type of varicocele.
The study of arteriovenous conflicts as a cause of varicose veins of the pelvic organs in men began relatively recently. The hypothesis about the role of venous congestion of the pelvic organs in the development of various urological diseases was expressed back in 1895 by the German urologist K. Posner in his monograph “Diagnostics of Genitourinary Diseases” [9]. V.V. Yakovenko (1955) was the first to suggest that the cause of the development of varicocele is venous congestion in the genitourinary plexus [10]. H. Sakamoto and Y. Ogawa (2008) examined the relationship between varicocele and the prostate venous plexus using scrotal Doppler and transperineal color Doppler. In all 209 men included in the study, the diameter of the veins of the prostatic plexus (PS) was positively correlated with the diameter of the veins of the right and left pampiniform plexuses. Thus, the authors found that varicoceles, especially bilateral ones, are associated with venous anomalies of the pancreas [11]. A.I. Neumark et al. (2013) proposed to distinguish 2 types of varicocele: 1) isolated, in which minimal hemodynamic disturbances are observed in the right testis, and the pancreas is not involved in the pathological process; 2) combined with pelvic congestion, in which disorders affect not only the left, but also the right testicle, as well as the pancreas [12]. Criteria for varicose veins of the small pelvis in men A.Yu. Tsukanov and R.V. Lyashev (2014) considered the diameter of the veins of the paraprostatic plexus to be >5 mm and/or the presence of retrograde blood flow during the Valsalva maneuver, recorded during duplex scanning of the veins using a rectal sensor [13]. A.A. Capto, O.B. Zhukov (2016) presented the world’s first review of the literature on varicose veins of the pelvic organs in men [14]. In 2017, we proposed an ultrasound classification of varicose veins of the PS as a marker of varicose veins of the pelvic organs in men [15].
Currently, there are neither specific nor general recommendations for the diagnosis and treatment of pelvic venous congestion and ileofemoral vascular conflicts in men with urological and andrological pathologies. In this regard, the purpose of this study is to study methods of diagnosis and treatment of iliac venous compression in men with urological and andrological pathology and varicose veins of the pelvic organs.
Materials and methods
From 2015 to 2021, 110 patients aged from 17 to 69 years (average 33.2 years) with bilateral varicocele, varicose veins of the pelvic organs and May-Turner syndrome were examined.
The diagnosis of varicocele was verified by examination and ultrasound examination (ultrasound) with Dopplerography of the scrotal organs. As an ultrasound criterion for diagnosing varicocele, we accepted the diameter of the veins of the pampiniform plexus >2 mm at rest in clinostasis, which reflects the generally accepted point of view [16–20].
The diagnosis of varicose veins of the pelvic organs was verified using transrectal ultrasound (TRUS) using the criteria and classification we proposed in 2017 (Table 2) [21].
Table 2. Ultrasound classification of prostate varicose veins according to A.A. Capto [21]
| Stage | Definition of varicose veins | Maximum vein diameter, mm | Blood flow speed, cm/s | Blood flow velocity during Valsalva maneuver, cm/s |
| I | Visible | <4 | <3 | <5 |
| II | Significant | 5–10 | 3–5 | 5–15 |
| III | Expressed | >10 | >5 | >15 |
May–Turner syndrome was diagnosed using contrast-enhanced magnetic resonance imaging (MRI) of the veins. The study was carried out using a GE Opima MR360 magnetic resonance imaging scanner (General Electric, USA). To diagnose aortomesenteric compression, we determined the value of the aortomesenteric angle (norm 28–65°) and aortomesenteric distance (norm 10–34 mm) [22, 23], to diagnose May–Turner syndrome – the value of the lower angle of the lumbar lordosis (lower lumbar lordosis angle) (norm >134.3°) and the diameter of the iliac vein tunnel (norm >4.2 mm). [24]. Variants of arteriovenous conflicts of the ileocaval segment were assessed using the classification we proposed in 2021 (Fig. 1) [25].
Rice. 1. Topographic-anatomical classification of options for compression of the iliac veins according to A.A. Capto [25]. Magnetic resonance imaging study of the inferior vena cava and pelvic vessels (modeling): a – central proximal (high bifurcation of the aorta, in which the right common iliac artery compresses the lower section of the inferior vena cava until it divides into the iliac veins); b – central distal (high bifurcation of the aorta, in which the right common iliac artery compresses the lower section of the inferior vena cava at the site of its division into the iliac veins); c – left proximal (the right common iliac artery compresses the left common iliac vein (May–Turner syndrome)); d – left distal (compression of the left external and/or left internal iliac artery by the left external iliac vein); e – right proximal (compression of the right common iliac artery by the right common iliac vein); e – right distal (compression of the right external and/or right internal iliac artery by the right external iliac vein)
To diagnose erectile dysfunction, the International Index of Erectile Function (IIEF-5) was used [26], Dopplerography of penile vessels was performed at rest and during pharmacologically induced erection. If venogenic erectile dysfunction was suspected, dynamic pharmacocavernosography was performed, which made it possible to verify the proximal, distal and mixed types of pathological venous outflow from the cavernous bodies of the penis.
In patients with urological and andrological diseases, the absolute indication for endovascular surgery was a combination of the following signs of iliac venous compression:
- severe symptoms from the pelvic organs (pain, dysuria, erectile dysfunction);
- bilateral and/or recurrent varicocele; 3) varicose veins of the pancreas of II–III degree (maximum diameter of the veins of the pancreas >5 mm);
- compression of the iliac veins according to MRI and venography;
- the presence of collateral branches of the iliac veins according to phlebography.
Isolated pathospermia without other clinical manifestations was extremely rare (n = 4) and was not considered an absolute indication for angioplasty and iliac vein stenting.
Surgical treatment of May–Turner syndrome was carried out under local anesthesia in a cath lab and included the following steps:
- puncture of the femoral or popliteal vein (under ultrasound guidance);
- multi-projection intraoperative venography;
- balloon angioplasty;
- stent implantation;
- postdilatation of the stented segment;
- control venography.
Pre- and postoperative care of the patient included:
- anticoagulant therapy with rivaroxaban at a dose of 20 mg/day for 1 week before surgery and 6 months after it;
- Ultrasound of the iliac vessels on the 1st day after surgery, after 2 weeks and after 1, 3, 6 months;
- Ultrasound of the scrotum with color Doppler mapping before surgery and 1, 3, 6, 9 and 12 months after it;
- TRUS of the pancreas and veins of the pancreas before surgery and 1, 3, 6, 9 and 12 months after it;
- assessment of the patient’s condition using IIEF-5, the International Prostatic Symptom Score (IPSS) [27], and the US National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) [28] before surgery and 1, 3, 6, 9 and 12 months after it.
Statistical processing of the results was carried out using statistical packages Microsoft Office and Excel. The statistical significance of differences in indicators having a normal distribution was assessed using the Student t test and the Mann–Whitney U test.
results
The ages of the 110 patients included in the study ranged from 17 to 69 years and averaged 33.2 years.
The reason for the patients' treatment was pain (in the lower abdomen, in the external genital area, in the lower back), dysuria (irritative and obstructive symptoms), erectile dysfunction (deterioration of morning, spontaneous and adequate erections), pathospermia (oligo-, astheno- and teratozoospermia), pyospermia (as a marker of the inflammatory process in the pancreas), recurrent and untreatable chronic prostatitis, bilateral and recurrent varicocele. Most often, patients complained of pain. Of the concomitant surgical pathologies, hemorrhoids and varicose veins of the lower extremities were found in most cases.
39 patients had a history of varicocelectomy: 1 operation in 23, 2 in 9, 3 in 2, 4 in 1, 5 in 1. Of these, 15 patients were previously operated on by us.
Ultrasound of the scrotal organs in all cases verified bilateral varicocele, more pronounced on the left side
According to TRUS, the volume of the pancreas varied from 6.6 to 76.6 cm3 and averaged 21.2 cm3. Pancreatic cysts were found in 2 patients. An increased mean prostate share was detected in 18 patients (aged 20 to 55 years, mean 36.3 years). The volume of the pancreas in patients with an increased average lobe varied from 9.3 to 37.9 cm3 and averaged 23.3 cm3 (Fig. 2).
Rice. 2. Transrectal examination of the prostate gland of patient X. , 39 years old, with May–Turner syndrome, recurrent bilateral varicocele (3 operations in history), stage III varicose veins of the pelvic organs, enlarged middle lobe of the prostate gland with its volume of 22.2 cm3 and severe dysuria (irritative and obstructive symptoms). According to uroflowmetry, the average urine flow rate was 6.2 cm/s
The veins of the PS were visualized by TRUS in all patients. The maximum diameter of the PS veins on the right is from 2.3 to 14.1 mm, with an average of 7.1 mm. The maximum diameter of the PS veins on the left is from 5.0 to 17.1 mm, with an average of 9.7 mm. Dopplerography of pancreatic vessels during the Valsalva maneuver revealed an increase in the velocity of blood flow through the veins from 5 to 15 cm/s. Thus, in all patients, bilateral dilatation of the veins of the PV was detected, which indicated the bilateral nature of the varicocele. The predominance of left-sided varicocele over right-sided varicocele was positively correlated with the predominance of the maximum diameter of the PS veins on the left over the maximum diameter of the veins on the right.
MRI of the inferior vena cava and pelvic vessels and verification of iliac venous compression were performed in 110 patients. Analysis of the data obtained allowed us to identify 4 stages of development (or forms) of May–Turner syndrome (Fig. 3).
Rice. 3. Stages of May–Turner syndrome according to magnetic resonance imaging of the inferior vena cava and pelvic vessels with 3D reconstruction: I – compression of the left common iliac vein; II – compression of the left common iliac vein with its dilatation; III – compression of the left iliac vein with closure of the walls of the vessel in its central part and with its dilatation; IV – pronounced and extended narrowing of the lumen of the left common iliac vein
Arteriovenous conflict was also diagnosed using the values of aortomesenteric angle, aortomesenteric distance, lower angle of lumbar lordosis and iliac vein tunnel diameter (Table 3).
Table 3. Indicators of arteriovenous conflict according to magnetic resonance imaging of veins, n = 110
| Index | Minimum | Maximum | Average | Norm |
| Aortomesenteric angle, degrees | 9,6 | 120,6 | 43,6 | 28–65 |
| Aortomesenteric distance, mm | 2,5 | 49,2 | 13,7 | 10–34 |
| Lower angle of lumbar lordosis, degrees | 112,2 | 134,3 | 123,9 | 134,33– 136,76 |
| Diameter of the iliac vein tunnel, mm | 0,9 | 5,1 | 2,5 | 4,18– 4,50 |
According to venous MRI, aortomesenteric compression (aortomesenteric tweezer syndrome, nutcracker syndrome) in combination with May–Turner syndrome was diagnosed in 32 (29.1%) patients. Retroaortic left renal vein (posterior nutcracker syndrome) in combination with May–Turner syndrome was identified in 4 (3.6%) cases. Compression of the iliac vein without combination with aortomesenteric compression was observed in 74 (67.3%) patients. Central proximal arteriovenous conflict was found in 4 patients, central distal – in 7, left proximal – in 90, left distal – in 43, right proximal – in 3, right distal – in 5 patients. Various combinations of arteriovenous conflicts of the iliac vessels were identified in 43 (39.1%) cases. Analysis of the data obtained allowed us to note that one or another conflict was diagnosed in 70 (63.6%) patients, and their combined forms – in 40 (36.4%) (Table 4).
Table 4. Prevalence of different types of arteriovenous ileal conflicts, n = 110
| Conflict type | Number of cases | |
| abs. | % | |
| Left proximal, or May–Turner syndrome | 56 | 51 |
| Left distal | 6 | 5 |
| Left proximal + left distal | 32 | 29 |
| Left proximal + right proximal | 2 | 2 |
| Left proximal + right distal | 1 | 1 |
| Central proximal | 3 | 3 |
| Central distal | 2 | 2 |
| Central distal + left distal | 4 | 3 |
| Right distal | 3 | 3 |
| Central distal + left distal + right distal | 1 | 1 |
| Total | 110 | 100 |
Complaints of insufficient erection were made by 78 (70.9%) of 110 patients. Dopplerography of penile vessels at rest and during pharmacologically induced erection was performed in 48 patients. In 40 cases, venogenic erectile dysfunction was verified. Dynamic pharmacocavernosography was performed in 22 patients; pathological venous drainage was found in 18: proximal type - in 10, distal - in 2, mixed - in 6.
Phlebography as a complex angiographic study to identify arteriovenous conflicts of the upper (aortomesenteric forceps syndrome) and lower (May–Turner syndrome) levels was performed in 41 patients. It included renocavagraphy and ileocavagraphy with phlebotonometry. In all cases, May–Turner syndrome was identified, and in 12 cases it was combined with aortomesenteric forceps syndrome.
Analysis of the data obtained from venography of the ileocaval segment allowed us to distinguish 4 stages of May–Turner syndrome depending on the presence and severity of collateral circulation (Fig. 4).
Rice. 4. Stages of May–Turner syndrome according to ileocavagraphy, depending on the presence and severity of collateral circulation: I – lack of contrasting of the pelvic veins; II – contrasting of pelvic veins; III – contrasting of the pelvic veins with contrast flow into the contralateral right common iliac vein; IV – contrasting of the pelvic veins with contrast flow into the right common iliac vein and the ascending lumbar veins on the left
We came to the conclusion that an increase in pressure in the common iliac vein during the Valsalva maneuver by more than 3 times may indicate the presence of iliac venous compression (Table 5).
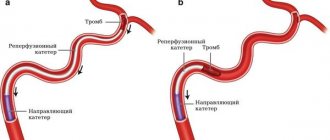
Surgical treatment of iliac venous compression syndrome was carried out in 26 patients. Considering that fibrous adhesions in the compressed iliac vein occur in most cases, being an inevitable pathogenetic link of this disease, we considered angioplasty before stenting mandatory (Fig. 5). For balloon angioplasty, we most often used Atlas Gold PTA Dilatation Catheters (Bard Peripheral Vascular).
Rice. 5. Balloon angioplasty (a) and stenting (b) of the left common iliac vein in the surgical treatment of May–Turner syndrome
Table 5. Phlebotonometry results in patients with iliac venous compression, n = 25
| Vein | Pressure, mmHg st | Pressure increase ratio | |
| at rest | with Valsalva maneuver | ||
| Bottom hollow | 5,25 | 10,35 | 1,97 |
| Right common iliac | 6,22 | 15,25 | 2,45 |
| Left common iliac | 8,35 | 26,28 | 3,14 |
From our point of view, balloon angioplasty without other methods is ineffective, and stent implantation is mandatory. For implantation, we used Wallstent-Uni Endoprosthesis venous stents (Boston Scientific) made of Elgiloy alloy (based on nickel, cobalt and chromium). The diameter and length of the stent were selected during the operation. The stent was implanted in such a way that its upper part fits tightly to the walls of the left common iliac vein, and its upper edge does not protrude into the lumen of the inferior vena cava. The installed venous stents are self-expanding. Despite this, in all cases of surgical treatment of iliac venous compression syndrome after stent implantation, we performed postdilatation of the stented area in the proximal and distal sections. After stenting, control venography and phlebotonometry were performed, which confirmed the absence of compression of the left common iliac vein, the absence of collateral circulation and venous iliac hypertension. In most cases, implantation of 1 stent was required, even if the iliac venous compression was combined (for example, left proximal + left distal conflicts). However, in 2 patients, 2 stents were installed: in 1 - in the left common and left external iliac veins, in 1 - in the left and right common iliac veins.
The most pronounced changes in complaints and objective data were observed by the 3rd month after angioplasty and stenting (Fig. 6). We obtained the following average values: IIEF-5 before surgery – 17.4, 3 months after it – 20.9; IPSS 21.7 and 15.3, respectively; NIH-CPSI – 28.4 and 22.3.
3 months after angioplasty and stenting, in all cases, the maximum diameter of the pancreatic veins decreased by 20–70% (on average by 45.6%) according to TRUS.
In 13 patients with isolated iliac compression (without combination with aortomesenteric forceps syndrome), varicocele reduction occurred by the 3rd month after angioplasty and stenting: in all cases, the diameter of the left and right testicular veins in clinostasis at rest became <2 mm.
In 4 patients with a combination of aortomesenteric forceps syndrome and iliac venous compression, 6 months after angioplasty and stenting of the left common iliac vein, bilateral varicocelectomy was performed from the midscrotal approach along the Wesling line with a positive result.
Discussion
BL Coolsaet in 1980, based on the results of an angiographic examination of 67 patients, identified 3 types of left-sided varicocele:
- reflux from the left renal vein into the internal testicular vein (renospermatic type);
- reflux from the left common iliac vein into the extrafunicular veins, which is caused by obstruction of the left common iliac vein into which they flow (ileospermatic type);
- a combination of the first 2 types (mixed type) [29].
Thus, BL Coolsaet was the first to identify the role of iliac venous compression, or, in a narrower sense, May–Turner syndrome, in the development of varicocele. MD Bomalaski et al. in 1993, they first described a varicocele resistant to surgical treatment, which developed as a result of compression of the left common iliac vein. This case demonstrated the need to abandon standard surgical techniques in such cases [30].
A previous analysis of the causes of varicocele recurrences in 14 (6.3%) of 223 patients who underwent surgery through a transscrotal approach along the Wesling line over a 10-year period allowed us to verify May–Turner syndrome in all cases [31]. However, in modern clinical guidelines in Russia, Europe and America there is no indication that the effectiveness of traditional surgical treatment may decrease due to May–Turner syndrome.
There are no conservative treatments for May–Turner syndrome. CA Binkert et al. in 1998, they first published a report of successful angioplasty and stenting of the left common iliac vein for May–Turner syndrome and thrombotic disease in 8 patients (7 women and 1 man) [32]. Since then, for 20 years, radiosurgeons have been providing similar treatment for thrombosis and pulmonary embolism in patients with May–Turner syndrome.
Despite the fact that the role of iliac venous compression in the development of varicocele was determined back in 1980 by BL Coolsaet, who proposed the hemodynamic classification that is currently used by most urologists, until 2021 angioplasty and stenting were not performed in patients with urological and andrological diseases. We performed the world's first stenting of the left common iliac vein in patients with ileospermatic varicocele on March 28, 2017 in Moscow [33]. After 4 months, JR Stern et al. in New York, stenting was performed in a patient with varicocele that developed as a result of May–Turner syndrome [34]. To date, we have performed angioplasty and stenting in 26 patients with May–Turner syndrome, ileospermatic varicocele and varicose veins of the pelvic organs and have obtained positive results.
The management of patients with a combination of aortomesenteric forceps syndrome and iliac venous compression included the following steps:
- phlebography, angioplasty and stenting of the left common iliac vein;
- assessment of the state of scrotal and pelvic venous congestion after 6 months in order to determine indications for surgical treatment of varicocele.
In all cases, a decrease in the intensity of pain and its disappearance were positively correlated with a decrease in the severity of pelvic venous hypervolemia and a decrease in the diameter of the pancreas veins. This observation allows us to consider the problem of chronic pelvic pain and chronic prostatitis from a new angle. Elimination of venous congestion of the pelvic organs as a result of angioplasty and stenting of the left common iliac vein was accompanied by improvement or complete restoration of erection without the use of phosphodiesterase type 5 inhibitors or any other therapy. This allowed us to evaluate venogenic erectile dysfunction as an integral part of the problem of varicose veins of the pelvic organs in men.
Conclusion
This paper presents the first and largest experience in the world of surgical treatment of ileofemoral compression syndrome as a cause of bilateral varicocele (including recurrent) and varicose veins of the pelvic organs in men.
Analysis of the data obtained allowed us to propose 2 new classifications: 1) 4 stages of development of May–Turner syndrome according to MRI of the inferior vena cava and pelvic vessels with 3D reconstruction; 2) 4 stages of compression of the left common iliac vein in May–Turner syndrome, depending on the presence and severity of collateral circulation during phlebography of the ileocaval segment.
Our data indicate that May–Turner syndrome may be a cause of recurrent varicocele. The modern diagnostic algorithm for patients with varicocele is not focused on identifying May–Turner syndrome. Indications for traditional surgical treatment of varicocele in patients with May–Turner syndrome need to be reconsidered.
Until recently, the diagnosis of recurrent varicocele due to ileofemoral compression, in particular May-Turner syndrome, was a “dead end” for urologists and andrologists in terms of treatment. The most popular operation recently according to JL Marmar et al. (subinguinal varicocelectomy) [35] is not able to solve the problem of ileofemoral compression, secondary pelvic venous hypervolemia, which in this case with a high degree of probability becomes recurrent.
Angioplasty and stenting of the iliac veins for arteriovenous conflicts is a highly effective method of treating patients with varicose veins of the pelvic organs in combination with varicocele.
Literature
- Virchow R. Uber die Erweiterung Kleinerer Gefasse. Arch Path Anat 1851;3(3):427–9. DOI: 10.1007/BF01960918.
- McMurrich JP The occurrence of congenital adhesions in the common iliac veins and their relation to thrombosis of the femoral and iliac veins. Am J Med Sci 1908;135:342–5. DOI: 10.1097/00000441-190803000-00004.
- Ehrich WE, Krumbhaar EB A frequent obstructive anomaly of the mouth of the left common iliac vein. Am Heart J 1943;26(6):737–50. DOI: 10.1016/S0002-8703(43)90285–6.
- May R., Thurner J. The cause of the predominantly sinistral occurrence of thrombosis of the pelvic veins. Angiology 1957;8(5):419–27. DOI: 10.1177/000331975700800505. PMID: 13478912.
- Usui N., Muraguchi K., Yamamoto H. et al. . Surgery 1978;40:983.
- Baron HC, Shams J., Wayne M. Iliac vein compression syndrome: a new method of treatment. Am Surg 2000;66(7):653–5. PMID: 10917476.
- Cockett FB, Thomas ML The iliacus compression syndrome. Br J Surg1965;52(10):816–21. PMID: 5828716.
- Cockett FB, Thomas ML, Negus D. Iliac vein compression – its relation to iliofemoral thrombosis and the postthrombotic syndrome. Br Med J 1967;2(5543):14–9. DOI: 10.1136/bmj.2.5543.14. PMID: 6020994.
- Big medical encyclopedia. Under. ed. ON THE. Semashko M.: Soviet Encyclopedia, 1933. T. 26. P. 70. .
- Yakovenko V.V. Venous formations of the testicle, spermatic cord and surgical treatment of varicocele. Abstract of dissertation. ...cand. honey. Sci. Leningrad, 1955. 15 p. .
- Sakamoto H., Ogawa Y. Is varicocele associated with underlying venous abnormalities? Varicocele and the prostatic venous plexus. J Urol 2008;180(4):1427–31. DOI: 10.1016/j. juro.2008.06.048. PMID: 18710746.
- Neimark A.I., Popov I.S., Gazamatov A.V. Features of microcirculation of the prostate gland and gonads in young men suffering from isolated varicocele and varicocele in combination with pelvic congestion. Experimental and Clinical Urology 2013;(2):56–60. .
- Tsukanov A.Yu., Lyashev R.V. Violation of venous blood flow as a cause of chronic abacterial prostatitis (chronic pelvic pain syndrome). Urology 2014;(4):37–42. [Tsukanov A.Yu., Lyashev RV Disorders of venous blood flow as a cause of chronic abacterial prostatitis (chronic pelvic pain syndrome). Urologiya = Urology 2014;(4):37–42. (In Russ.)].
- Kapto A.A., Zhukov O.B. Varicose veins of the pelvis in men (literature review). Andrology and Genital Surgery 2016;17(2):10–9. .
- Kapto A.A. Varicose veins of the prostate gland in patients with varicocele. Experimental and Clinical Urology 2017;(1): 98–103. .
- Wolverson MK, Houttuin E., Heiberg E. et al. High-resolution real-time sonography of scrotal varicocele. AJR Am J Roentgenol 1983;141(4):775–9. DOI: 10.2214/ajr.141.4.775. PMID: 6604430.
- Rifkin MD, Foy PM, Kurtz AB et al. The role of diagnostic ultrasonography in varicocele evaluation. J Ultrasound Med 1983;2(6):271–5. https://doi. org/10.7863/jum.1983.2.6.271. PMID: 6876259.
- Gonda RL Jr, Karo JJ, Forte RA, O'Donnell KT Diagnosis of subclinical varicocele in infertility. AJR Am J Roentgenol 1987;148(1):71–5. https://dx.doi.org/10.2214/ajr.148.1.71. PMID: 3024475.
- Gerscovich EO High-resolution ultrasonography in the diagnosis of scrota1 pathology. I. Normal scrotum and benign disease. J Clin Ultrasound 1993;21(6):355–73. PMID: 8227378.
- Kocakoc E., Serhatlioglu S., Kiris A. et al. Color Doppler sonographic evaluation of inter-relations between diameter, reflux and flow volume of testicular veins in varicocele. Eur J Radiol 2003;47(3):251–6. DOI: https://doi. org/10.1016/S0720-048X(02)00182-1. PMID: 12927671.
- Kapto A.A. Varicose veins of the pelvic organs in men. In the book: Diagnosis and treatment of venogenic erectile dysfunction. Ed. D.G. Kurbatova. M.: Medpraktika-M, 2021. pp. 140–166. [Kapto AA Varicose disease of the small pelvic organs in men. In: Diagnosis and treatment of venogenic erectile dysfunction. Ed. by DG Kurbatov. M.: Medpraktika-M, 2021. Pp. 140–166. (In Russ.)].
- Felton BM, White JM, Racine MA An uncommon case of abdominal pain: superior mesenteric artery syndrome. West J Emerg Med 2012;13(6):501–2. DOI: 10.5811/westjem.2012.6.12762. PMID: 23358897.
- Vulliamy P., Hariharan V., Gutmann J., Mukherjee D. Superior mesenteric artery syndrome and the “nutcracker phenomenon”. BMJ Case Rep 2013;2013. DOI: 10.1136/bcr-2013-008734. PMID: 23524345. 24.
- Ou-Yang L., Lu GM Underlying anatomy and typing diagnosis of maythurner syndrome and clinical significance an observation based on CT. Spine(Phila Pa 1976) 2016;41(21):E1284–91. DOI: 10.1097/BRS.0000000000001765. PMID: 27379417.
- Kapto A.A. Endovascular surgery of the iliac veins for bilateral varicocele and varicose veins of the pelvic organs in men. Urological Journal 2018;8(1):11–7. . DOI: 10.17816/uroved8111-17.
- Rosen RC, Riley A., Wagner G. et al. The international index of erectile function(IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997;49(6):822–30. PMID: 9187685.
- International Prostate Symptom Score (I-PSS). Available at: https://www.urospec. com/uro/Forms/ipss.pdf.
- Litwin MS, McNaughton-Collins M, Fowler FJJ et al. The National Institutes of Health chronic prostatitis symptom index: development and validation of a new outcome measure. J Urol 1999;162(2):369–75. PMID: 10411041.
- Coolsaet BL The varicocele syndrome: venography determining the optimal level for surgical management. J Urol 1980;124(6):833–9. DOI: 10.1016/S0022-5347(17)55688-8. PMID: 7441834.
- Bomalaski MD, Mills JL, Argueso LR et al. Iliac vein compression syndrome: an unusual cause of varicocele. J Vasc Surg. 1993;18(6):1064–8. DOI: 10.1016/0741–5214(93)90564-3. PMID: 8264037.
- Kapto A.A. Transscrotal access along the Wesling line for surgical treatment of varicocele (10 years of experience). Urological Journal 2018;8(Special Issue):53–4. .
- Binkert CA, Schoch E., Stuckmann G. et al. Treatment of pelvic venous spur (May–Thurner syndrome) with self-expanding metallic endoprostheses. Cardiovasc Intervent Radiol 1998;21(1):22–6. DOI:10.1007/ s002709900205. PMID: 9473541.
- Kapto A.A., Vinogradov I.V., Kharpunov V.F., Mamedov R.E. X-ray endovascular angioplasty and stenting in a man with May–Thurner syndrome. In the book: Collection of abstracts of the 12th Congress of the Professional Association of Andrologists of Russia. Sochi, 2021. P. 62. .
- Stern JR, Patel VI, Cafasso DE et al. Left-sided varicocele as a rare presentation of May–Thurner syndrome. Ann Vasc Surg 2017;42:305.e13–6. DOI: 10.1016/j.avsg.2016.12.001. PMID: 28258018.
- Marmar JL, DeBenedictis TJ, Praiss D. The management of varicoceles by microdissection of the spermatic cord at the external inguinal ring. Fertil Steril 1985;43(4):583–8. PMID: 3987926.
The article was published in the journal “Andrology and Genital Surgery”, issue No. 4 2021, pp. 28-38
Magazine
Andrology and genital surgery 2021 No. 4
Comments
To post comments you must log in or register
Angioplasty and stenting
The chronic form of NVC syndrome is more difficult to treat. When the venous outflow is decompensated, it becomes necessary to restore the patency of the vessel. Open operations involving the isolation of the IVC and its replacement with a vascular prosthesis are feasible, but very traumatic and ineffective. The artificial vena cava prosthesis often thromboses again and the complex operation becomes completely useless. With the advent of new composite materials for large-diameter stents, our clinic began to perform endovascular methods for restoring the patency of the vena cava. Angioplasty and IVC stenting are performed by experienced endovascular surgeons at the Innovative Vascular Center. The point of the intervention is to restore the patency of the closed segment of the inferior vena cava with a special conductor, a high-pressure balloon and the installation of a metal frame - a stent.
Diagnosis of superior vena cava syndrome
On the one hand, the diagnosis of superior vena cava syndrome is simple: the appearance is so specific that the diagnosis is made immediately, and at first glance it is enough to look at the patient. If the patient has an anamnesis - a history of oncohematological or oncological disease, when there is data from histological examination. Then they limit themselves to identifying all areas of tumor damage and begin therapy. But in half of the cases, superior vena cava syndrome develops at the onset of the disease, that is, SPVP is the first and only obvious sign of a malignant tumor.
It is necessary to find out what causes the syndrome, and only then treat it. The presence of a malignant tumor is indicated by a morphological examination of a piece of the tumor; chemotherapy and radiation therapy are carried out only if there is morphological confirmation of cancer. Exceptions to this unshakable rule include severe manifestations of the superior vena cava syndrome, in which case treatment is carried out according to vital indications until a cellular analysis is obtained. However, in specialized clinics today it is possible to urgently verify and obtain morphological confirmation of cancer.
A chest x-ray with layered tomography of the mediastinum is always performed, but it is better to do a computed tomography. Research helps to navigate the subsequent diagnosis - where to do a puncture or take a biopsy. If lung cancer is suspected, a sputum test for cancer cells is performed, a biopsy during bronchoscopy, a puncture biopsy of the mediastinal lymph node, an endoscopic examination of the mediastinum is possible, and if malignant lymphoma is suspected, a puncture is taken from the ilium or sternum.
Obtaining histological material begins with a simple diagnostic technique; if unsuccessful, they turn to a more complex one. It’s easy to figure out the diagnosis if there are other visual tumors or enlarged peripheral lymph nodes from which cells can be taken for microscopic examination. Without understanding what malignant process caused the syndrome of compression of the superior vena cava, it is impossible to choose the optimal treatment, although in extremely severe cases, when life is at risk and death is delayed, a wide range of chemotherapy drugs are administered that act on all possible causes of SVVC.
Conservative therapy
The most common treatment option is conservative therapy with anticoagulants. Drugs used for inferior vena cava syndrome include the anticoagulant warfrin or Xarelto. Detralex or phlebodia are used to improve blood flow from the legs. The main means of conservative treatment is the constant wearing of compression tights of compression class 2-3. They need to be changed every 3 months, as they lose their properties with prolonged wear.
Treating the symptoms of inferior vena cava syndrome with medications and compression can reduce chronic venous insufficiency. Considering the technical complexity of surgical treatment, conservative methods are predominant in modern medical practice.
Symptoms of superior vena cava syndrome
Depending on the cause of the syndrome, symptoms may develop slowly or quickly. Fast - for aggressive tumors such as malignant lymphoma and small cell lung cancer. Clinical signs gradually appear with metastases to the lymph nodes of cancer and venous thrombosis. The severity of the signs of superior vena cava syndrome depends on the level at which the vein is partially blocked and the degree of narrowing of its lumen.
At any rate of development of superior vena cava syndrome, there comes a time when it is impossible to do without emergency medical care.
Book a consultation around the clock +7+7+78
Until the middle of the last century, superior vena cava syndrome (SVVC), or to be precise, its compression syndrome, caused only tertiary syphilis, when gummas destroyed the wall of the thoracic aorta with the formation of aneurysmal sacs that compressed the mediastinal organs and the superior vena cava as well. Tertiary syphilis was eradicated with antibiotics, but since the beginning of the twentieth century, smoking has spread, and with it the incidence of lung cancer has increased a thousandfold, which became the main cause of an emergency condition caused by a violation of the blood circulation of a very large vein and the vessels flowing into it.
It is not known exactly how many people are affected by SVPV every year; medical statistics take into account only the etiological cause - lung cancer, but not its complications; however, in recent years, patients with SVPV are increasingly ending up in oncological intensive care due to the life-threatening severity of the condition. The majority of all diagnosed SVPVs are due to advanced lung cancer, with eight out of ten cases caused by a tumor in the right lung. If you look at the morphology, then predominantly venous syndrome initiates small cell lung cancer, less often squamous cell cancer, and very rarely adenocarcinoma. The last two are classified as non-small cell lung cancer.
In second place in terms of the frequency of induction of SVPV are oncohematological diseases - high-grade lymphomas or lymphosarcomas affecting the anterior mediastinum, most often lymphoblastic and diffuse large cell. As a rule, these are very aggressive tumors that grow in just a few days. The syndrome develops with metastases in the mediastinal lymph nodes of any cancer, but more often these are organs whose lymph collectors are located in the mediastinal tissue: the mammary gland, esophagus and stomach. Metastases of testicular germ cell tumors spread from the retroperitoneum to the supraclavicular zones mainly through the lymphatic tract, but they account for a small number of cases of SVST.
Diagnosis of pathology
Activities for diagnosing pathology include:
- X-ray examination;
- Venocavagraphy;
- CT;
- MRI;
- Endoscopy with biopsy.
The doctor needs to determine what caused the pathology. This is important for the selection of treatment methods. So chemotherapy is prescribed only if cancer is confirmed.
Lung cancer: symptoms
Treatment of May-Turner syndrome
The syndrome requires surgical intervention because it is caused by a mechanical cause (i.e., compression). The goal of the operation is to eliminate it through stenting. To do this, a self-expanding stent is delivered to the area affected by the syndrome, with the help of which the narrowed area is opened.
The stent itself will serve as a framework for the affected area and will be able to provide the required level of vein patency. In order to achieve the desired result, special reinforced stents are used, since conventional ones are not able to cope with high pressure. If, in addition to the syndrome, the patient suffers from severe varicose veins, he undergoes microphlebectomy - an operation aimed at removing damaged areas of blood vessels.
You can make an appointment with specialists from the phlebology department of CELT for the treatment of May-Turner syndrome online or by contacting our operators: +7 (495) 788-33-88
Make an appointment through the application or by calling +7 +7 We work every day:
- Monday—Friday: 8.00—20.00
- Saturday: 8.00–18.00
- Sunday is a day off
The nearest metro and MCC stations to the clinic:
- Highway of Enthusiasts or Perovo
- Partisan
- Enthusiast Highway
Driving directions
Our doctors
Drozdov Sergey Alexandrovich
Cardiovascular surgeon, phlebologist, Doctor of Medical Sciences
47 years of experience
Make an appointment
Malakhov Yuri Stanislavovich
Doctor - cardiovascular surgeon, phlebologist, Honored Doctor of the Russian Federation, Doctor of Medical Sciences, doctor of the highest category
Experience 36 years
Make an appointment
Causes of pathology
Pathology can develop due to:
- Compression of the vein of an extravasal nature;
- Spread of cancer cells to neighboring organs;
- Formation of blood clots in a vein;
- Lung oncology (up to 90% of diseases);
- Lymphogranulomatosis;
- Lymphoma;
- Metastasis of the tumor from other organs, for example, from the breast;
- Sarcomas and in a number of other cases.
Benign neoplasms, such as cysts, can also become a source of pathology.
In addition, some diseases, for example, tuberculosis, become the “culprit” for the occurrence of pathology.
Pathology can develop slowly or rapidly, it all depends on the cause that caused it. The symptoms are directly affected by the location of the vein blockage and its degree. Sooner or later, a situation arises in which emergency medical care is needed.