Complexes with this research
Pregnancy planning.
Clinical indicators 6,630 ₽ Composition Examination during pregnancy. 3rd trimester 9,620 ₽ Composition
Male check-up No. 1 39 studies for annual preventive examination 18,570 ₽ Composition
IN OTHER COMPLEXES
- Extended coagulogram RUB 4,150
- Coagulogram RUB 2,020
- Female infertility RUB 16,210
- Examination during pregnancy. 1st trimester 16,690 RUR
- Preventative check-up RUB 11,960
Fibrinogen
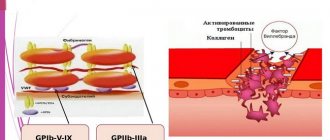
Determination of fibrinogen content in human blood plasma is one of the main tests in the study of hemostasis. Elevated fibrinogen levels have been associated with an increased risk of thrombosis in some epidemiological studies. In addition to its coagulological function, fibrinogen is involved in wound healing and the pathogenesis of malignant neoplasms. Fibrinogen is a euglobulin, an acute phase protein and a glycoprotein with a molecular weight of 340,000 D.
Fibrinogen is the only substrate from which, under the influence of thrombin, the fibrin basis of a blood clot (thrombus) is formed, which ensures complete hemostasis. Fibrinogen is synthesized in the parenchymal cells of the liver and enters the blood.
Fibrinogen defects can be quantitative (hypo- or hyperfibrinogenemia) or qualitative (dysfibrinogenemia). Hereditary dysfibrinogenemia is extremely rare, but the acquired form is more common, especially in liver diseases.
Fibrinogen consists of three pairs of polypeptide chains: two Aa, two Bβ and two ƴ. They are linked together by disulfide bonds in such a way that the N-terminal portions of the 6-polypeptide chains meet to form the central E domain. The C-terminal regions (Aa, Bβ and γ) for the D domain are connected by alpha helical cables to the central E domain to give the characteristic structure of fibrinogen.
Under the influence of thrombin, fibrinogen is converted into fibrin. At the proteolytic stage of this transformation, fibrinopeptides A and B are cleaved from fibrinogen at the arginyl bonds in the N-terminal disulfide unit to form α- and β-chains of fibrin monomers. In the next polymerization phase, the monomers join together to form a fibrin network. At the final stabilization stage, under the influence of FXIII, covalent bonds are formed to form a stable fibrin network. The main mechanism of fibrin self-assembly is the non-covalent intermolecular binding of the complementary centers D1-E1 and D2-E2 of domains D and E.
Principles of methods:
There are a number of methods for determining plasma fibrinogen levels, although in practice most laboratories use the Clauss method.
| Method | Characteristic |
| Clauss method | Functional analysis based on the time of formation of a fibrin clot (see instructions for the Fibrinogen test. The clotting time of diluted* citrated blood plasma is measured by adding excess thrombin**. In this case, the time of formation of a fibrin clot depends only on the concentration of fibrinogen in the plasma, determined by the calibration chart of dilutions of the calibrator plasma with the established fibrinogen content. *Plasma is diluted, usually 10 times, to minimize the effect of “inhibiting substances.” This value depends on the coagulometer used and may change at very low or very high fibrinogen concentrations.* *The use of high activity thrombin ensures that clotting time is independent of thrombin activity over a wide range of fibrinogen concentrations. Most laboratories use an automated method in which clot formation occurs when the optical density of the mixture exceeds a certain threshold. |
| Analysis based on determination of prothrombin time. | In the PT test, a calibration graph is constructed of the dependence of the concentration of fibrinogen in dilutions of standard plasma on the change in optical density recorded by an automatic analyzer when recording clot formation. The concentration of fibrinogen in the test samples is calculated from the calibration graph. Since the method is an indirect measurement of fibrinogen, the choice of standard is very important. |
| Immunological method | The immunological method measures the level of fibrinogen antigen. It is advisable to use it with high levels of fibrinogen, or with congenital dysfibrinogenemia, where there is a discrepancy between functional activity and the level of fibrinogen antigen. |
| Gravimetric method | A method based on measuring the weight of a fibrin clot (according to Rutberg et al.), which is formed by adding either thromboplastin or thrombin and calcium to the plasma. To determine the fibrinogen concentration, the clot is blotted with filter paper, separating plasma and unused reagents, washed, and then weighed. This analysis is outdated, technically complex, time-consuming and not standardized. |
In this cascade diagram of blood coagulation, the factors of hemostasis that are reflected in determining the fibrinogen content using the Clauss method are highlighted in purple:
Reference values
normal range for fibrinogen is usually 2.0-4.0 g/L.
Interpretation of results:
A decrease in fibrinogen levels is observed:
— with DIC syndrome in the consumption stage (usually in combination with prolongation of APTT, PT, TV),
- for liver diseases due to decreased synthesis of blood clotting factors,
- with massive blood transfusions leading to dilution coagulopathy, - after thrombolytic therapy,
- with hereditary deficiencies of fibrinogen - hypo- or afibrinogenemia or dysfibrinogenemia, the latter is often associated with a decrease in fibrinogen levels.
Dysfibrinogenemia may also occur in patients with liver disease associated with abnormal increases in sialic acid. A decrease in fibrinogen levels below 1.0 g/l is a risk factor for bleeding.
An increase in fibrinogen levels is observed:
- in a healthy person, fibrinogen can increase after 60 years of age, during pregnancy, when taking oral contraceptives, during postmenopause,
- among smokers,
- in the intra- and postoperative period,
- for obesity, diabetes mellitus, atherosclerotic vascular lesions,
- with ischemic heart disease, especially with myocardial infarction,
- with DIC syndrome (in the hypercoagulation stage - often in combination with a shortening of APTT, PT, TV, etc.), with vascular thrombosis,
- for oncological, infectious, inflammatory, autoimmune and other conditions.
A high level of fibrinogen in the blood is an independent thrombogenic risk factor. An increase in fibrinogen concentration above 4.0 g/l is a prognostic indicator for the development of myocardial infarction, stroke, and peripheral arterial diseases.
What you need to know about Fibrinogen:
- Fibrinogen levels are usually interpreted in conjunction with other screening tests, such as APTT.
- If there is no obvious reason for the decrease in fibrinogen levels (for example, thrombolytic therapy) and there are certain clinical or anamnestic data (for example, a family history of bleeding diathesis, poor wound healing, umbilical cord bleeding), then to determine the nature of these disorders, use immunological analysis of fibrinogen.
- Fibrinogen blood testing, as with other baseline tests, should not be performed on specimens collected within 4 hours of therapeutic doses of unfractionated heparin (UFH) to avoid false results.
- High concentrations of UFH (>0.8 IU/ml) may lead to an underestimation of the true fibrinogen level.
When preparing this section, data from the scientific website www.practical-hemostasis.com was used.
Detailed description of the study
Fibrinogen, or factor I, is a plasma protein that is produced in the liver and plays an important role in the blood clotting process (hemostasis). Through various interactions, it plays an important role in stopping bleeding.
Fibrinogen is able to dissolve in plasma and serves as a precursor to the insoluble protein fibrin. The process of converting fibrinogen into fibrin, as well as the adhesion (aggregation) of platelets, is carried out with the help of three main enzymes that regulate hemostasis - thrombin, plasmin and factor XIIIa.
As a result, a blood clot is formed, consisting of fibrin strands and an accumulation of platelets. This helps stop bleeding effectively. The fibrin clot also activates the fibrinolytic system. This maintains a balance between blood clotting and protection against blood clots.
The amount of fibrinogen in the body may vary among individuals depending on age and gender. A physiological increase in the concentration of this protein is observed in pregnant women. Its level may also increase with age.
Pathological conditions that change the fibrinogen content in the body are divided into congenital and acquired. The first group of diseases is associated with mutations in genes. Some of them lead to fibrinogen deficiency in the blood, others increase its level. The presence of single mutations in the genes encoding the formation of this protein may not have any symptoms, but increases the risk of thrombosis with unfavorable concomitant factors.
Acquired diseases in which fibrinogen concentration increases primarily include infectious or other diseases accompanied by inflammation. Among them one can also note diabetes mellitus and obesity. Injuries and burns increase fibrinogen production, which is necessary to stop bleeding. An increased content of this protein is observed during pregnancy, as well as when taking estrogen hormones.
A decrease in fibrinogen levels is caused by a violation of its formation in the liver or accelerated destruction in the blood (fibrinolysis). However, in most pathological conditions (acute and chronic inflammation, trauma, burns, surgical interventions), this indicator becomes higher than normal.
Determining the amount of fibrinogen in plasma is necessary in order to assess blood clotting ability and, if necessary, carry out treatment to normalize hemostasis.
COVID-19 is associated with increased blood clotting. Patients with COVID-19 often have elevated levels of D-dimer, high concentrations of which are a predictor of death. Experts from the International Society of Thrombosis and Hemostasis (ISTH) believe that an increase in D-dimer levels by 3-4 times in a patient with COVID-19 is an independent indication for hospitalization.
Patients with COVID-19 are often diagnosed with both obvious thrombotic complications with the identification of large blood clots (not only in the veins and pulmonary arteries, but also in the heart, brain vessels, kidneys, liver), and signs of thrombosis at the microcirculatory level, which is intravital quite difficult to prove. Some researchers suggest that in COVID-19, thrombosis of the microvasculature may underlie damage to many organs, including multiple organ failure. For example, thrombosis of the renal vessels can lead to increasing renal failure, and of the microvasculature of the lungs - to worsening respiratory failure. Interestingly, when the myocardium is damaged by signs of inflammation and interstitial fibrosis, viral particles are not detected directly in the myocardium. Researchers suggest that myocardial damage may develop against a background of hypoxia, microvascular thrombosis, and a systemic inflammatory response.
The mechanism of hypercoagulation in patients with COVID-19 is presumably associated with severe endothelial dysfunction and induction of platelet aggregation (the endothelium carries ACE2 receptors and is a target for the SARS-COV-2 virus). Separate series of studies have also been published in which an increase in titers of antibodies to phospholipids was detected in patients with COVID-19 and massive thrombosis (https://www.nejm.org/doi/full/10.1056/NEJMc2007575), however, such transient changes may be nonspecific nature, since they are often detected during a pronounced inflammatory reaction.
Now the prescription of anticoagulants in a prophylactic dose in hospitalized patients with severe COVID-19 to prevent venous thromboembolism has become almost universal practice; This therapy has been shown to reduce mortality in patients with COVID-19. Clear indications for prescribing anticoagulants have not been determined, and the question of which drugs are best to use has not been fully resolved. Theoretically, it appears that unfractionated heparin, which has its own anti-inflammatory effects, may have some benefits. Moreover, it is believed that it may reduce the binding of viral particles to target cells. On the other hand, the use of UFH requires more frequent nurse visits to the patient (several times a day), which exposes staff to greater risk. Therefore, many clinics in the United States use direct oral anticoagulants to prevent VTE.
However, to date, expert communities recommend the use of low molecular weight heparins in severe patients with COVID-19. Thus, ISTH recommends prescribing LMWH in a prophylactic dose to all patients hospitalized due to COVID-19, even if they are not in intensive care (contraindications - active bleeding, thrombocytopenia <25 -="" 1500="" 8="" p= "">The latest version of the temporary recommendations of the Ministry of Health of the Russian Federation for the treatment of patients with COVID-19 says the following in this regard: "It is recommended to include low molecular weight heparin drugs in the treatment regimens of such patients. The criterion for prescribing drugs may be cumulative changes in the general blood count (thrombocytopenia) and coagulogram (increased D-dimer level, prothrombin time) or the risk of developing coagulopathy, which was stratified according to the sepsis-induced coagulopathy (SIC) scale" (https://static-1.rosminzdrav.ru/system/attachments/attaches/000/ 049/951/original/09042020_%D0%9C%D0%A0_COVID-19_v5.pdf).
In severe patients with COVID-19, the frequency of thrombotic complications remains high even with the use of anticoagulants in a prophylactic dose, so some experts are discussing the possibility of prescribing anticoagulants in a full, therapeutic dose.
Given the lack of an adequate evidence base, the management of anticoagulant therapy in each severe patient with COVID-19 should be discussed individually, taking into account the risk of thrombosis and bleeding.
Experts recommend monitoring indicators that characterize blood coagulation, such as prothrombin time, D-dimer, fibrinogen levels, and platelet count.
Patients with severe COVID-19 often develop thrombocytopenia, but hemorrhagic complications are rare. Laboratory signs of hypocoagulation without bleeding do not require any correction.
If patients develop major bleeding, empirically fresh frozen plasma (FFP) should be used, Ermassa should be used if indicated, then the tactics depend on laboratory parameters:
- if INR>1.5 or increase in aPTT by more than 1.5 times - use FFP
- if fibrinogen is less than 1.5 g/l - cryoprecipitate or fibrinogen concentrate
- if platelets are less than 50 thousand / μl - platelet transfusion
- in the absence of signs of disseminated intravascular coagulation, tranexamic acid can also be used; - recombinant factor VIIa preparations should not be used.
Based on materials:
- ACC/Chinese Cardiovascular Association COVID-19 Webinar 1. https://www.youtube.com/
- Thachil J et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. published: 25 March 25, 2020 (link)
- Hunt B et al. Practical guidance for the prevention of thrombosis and management of coagulopathy and disseminated intravascular coagulation of patients infected with COVID-19. March 25, 2021. Published on
Text: Shakhmatova O.O.