Sofia Kasatskaya “Nature” No. 2, 2016
about the authorSofya Alekseevna Kasatskaya — Junior Researcher, Laboratory of Genomics of Adaptive Immunity, Institute of Bioorganic Chemistry named after. Academicians M. M. Shemyakin and Yu. A. Ovchinnikov RAS. Area of scientific interests: T-cell immunity, neuro- and oncoimmunology. Winner of the “Bio/mol/text” competition 2015 in the category “Best article on immunology.” |
The body’s adequate defense response to the invasion of viruses, bacteria and other pathogens is to destroy the affected cells, preventing the spread of infection and the death of a large number of its own cells. If a cell infected with a virus notices it, innate immune processes are launched: autophagy (utilization of internal cell components using lysosome enzymes) and apoptosis (programmed cell death). However, there are a lot of pathogenic viruses and bacteria, and they are constantly changing beyond recognition. To cope with them, the adaptive immune system and its main participants - lymphocytes - are activated. The pinnacle of the evolution of adaptive immunity was the cytotoxic T-lymphocyte, or killer T-lymphocyte. To recognize a fragment of the virus (antigen) on an infected cell, it uses the T cell receptor
, TCR), randomly and independently assembled on each T cell in the thymus gland. The mechanism of TCR assembly is unique and is characteristic only of the immune system of vertebrates. It is believed that these advantages were first obtained by primitive fish about 500 million years ago, when, as a result of a retroviral infection, genes encoding special proteins (recombinases) responsible for the recombination of TCR genes were introduced into their gametes.
Classical human immunology is based on the study of immune cells in the blood simply because a blood sample can be taken from any patient and examined in normal and pathological conditions. It is on blood cells that the classification of T-lymphocytes was built - division into T-killers and T-helpers, which check the antigenic specificity of T-killers, give them a “license to kill” and are able to control the entire course of the immune response through soluble signaling molecules and cytokines. Later, a group of regulatory T cells was isolated from the T-helper branch, suppressing excess adaptive immunity.
But as yogurt commercials remind us, a significant portion of the immune system's cells are concentrated around the lining of the digestive tract and in other tissues. While there are about 6–15 billion T-lymphocytes in 5–6 liters of blood in an adult, there are 20 billion T-cells in the epidermis and skin [], and another 4 billion in the liver []. Is studying blood samples enough to fully characterize T cell function if there are more T cells in peripheral organs than in the bloodstream? And are classical subpopulations sufficient to describe all the types of T cells found in the human body?
Detailed description of the study
The immune system (immunity) helps protect the human body from various inflammatory (infectious and non-infectious), cancer and other diseases. Lymphocytes are representatives of leukocytes that are responsible for specific immunity - an immune reaction that specifically acts on a specific foreign agent - antigen.
This comprehensive analysis determines the total number of T lymphocytes (T cells) and their main subpopulations, as well as the proportion of activated T cells from the total number.
Under physiological conditions, all T lymphocytes sequentially undergo maturation stages and gradually become mature cells that can participate in immune reactions. When a mature T cell comes into contact with a foreign antigen, an activation process occurs: the T lymphocyte turns into the type that is necessary for the body’s immune defense at the moment.
The following types (subpopulations) of T-lymphocytes are distinguished:
- Cytotoxic (T-killer);
- Helpers;
- Regulatory (T-suppressors).
The function of cytotoxic T-lymphocytes is the destruction of any foreign molecules: microorganisms, toxins, tumor cells, etc. T-helpers enhance the degree of the immune response and participate in the activation of other immune cells (T-killers, B-lymphocytes, monocytes). T-regulatory lymphocytes prevent an excessive immune response and inhibit the activity of T-helper cells.
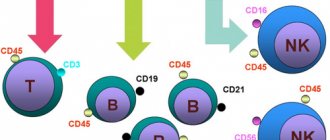
All subpopulations of T cells differ from each other in a set of CD (cluster of differentiation) markers. These are chromosomal markers (antigens) of differentiation, presented mainly on the surface of T cells. Each CD carries not only an identification function, but also a certain biological meaning. The CD3 antigen (CD3+) is present on all mature T lymphocytes and can be used to determine the absolute number of T cells. CD4+ is found on helper T cells, CD8+ on killer T cells.
There are also CD antigens, which indicate not the type of T cell, but its functional state - whether it is activated or not. They are conventionally divided into “early” and “late” CD activations. Early ones include: CD69+, CD25+, etc.; to late: HLA-DR, etc.
The analysis evaluates the total number of activated T-lymphocytes with CD25+ and (separately) activated T-helper cells (CD3+CD4+CD25+) and T-killer cells (CD3+CD8+CD25+).
This study is a comprehensive diagnostic method and, as a rule, complements the results of the immune status test (a comprehensive study of the state of the immune system). The test may be recommended for the evaluation of patients with a potential immunopathological problem in the following groups of diseases:
- Infectious diseases of any location and any nature (bacterial, viral, fungal, parasitic);
- Allergic pathologies with severe course (bronchial asthma, atopic dermatitis, allergic rhinitis, etc.);
- Autoimmune nosologies (rheumatic diseases, autoimmune thyroiditis, inflammatory bowel diseases, etc.);
- Malignant (mostly) and benign (less often) oncological diseases of various origins (hematopoietic system, internal organs associated with immunodeficiency, etc.).
Composition of the complex “Early activation of T-cells and T-regulatory lymphocytes”:
- T lymphocytes;
- T helpers;
- T-regulatory cells (CD4+CD25+CD127neg);
- Leukocyte formula;
- T-activated cells with an early activity marker (CD3+CD25+);
- T-helpers activated with an early activity marker (CD3+CD4+CD25+);
- T-cytotoxic activated cells (CD3+CD4-CD25+).
Life cycle of a T lymphocyte
Each T cell, after TCR assembly, is tested for the functionality of the randomly assembled receptor (positive selection) and for lack of specificity for the body's own antigens, i.e. for the absence of an obvious autoimmune threat (negative selection). The stages of selection occur in the thymus gland; in this case, more than 90% of precursor cells die, failing to correctly assemble the receptor or undergo selective selection. The surviving T cells proliferate and exit the thymus into the bloodstream - these are naïve T lymphocytes that have not yet encountered the antigen. A naive T cell circulates in the blood and periodically enters the lymph nodes, where in the T-cell zone it contacts specialized cells that present it with a foreign antigen.
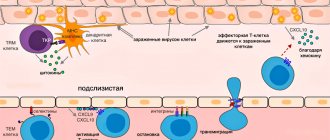
Effector T cell migration into tissue during viral infection []. Inflammatory signals from infected epithelial cells, with the participation of resident cells, are transmitted to the vascular endothelium, whose cells attract effector T cells with the chemokines CXCL9, CXCL10. Rolling:
When moving along the postcapillary venule in the tissue, the effector cell slows down, forming temporary contacts with E-selectins and P-selectins on endothelial cells.
Arrest:
The effector cell attaches tightly to the endothelium when LFA-1 and other alpha integrins interact with ICAM-1/VCAM-1/MAdCAM-1 (on the endothelium).
Transmigration:
effector T cell binds endothelial JAM-1 with molecules PECAM, CD99, LFA-1 and penetrates through endothelial cells into the submucosa
After encountering an antigen in the lymph node, the T cell acquires the ability to divide again - it becomes the precursor of memory T cells ( Stem Cell Memory T cells
, TSCM).
Among its descendants, central memory cells ( Central Memory T cells
, TCM) and effector progenitor cells (
Effector Memory T cells
, TEM) appear, which, when divided, give rise to short-lived effector cells that carry out the immune response (TEMRA cells) []. All these cells leave the lymph node and travel through the blood. The effector cells can then leave the bloodstream to carry out an immune response in the peripheral tissue of the organ where the pathogen resides. What then - another journey through the blood and lymph nodes?
Scheme of transition of descendants of activated T-lymphocytes between populations []. Explanations in the text
Stroma cells, i.e. the bases of the lymph node secrete signaling substances (chemokines) in order to invite the T cell to the lymph node. Lymph node chemokines are recognized by homing receptors CCR7 and CD62L. But both receptors are absent on effector cells. Because of this, it has long been a mystery how effector cells can get from peripheral tissue back to the secondary lymphoid organs - the spleen and lymph nodes.
Difficult choice of effector cell.
To home
is the process of homing, or migration of T cells, for example, to the place most familiar to naive cells - the lymph node. The alternative is not to travel through the body and become a resident tissue cell.
At the same time, data began to accumulate (on differences in TCR repertoires and transcription profiles between TEMs in the blood and in other tissues) that did not fit into the concept of constant migration of T cells between tissues and blood. It was decided to isolate a new subpopulation - resident memory cells ( Resident Memory T cells
, TRM), which inhabit a specific organ and do not recycle [].
Where do tissue resident cells first appear? These are the descendants of effector cells that have lost their ability to recycle. Some tissues peripheral to the immune system, such as the mucosa of the small intestine and the abdominal cavity, allow effector T lymphocytes to penetrate freely, while others allow very limited penetration. A large influx of effector T cells into these tissues is observed only during an inflammatory response. The second type of tissue includes the brain and spinal cord, separated by a barrier from the immune system, as well as many other tissues: peripheral ganglia, mucous membranes of the genitals and intestines, lungs, epidermis, eyes. The difference between the two tissue types is the expression of additional homing molecules for effector T cells, such as the adhesion molecule MadCAM-1 for penetration into the epithelium [].
What else is prescribed with this study?
Activated lymphocytes (T-lymphocytes, T-helpers, T-cytotoxic cells, immunoregulatory index, T-activated, NK- and B-activated cells)
17.54. Ven. blood 3 days
6,500 ₽ Add to cart
Humoral immunity (immunoglobulins IgA, IgM, IgG, IgE, circulating immunocomplexes, complement components C3, C4)
17.51. Ven. blood 8 days
3,890 ₽ Add to cart
Immune status (screening) (Phagocytic activity of leukocytes, cellular immunity, total immunoglobulin IgE, immunoglobulins IgA, IgM, IgG)
27.960. Ven. blood 3 days
7,640 ₽ Add to cart
Immune status expanded
17.61. Ven. blood 14 days
22 310 ₽ Add to cart
Cellular immunity (T-lymphocytes, T-helpers, T-cytotoxic cells, Immunoregulatory index, B-lymphocytes, NK-T cells, NK cells, Leukocyte formula)
17.50. Ven. blood 3 days
5 200 ₽ Add to cart
Killer T cells:
Killer T cells are the most famous subpopulation of lymphocytes. They have the ability to destroy defective cells of the body by coming into direct contact with them. They are also called cytotoxic lymphocytes: “cyto” in translation means “cell”, the meaning of the word “toxic” does not need to be explained.
Killer T cells, which strictly carry out immune surveillance, react aggressively to foreign proteins. They are the ones who cause graft rejection during organ transplantation. For this reason, when a person is transplanted with any organ, he is given special medications for some time that suppress the immune system: they reduce the increased content of lymphocytes and disrupt their interaction. Otherwise, any such operation would end in the rejection of a new organ or tissue, and perhaps even in the death of the patient undergoing such an intervention.
The mechanism of operation of these cells is interesting. Unlike phagocytes, which actively attack, devour and digest foreign particles, T-killers behave at first glance quite restrained. With their appendages they touch the object, and then break contact and “go about their business.” The cell that the lymphocyte touches dies after some time... Why?
The fact is that during their “kiss of death” T-killers leave particles of their membrane on the surface of the cell they destroy. At points of contact, the particles “corrode” the surface of the object of attack. As a result, a through hole is actually formed in a cell doomed to death. It loses potassium ions, sodium ions and water enter it - since the cell barrier is broken, its internal environment begins to communicate directly with the external one... As a result, the cell swells with water that has penetrated inside it, cytoplasmic proteins come out of it, organelles are destroyed... It dies, and then phagocytes approach it and devour its remains. This is the terrible punishment the body prepares for all cells that were recognized by the immune system as “wrong” or foreign.
References
- Litvinova, L.S., Gutsol, A.A., Sokhonevich N.A. and others. Basic surface markers of the functional activity of T-lymphocytes. Medical immunology, 2014. - No. 1.
- Histology (introduction to pathology) / ed. E.G. Ulumbekova, Yu.A. Chelysheva, 1997. - P. 15-17, 532 -546.
- Encyclopedia of clinical laboratory tests / ed. WELL. Titsa. - M.: Labinform, 1997. - P. 214-215.
- Saraiva, D., Jacinto, A., Borralho, P. et al. HLA-DR in Cytotoxic T Lymphocytes Predicts Breast Cancer Patients' Response to Neoadjuvant Chemotherapy. Front Immunol., 2021. - Vol. 9. - P. 2605.
Memory T cells:
Having coped with the next threat, lymphocytes remember it. A special clone of cells is formed in the human body, which store these “memories”. Each clone carries information about a certain type of threat. If some aggressor that the immune system has already encountered enters the body, the corresponding clone multiplies and quickly forms a secondary immune response.
The conversation about the types of lymphocytes and their functions is quite long. Here this topic was presented in the most acceptable and simple form, without loading it with specific terms and unclear names. Let's hope that any reader, even without a medical education, has roughly understood how different types of T-lymphocytes function in his body.
From all this we can draw an obvious conclusion: in order to live a full, healthy life, you must have a strong immune system. It is necessary that processes that many people do not think about, and even more people do not even know, happen as they should.
If nature has not rewarded you with stable immunity, you should think about strengthening it yourself. To do this, you can start taking Transfer Factor. It contains information molecules with the help of which lymphocytes normally communicate with each other, manage and coordinate various processes. Compensating for the lack of natural information molecules, the product is one of the most recommended and effective drugs for normalizing the immune system, improving health and preventing diseases.
Immune cells have memory and transmit information to each other