Arteriovenous malformation is a cerebral vascular developmental anomaly. It is characterized by the formation in some parts of the brain or spinal cord of a vascular tangle, consisting of arteries and veins connecting directly to each other, that is, without the participation of the capillary network.
The disease occurs with a frequency of 2 cases per 100,000 population, and men are more susceptible to it. More often it clinically manifests itself between the ages of 20 and 40, but sometimes it debuts after 50 years.
The main danger of arteriovenous malformation is the risk of intracranial hemorrhage, which can lead to death or cause permanent disability.
Vascular tangle in the brain with arteriovenous malformation
Causes and risk factors
Arteriovenous malformation is a congenital pathology that is not hereditary. Its main reason is negative factors influencing the process of formation and development of the vascular network (in the first trimester of pregnancy):
- intrauterine infections;
- some common diseases (bronchial asthma, chronic glomerulonephritis, diabetes mellitus);
- use of drugs that have a teratogenic effect;
- smoking, alcoholism, drug addiction;
- exposure to ionizing radiation;
- intoxication with salts of heavy metals.
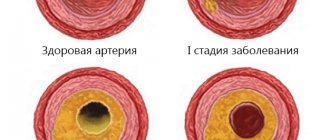
Arteriovenous malformations can be located in any part of the brain or spinal cord. Since there is no capillary network in such vascular formations, blood is discharged directly from the arteries into the veins. This causes the pressure in the veins to increase and their lumen to expand. Arteries with this pathology have an underdeveloped muscle layer and thinned walls. All together increases the risk of developing rupture of an arteriovenous malformation with life-threatening bleeding.
Intracranial hemorrhage associated with rupture of an arteriovenous malformation kills every tenth patient.
The direct discharge of blood from the arteries into the veins, bypassing the capillaries, entails disturbances in breathing and metabolic processes in the brain tissue in the area of localization of the pathological vascular formation, which becomes the cause of chronic local hypoxia.
Arteriovenous malformation: causes, symptoms, diagnosis
Arteriovenous malformation (AVM) is a congenital condition that occurs due to the appearance of abnormal blood discharge through arteriovenous shunts. The pathology is localized mainly in the occipital region. The anatomical structure is typical - several arteries, a glomerular plexus, a vein. The absence of an abnormal capillary bed between the vascular block is accompanied by dilation of the arteries, which determines the characteristics of clinical symptoms. Dysplastic tissue increases the likelihood of intracerebral hemorrhages of the torpid or hemorrhagic type.
Forms of the disease
Arteriovenous malformations are classified by size, location, and hemodynamic activity.
By localization:
- Superficial. The pathological process occurs in the cerebral cortex or in the layer of white matter located directly below it.
- Deep. The vascular conglomerate is located in the subcortical ganglia, in the area of the gyri, in the trunk and (or) ventricles of the brain.
According to the diameter of the ball:
- tiny (less than 1 cm);
- small (from 1 to 2 cm);
- medium (from 2 to 4 cm);
- large (from 4 to 6 cm);
- gigantic (over 6 cm).
Depending on the hemodynamic characteristics, arteriovenous malformations can be active or inactive.
Arteriovenous malformation can be localized in any part of the body, but most often in the vessels of the neck and head
Active vascular formations are easily detected by angiography. In turn, they are divided into fistula and mixed.
Inactive malformations include:
- some types of cavernomas;
- capillary malformations;
- venous malformations.
In what part of the head are brain AVMs located?
A common location for arteriovenous anomaly is the supratentorial space (upper brain) passing over the cerebellar tent. To make it clearer, let's put it simply: a vascular defect in approximately 85% of cases is found in the cerebral hemispheres. Lesions of the vascular units of the parietal, frontal, occipital, and temporal lobes of the cerebral hemispheres predominate.
In general, AVMs can be located in any pole of the brain, both in the superficial parts and in the deep layers (thalamus, etc.). It is possible to reliably determine the exact localization of the lesion only after undergoing a hardware examination with the ability to visualize soft tissues. The basic principles of diagnosis include MRI and angiography. These methods make it possible to qualitatively assess the order of branching of arteries and the construction of veins, their connection with each other, the caliber of the AVM core, afferents of arteries, draining veins.
Symptoms
Arteriovenous malformation is often asymptomatic and discovered accidentally during an examination for another reason.
With a significant size of the pathological vascular formation, it puts pressure on the brain tissue, which leads to the development of cerebral symptoms:
- bursting headache;
- nausea, vomiting;
- general weakness, decreased ability to work.
In some cases, the clinical picture of arteriovenous malformation may also include focal symptoms associated with impaired blood supply to a certain area of the brain.
When the malformation is located in the frontal lobe, the patient is characterized by:
- motor aphasia;
- decreased intelligence;
- proboscis reflex;
- unsure gait;
- convulsive seizures.
For cerebellar localization:
- muscle hypotension;
- horizontal large-scale nystagmus;
- unsteadiness of gait;
- impaired coordination of movements.
For temporal localization:
- seizures;
- narrowing of visual fields, up to complete loss;
- sensory aphasia.
When localized at the base of the brain:
- paralysis;
- visual impairment up to complete blindness in one or both eyes;
- strabismus;
- difficulty moving the eyeballs.
Arteriovenous malformation in the spinal cord is manifested by paresis or paralysis of the limbs, a violation of all types of sensitivity in the limbs.
When the malformation ruptures, hemorrhage occurs in the tissue of the spinal cord or brain, which leads to their death.
The risk of rupture of an arteriovenous malformation is 2–5%. If hemorrhage has already occurred once, the risk of recurrence increases 3-4 times.
Signs of malformation rupture and cerebral hemorrhage:
- sudden sharp headache of high intensity;
- photophobia, visual impairment;
- speech dysfunction;
- nausea, repeated vomiting that does not bring relief;
- paralysis;
- loss of consciousness;
- convulsive seizures.
Rupture of an arteriovenous malformation in the spinal cord leads to sudden paralysis of the limbs.
The concept of arteriovenous malformation - what is it?
The most dangerous manifestation of the nosology is wall rupture. Any increase in intracranial pressure (traumatic brain injury, hypertension) increases the permeability of the arterial wall and deformation of the endometrium.
The frequency of bleeding in patients with essential hypertension is combined with increased blood pressure and crises. The lack of oxygen supply to the brain during hemorrhages is explained by the venous composition.
The chance of an AVM rupture is 15%.
In contrast to an aneurysm, with an arteriovenous malformation, predominantly vasospasm with subarachnoid hemorrhage occurs. The initial narrowing of the arteries is a compensatory reaction aimed at preventing massive hemorrhage.
The first signs of rupture of an arterial malformation:
- Neurological disorders;
- Headache, dizziness;
- Epileptic convulsions.
Initially, intracellular edema develops; tissue swelling contributes to hypoxia. Tissue compression by hematoma and excessive accumulation of cerebrospinal fluid are provoking factors.
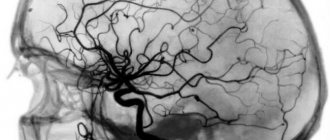
Diagnostics
A neurological examination reveals symptoms characteristic of spinal cord or brain damage, after which patients are referred for angiography and computed tomography or magnetic resonance imaging.
Arteriovenous malformation detected by CT angiography
The disease occurs with a frequency of 2 cases per 100,000 population, and men are more susceptible to it. More often it clinically manifests itself between the ages of 20 and 40, but sometimes it debuts after 50 years.
Incidence (per 100,000 people)
| Men | Women | |||||||||||||
| Age, years | 0-1 | 1-3 | 3-14 | 14-25 | 25-40 | 40-60 | 60 + | 0-1 | 1-3 | 3-14 | 14-25 | 25-40 | 40-60 | 60 + |
| Number of sick people | 0.01 | 0.1 | 0.5 | 15 | 15 | 19 | 7 | 0.01 | 0.1 | 0.5 | 12 | 12 | 16 | 5 |
Treatment
The only method to eliminate arteriovenous malformation and thereby prevent the development of complications is surgical intervention.
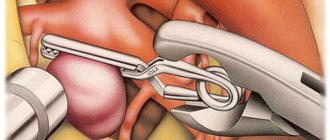
If the malformation is located outside a functionally significant area and its volume does not exceed 100 ml, it is removed using the classic open method. After craniotomy, the surgeon ligates the adductor and efferent vessels of the vascular tangle, then isolates it and removes it.
Classic method of removing arteriovenous malformation
When an arteriovenous malformation is located in the deep structures of the brain or functionally significant areas, it can be difficult to remove it transcranially. In these cases, preference is given to the radiosurgical method. Its main disadvantages:
- the long period of time required for obliteration of the vessels of the malformation;
- low efficiency in removing choroid plexuses whose diameter exceeds 3 cm;
- the need for repeated irradiation sessions.
Another way to remove an arteriovenous malformation is x-ray endovascular embolization of the feeding artery. This method can only be used if there is a blood vessel accessible for catheterization. Its disadvantages are the need for step-by-step treatment and low efficiency. Statistics show that X-ray endovascular embolization allows for complete embolization of malformation vessels only in 30–50% of cases.
Currently, most neurosurgeons prefer combined removal of arteriovenous malformations. For example, if their size is significant, X-ray endovascular embolization is first used, and after the vascular conglomerate has been reduced in size, its transcranial removal is carried out.
Treatment methods for cerebral vascular malformations
Therapy consists of complete resection or complete surgical obliteration of the vascular defect. There are 3 types of high-tech operations that are used for these purposes: endovascular treatment, stereotactic radiosurgery, microsurgical intervention.
- Endovascular surgery
. The method is suitable for the treatment of deeply located and large formations. The intervention is performed under X-ray control, anesthesia is provided by general anesthesia. This minimally invasive tactic is often the initial stage of treatment before the upcoming open surgery.
- A thin catheter tube is brought to the pathological part of the brain through the femoral artery through the vessels.
- Through an installed guide, a special adhesive biomaterial, similar to polyurethane foam, is supplied to the area of the malformation.
- The neurosurgeon uses a foam composition to cover the affected areas, that is, thromboses the abnormally developed vessels while maintaining healthy ones.
- Embolization allows you to “turn off” the pathological plexus from the general cerebral circulatory system.
- After the operation, the patient is usually under inpatient observation for 1-5 days.
- Stereotactic radiosurgery.
The therapeutic tactics, although related to angioneurosurgery, are not traumatic. This means that there will be no incisions or insertion of intravascular probes at all. Suitable for the treatment of small vascular defects (up to 3.5 cm) or in cases where the lesion is located in an inoperable section of the brain.
- Radiosurgery involves the destruction of angiomas using systems such as CyberKnife or Gamma Knife.
- The devices operate on the principle of targeted exposure to radiation to an anomaly.
- The rays are emitted from different directions and converge at one point only in the defective zone; healthy structures are not affected. As a result, the AVM vessels grow together and the focus is suppressed.
- Cyber or Gamma Knife procedures are absolutely painless, and the patient remains conscious during treatment. The devices, on the couch of which you just need to lie motionless (from 30 minutes to 1.5 hours), resemble traditional tomographs.
- When treating with a Gamma Knife, a special helmet is put on the head and firmly fixed. To ensure that the patient receiving the helmet does not experience any discomfort, superficial local anesthesia is given to individual areas of the head. CyberKnife surgery does not require anesthesia or placing the head in a rigid structure.
- There is no need for hospitalization. But it may be necessary to undergo more than one radiosurgery session in order to finally eliminate the residual effects of AVM of the brain. Sometimes the obliteration process lasts 2-4 years.
- Direct microsurgical removal.
Microsurgery for this diagnosis is the only method that gives the highest chance of radical cure of the pathology, minimizing the risk of relapse. It is the “gold standard” in the treatment of this disease with superficial localization and compact forms of the node.
- A microsurgical operation is not complete without a typical craniotomy; an economical opening of the skull is necessarily performed to carry out basic surgical manipulations on the brain.
- The intervention takes place under general endotracheal anesthesia, under the control of a high-power intraoperative microscope and ultrasound equipment.
- To prevent blood discharge through the supplying arterial vessel and vein, the bipolar coagulation method is used, that is, cauterization is performed.
- Next, in a single block through the trepanation window, a single-stage excision of the entire body of the malformation is performed with minimal blood loss.
- At the end of the surgical session, the hole in the skull is closed with a bone flap, and a suture is placed on the skin.
- Discharge is possible approximately 14 days after surgery. Next, you need to continue postoperative recovery in a specialized rehabilitation center. The duration of rehabilitation is determined individually.
A video of the open operation can be viewed at the link: https://www.youtube.com/watch?v=WA2FTX1NK1Y
In certain situations, it is impossible to immediately proceed with direct microsurgery due to high intraoperative risks, especially with large AVMs. Or another option: the angioma after stereotaxy or catheter embolization is only partially compensated, which is extremely bad. Therefore, sometimes it is advisable to resort to staged treatment, using a sequential combination of several angioneurosurgical methods.
Anatomy and types of pathology of the great cerebral vein
The vein of Galen is called the great vein of the brain, since it is one of the largest venous trunks, carrying blood saturated with carbon dioxide and metabolic products from the internal parts of the brain - the subcortical nuclei, visual thalamus, transparent septum, plexus of the lateral ventricles.
The great cerebral vein belongs to the deep venous system and runs in the subarachnoid space (it is also called the cistern of the vein of the same name in this area), connecting with the inferior sagittal venous sinus to form the straight sinus. The large vein of Galen flows into the straight sinus.
The anatomy of the vein of Galen depends on the shape of the head. Its length is about 12-14 mm, while in people with a dolichocephalic type of skull (with a long and narrow head) it can reach 2 centimeters, and in brachycephalics with a short and wide head - a little more than a centimeter.
The diameter of the vessel is not related to the shape of the skull and averages 5-7 mm, however, it has been noted that brachycephals have a relatively short but wider trunk than dolichocephals with a long and narrower vein.
The normal speed of blood flow in the vein of Galen in children under one year of age is 4-18 cm/sec.
Among the changes that are most often detected in the vessel in question are:
- Aneurysm of the vein of Galen;
- Arteriovenous malformation of the great cerebral vein.
Why does it occur?
The issue of genetic predisposition to this pathology has been discussed for a long time, but no reliable evidence has been found. It is a congenital disease. The processes of vascular disruption that lead to this pathology occur in the first or second month of intrauterine fetal formation. Currently, predisposing factors are considered:
- uncontrolled use of medications, especially those affecting the fetus (with a teratogenic effect);
- past illnesses that occur in the first trimester of pregnancy, of a viral or bacterial nature;
- rubella disease during pregnancy;
- smoking and drinking alcohol during pregnancy. Not all girls, even when “in position”, give up these habits;
- ionizing radiation;
- pathology of the uterus;
- severe poisoning with chemicals or other toxic agents;
- intrauterine fetal injuries;
- exacerbation of a chronic pathology in a pregnant woman (diabetes mellitus, glomerulonephritis, bronchial asthma, etc.)
As a result, the vascular bundles connect incorrectly, become intertwined, and arteriovenous malformation occurs. When it forms to a significant size, cardiac output increases, the walls of the veins hypertrophy (compensatory enlargement), and the formation takes on the image of a large pulsating “tumor.”
Arteriovenous malformations of the vein of Galen (AVMvG) are a special type of arteriovenous malformations (AVMs), characteristic mainly of pediatric patients. AVMvH account for approximately 30% of AVMs detected in childhood. The pathology is congenital and is characterized by the formation of an arteriovenous shunt in the area of one of the main venous collectors - the great cerebral vein (vein of Galen) [3-5, 7, 9, 11].
The main clinical manifestations of AVMvH are: 1) hypertensive-hydrocephalic syndrome, which develops as a result of occlusion of the cerebral aqueduct, impaired cerebrospinal fluid resorption; 2) focal neurological symptoms caused by secondary disorders of cerebral circulation of the ischemic type; 3) delayed psychomotor development of a child of a younger age group [5-7, 10, 12].
At the first stages of the development of vascular neurosurgery, treatment of AVMvH was palliative and limited to shunt operations for occlusive hydrocephalus, which did not solve the problem of the underlying disease. In cases of the natural course of the disease, i.e. without medical support, up to 90-96% of children died during the 1st year of life [14].
In connection with the modernization and fundamental changes in approaches to the treatment of AVMvH, the issue of early diagnosis of this pathology is of particular relevance. Currently, the standard for diagnosing AVMvH is ultrasound examination, which makes it possible to establish an accurate diagnosis already in the third trimester of pregnancy [1, 2, 15].
Attempts at open interventions for AVMvH, carried out before the era of endovascular surgery, were associated with high postoperative mortality, reaching 60%. Currently, this option of open interventions is performed only for thrombosed vein of Galen, which has a volumetric effect on the midbrain [8].
The question of the need, timing, and clear indications for performing CSF shunt operations as part of the complex treatment of this pathology remains debatable; This refers to the combination and sequence of endovascular and liquor shunt operations [16].
The small amount of data in the world and domestic literature and the inconsistency in approaches to complex treatment became the reason for conducting this study, the purpose of which is to develop an optimal algorithm for the diagnosis and treatment of patients with AVMvH.
Material and methods
The work is based on an analysis of the results of examination and treatment of 90 patients with AVMvH at the Research Institute of Neurosurgery named after. acad. N.N. Burdenko RAMS for the period from 1987 to 2009. Over the entire analyzed period, there was a gradual annual increase in the number of patients with AVMvH, mainly children of the younger age group, which is associated with improved early diagnosis and expanded opportunities for effective treatment of this pathology. The largest number of observations (38) occurred in the period from 2006 to 2009. Patients examined and operated on during this period constituted a prospective group.
The age of the patients ranged from 3 weeks to 38 years. All patients (27 (30%) female and 63 (70%) male) were divided into four age groups: group 1 (≤1 year) - 35 (39%), group 2 (1- 3 years old) - 15 (17%), 3rd group (3-10 years old) - 19 (21%), 4th group (over 10 years old) - 21 (23%), including 11 aged up to 16 years old, 10 - adults. The average age of patients under 16 years was 3.5 years, for adults - 22.9 years.
The examination of patients included the collection of anamnesis and general clinical data (term and method of diagnosis, the presence of hydrocephalus, delayed psychomotor and speech development, epileptic seizures, a history of hemorrhages, cerebral symptoms), indicators of physical development (weight, height, head circumference), ophthalmological, otoneurological , cardiac examination (echocardiography), CT and MRI of the brain at the pre- and postoperative stages. Angiographic examination (AG) was performed in all 90 patients; in most cases, 75 (83.3%) were performed directly during endovascular surgery. The division of AVMvH into angioarchitectonic types - mural and choroidal - was carried out according to total selective cerebral angiography in accordance with the classification of P. Lasjaunias et al. [10].
Anesthesia for all operations in patients of younger age groups (younger than 10 years) was carried out under endotracheal anesthesia and constant monitoring of ECG, blood pressure, partial pressure of CO2 and respiratory rate. In patients older than 10 years, intravenous sedation or neuroleptanalgesia was most often used.
Of the 90 patients, 78 (86.7%) used various treatment options for AVMvH. In 12 (13.3%) patients, the tactics of dynamic observation were chosen. In this group, 3 patients underwent only cerebrospinal fluid shunt surgery.
Endovascular treatment was performed in 75 (94.9%) patients out of 78 operated on. They performed a total of 131 endovascular operations. In the vast majority of cases - 129 (98.5%) - operations were performed using transarterial access; in a few cases - 3 (2.3%) transvenous access was used. The tactics of endovascular treatment depended on the type of AVM, the caliber of afferents and the age of the patient.
Microsurgical treatment of AVMvH was performed in 4 (5%) patients. The purpose of direct surgical interventions was the excision of the thrombosed ampulla of the vein of Galen after endovascular occlusion or after spontaneous thrombosis of the dilated vein of Galen, which had a local volumetric effect on the adjacent brain structures, in combination with occlusive hydrocephalus.
Radiosurgical treatment was performed in 8 (10.1%) patients.
CSF shunt operations were performed in 24 (26.7%) patients. In 20 patients, ventriculoperitoneostomy was performed, in 4 cases endoscopic perforation of the floor of the third ventricle was performed. In 3 cases, endoscopic ventriculostomy of the third ventricle and ventriculoperitoneostomy were performed sequentially.
The natural history group included 12 (13.3%) patients. In 9 cases there was a functioning AVMvH; in 3 (3.3%) patients, according to hypertension, spontaneous thrombosis of the AVMvH was detected.
The dynamics of the disease picture was assessed on the basis of clinical and radiological examination of patients in the immediate postoperative period, as well as 3 and 6 months after endovascular surgery, depending on the age, dynamics of psycho-speech and motor development of the child in a prospective group of patients. In the retrospective group, the analysis was carried out using records of control examinations of patients in the clinic of the Institute of Neurosurgery.
Clinical picture and angioarchitecture
The primary diagnosis of AVMvH was established on the basis of fetal ultrasound in utero in 22 (22.2%) patients, in newborns - according to neurosonography - in 16 (17.8%), CT - in 23 (25.5%), MRI - in 30 (33.3%), hypertension - in 1 (1.1%).
The leading clinical picture in children of all age groups with AVMvH was hydrocephalic syndrome; in the group of children aged 1 to 3 years, it was detected in 80% of cases. Hydrocephalus was the result of hyporesorption of cerebrospinal fluid due to impaired venous circulation, or was of an occlusive nature due to compression of the cerebral aqueduct by the dilated ampulla of the vein of Galen. An increase in head circumference occurred mainly in patients of the younger age group - up to 3 years (60%). Among patients over 10 years of age (47%) who were diagnosed with hydrocephalus, according to CT data, craniomegaly was found in 4% of patients. In group 1, hydrocephalus was detected in 59% of children under 6 months of age, and in 75% of children aged 6 months to 1 year (Table 1).
Other symptoms were less common: symptomatic epilepsy - in 19 (21.1%), dysfunction of cranial nerves (II, III, VI, VII) - in 19 (21.1%) and intracerebral hemorrhages - in 8.9% . Congenital heart defects were observed in 34.4% of patients, of which 3 underwent surgical treatment for this pathology.
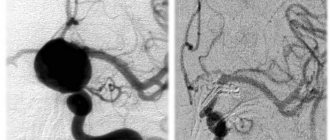
According to angiography, AVMvH of the choroidal type was detected in 67 (74.4%) patients, the mural type of AVM was verified in 20 (22.2%) patients (Fig. 1, a, b).
Figure 1. Angiogram of patients with AVMvH. a — choroidal type; b - mural type.
In 3 (3.3%) patients, spontaneous thrombosis occurred at the time of AG.
The afferents of AVMvH were most often the arteries of the vertebrobasilar system, less often - the arteries of the carotid system. In most cases, adjacent blood supply was detected (Table 2).
Results and its discussion
Results of endovascular treatment
The main goal of endovascular intervention was to stop or reduce the discharge of arterial blood into the cerebral venous system, which was achieved mainly through occlusion of afferent vessels using transarterial access. Only 3 patients had occlusion of the great cerebral vein due to the complete failure of transarterial embolization for the choroidal type of AVM. To occlude the afferents of AVMs of the mural type, microcoils and balloon catheters were used; for the choroidal type, microcoils, adhesive compositions, or a combination thereof were used.
Microspirals were used as occluding materials in 45 (60%) cases, detachable balloon catheters were used in 21 (28%) patients, adhesive compositions were used in 19 (24.9%) cases, including ONYX composition in 2 cases ( MTI, EV3, USA). In some observations - 10 (13.3%) a combination of different occlusive materials was used.
According to control cerebral hypertension, complete cessation of arterial inflow was achieved in the mural type of AVMvH in 61% of cases, while in choroidal type malformations this figure did not exceed 7%.
Complications after endovascular operations were noted in 10 (16%) cases. Of these, clinically significant complications - persistent neurological deficit caused by ischemic cerebral circulatory disorders in the choroidal arteries - occurred in 6 (10.7%). In 3 patients, intraventricular hemorrhage developed, in 1, a retroperitoneal hematoma was detected after endovascular surgery.
Death in the early period after endovascular surgery occurred in 2 (2.7%) cases. In one case, the cause of death was massive intraventricular hemorrhage, in the second - acutely developed occlusion at the level of the Sylvian aqueduct. Despite urgent external drainage of the lateral ventricles, the child died from axial herniation of the brain stem.
When assessing the dynamics of clinical symptoms 3 and 6 months after endovascular treatment, approximately the same results were obtained in all age groups; in the vast majority of observations, a positive result was noted (Fig. 2).
Figure 2. Dynamics of the clinical picture in different age groups 1 year after endovascular treatment.
In the long-term period (follow-up from 1 to 15 years) after endovascular treatment, improvement occurred in all age groups, the best result (82.8%) was observed in the group of patients under 1 year (Fig. 3).
Figure 3. Disease outcome in different age groups after endovascular treatment.
The “recovery” group included patients who had no clinical symptoms of the disease, and neuroimaging data did not reveal signs of functioning AVMvH and hydrocephalus (Fig. 4).
Figure 4. AVMvH of mural type. Endovascular occlusion of afferents by microspirals. Patient M. 4 months of age. a — MRI before surgery; b — initial hypertension of the vertebral artery; c — MRI 1 year after surgery; d — control hypertension of the vertebral artery. A comparative analysis of the outcome of the disease in different age groups found that improvement occurred in all age groups. The rate of recovered patients is significantly higher in groups 1-2 compared to groups 2-3. These trends reflect the degree of influence of endovascular operations on the clinical course of the disease. Thus, even a partial decrease in the degree of arteriovenous discharge creates conditions for improving cerebral hemodynamics, as a result of which the clinical status of patients improves (Fig. 5).
Figure 5. Choroidal type AVMvH. Occlusion of AVMvH afferents with microspirals (2 stages). Patient G. 3 months of life. a — MRI before surgery; b — initial hypertension of the vertebral artery; c — MRI 2 years after the 2nd stage of the operation; d — control hypertension of the vertebral artery. Total switching off of the pathological arteriovenous shunt (more often with mural-type AVMvH or with continued thrombosis after embolization of choroidal-type malformations) creates conditions for complete normalization of cerebral blood flow and leads to recovery of patients. Moreover, the earlier the endovascular operation is performed, the more obvious the overall positive effect. In addition, it is technically easier to perform endovascular interventions at an earlier age, when there is still no pronounced hypertrophy of afferent vessels and the volume of arteriovenous shunting is relatively low.
The probability of an increase in neurological symptoms is greatest in group 2 - 23.1%, and in the group of patients under 1 year of age the negative result was minimal - 3.2%.
The overall mortality rate among all 90 patients with AVMvH was 6.7%. In the early period after endovascular surgery, 2 (2.7%) patients died. In the long-term period, after partial occlusion of the afferents of the malformation, 2 (2.7%) patients died. Among patients who did not receive any treatment for AVMvH, 2 (16.7%) patients died from intraventricular hemorrhage.
Results of microsurgical and radiosurgical treatment
Microsurgical total excision of the thrombosed vein of Galen was performed in 4 patients. 3 of them experienced disability with the development of severe pyramidal symptoms.
Radiosurgical treatment was performed in 8 patients over 5 years of age with choroidal AVMvH. In 7 (87.5%) cases, radiation therapy was preceded by endovascular partial occlusion of accessible AVM afferents. When analyzing the long-term results of radiosurgical treatment, stabilization in the clinical and radiological picture of the disease was revealed in the majority of patients. In 2 patients, an increase in symptoms in the neurological status was noted. In 7 cases, according to control CT and MRI data, performed at different times after irradiation, the x-ray picture remained unchanged. Only in 1 observation during control hypertension was complete thrombosis of the AVM detected. However, this patient, a year after proton irradiation, developed symptoms of damage to subcortical structures, hemiparesis, paresis of the third nerve on the left, and emotional and personal disorders caused by the development of radiation necrosis.
Results in the natural history group
The natural course of the disease group included patients with spontaneous thrombosis of AVMvH, patients who at the time of treatment did not have the technical capabilities to perform endovascular surgery, as well as patients who refused surgical intervention, a total of 12 (13.3%) observations. When assessing the neurological status after 1 year of observation, stabilization of the clinical symptoms of the disease was noted in 8 (66.7%) cases. In 4 (33.3%) there was an improvement in neurological status. It should be noted that in 3 of these 4 patients spontaneous thrombosis of AVMvH was detected according to hypertension.
When analyzing the frequency of cases of stabilization and improvement in the groups of endovascular treatment and the natural course after 1 year, a statistically significant (p < 0.05) increase in positive results was shown in the group of patients who underwent endovascular treatment.
Evaluation of long-term results in the group with the natural course of the disease revealed a high mortality rate - 16.7% (2 observations) - in comparison with the endovascular treatment group (5.3%). Recovery occurred only in 3 patients with spontaneously thrombosed malformation; in the remaining 5 (41.7%) cases, the clinical status remained relatively stable.
Results of treatment of AVMvH against the background of liquor shunt operations
CSF shunt operations were performed in 24 patients. In 9 cases, shunt surgery was performed before endovascular treatment, in 13 patients - after partial occlusion of AVMvH, in 2 cases - with spontaneously thrombosed malformation. In no case was there any intracranial hemorrhage during CSF shunt surgery.
Complications after bypass operations developed in 9 (37.5%) patients. They were not specific in nature and were associated with inflammatory changes, as well as hyperdrainage syndrome and the development of subdural accumulations of cerebrospinal fluid.
Thus, AVMvH has a complex angioarchitecture, which is largely individual for each patient. There is no doubt that it is on the basis of a thorough study of angioarchitecture that adequate planning of all stages of surgical treatment of patients with AVMvH is possible.
Summarizing the results of our work, it should be noted that the most effective method of treating AVMvH is the endovascular method. The high percentage of satisfactory results obtained indicates the correct selection of optimal types of treatment depending on the anatomical type of AVMvH and the age of the patient.
A wait-and-see approach for asymptomatic AVMvH is unjustified, since successful endovascular treatment in the first year of life, before the onset of irreversible neurological disorders, allows one to achieve good clinical results. The natural history of AVMvH is associated with a higher risk of intracranial hemorrhage.
With mural-type AVMvH, endovascular treatment can achieve complete recovery of the patient, while with choroidal-type AVMvH, by occlusion of the largest afferents, stabilization of the clinical manifestations of the disease can be achieved by improving cerebral circulation.
Radiation therapy for AVMvH with large afferents and high shunting rates is ineffective. Indications for radiosurgical treatment can be determined in patients with AVMvH of the choroidal type with numerous small-caliber afferents and a relatively low volume of arteriovenous discharge, as well as after partial embolization upon achieving maximum blood flow reduction.
The assumption existing in some literature sources [1, 13] that CSF shunt operations against the background of a functioning AVMvH are associated with a high risk of hemorrhagic complications was not confirmed in our observations. For progressive occlusive hydrocephalus, the method of choice is endoscopic ventriculostomy, for aresorptive hydrocephalus - ventriculoperitoneal shunting.
conclusions
1. Prenatal diagnosis of AVMvH using ultrasonography and fetal MRI makes it possible to establish a diagnosis already in the third trimester of pregnancy, which allows adequate planning of delivery and further treatment of the child.
2. The endovascular method is a highly effective and low-traumatic method of treating AVMvH with a low level of disability and mortality. Microsurgical excision of AVMvH is associated with a high risk of complications and disability for the patient.
3. The optimal age for treatment is 4-5 months. Heart failure is not an absolute contraindication for endovascular surgery, except for decompensated heart failure requiring surgical treatment.
4. The presence of progressive hydrocephalus with corresponding symptoms is an indication for CSF shunt surgery before endovascular treatment.
Review
Treatment of patients with arteriovenous malformations of the vein of Galen (AVMvG) continues to be one of the important problems of modern neurosurgery. Due to its relatively rare occurrence, there is no uniform standard for the treatment of this pathology.
The article provides convincing data in favor of the endovascular method of treating this pathology, based on retrospective and prospective analysis of a large number of patients, even according to global data. At the same time, the authors prove the low effectiveness of radiation therapy and justify performing CSF shunt operations for progressive occlusive and aresorptive hydrocephalus.
The work shows that endovascular treatment of AVMvH in the first year of life makes it possible to achieve complete recovery in a large number of patients, so wait-and-see tactics for this pathology are unjustified.
Undoubtedly, the presented article contains novelty and certain value in solving the problem of treating AVMvH and will be useful for both scientific and practical neurosurgeons.
V.E. Ryabukhin
(Moscow)
Principles of microsurgical removal of AVM
| AVM. View of operations |
Direct surgical interventions are preferably performed in the cold period after hemorrhage, since concomitant brain changes make it difficult to isolate the AVM, which leads to worsening brain injury and is more difficult for patients to tolerate. In the acute period of parenchymal hemorrhage, the tactic of removing large hematomas without attempting to excise the AVM is justified. An operation to simultaneously remove a hematoma and malformation can be performed for small lobar AVMs. It should be remembered that in the presence of a hematoma and cerebral edema, the contrast of the AVM may differ significantly from the true one due to compression of the vessels of the malformation. With deep AVMs, as well as with large AVMs, it is advisable to wait for the hematoma to resolve. In case of ventricular hemorrhage, installation of external ventricular drainage is indicated. Trephination should be performed in such a way that, in addition to the AVM node, it provides visualization of the afferent arteries and main drainage veins of the malformation throughout. When isolating the feeding vessels of the AVM, in order to reduce trauma to the medulla, it is important to use natural grooves and spaces (for example: wide preparation of the lateral fissure when isolating afferents coming from the middle cerebral artery; interhemispheric approaches to control afferents coming from the pericallosal or posterior cerebral arteries, etc.). P.). Switching off the afferent arteries must be done at a minimum distance from the AVM tangle. This is due to the fact that branches from the arteries feeding the AVM can extend to the brain parenchyma. This situation is especially relevant when excision of AVMs of deep and para-trunk localization, when branches supplying blood to functionally important structures may be damaged. To avoid injury to the AVM vessels, its isolation should be carried out as a single block along the perifocal zone. In patients after hemorrhage, one of the poles of the AVM tangle can be clearly demarcated from the brain by an intracerebral hematoma or post-hemorrhagic cyst, which simplifies surgical manipulations. It should be remembered that in some cases the hematoma can fragment the AVM, and therefore revision of the walls of the cavity of the removed AVM is mandatory. Transection of the main drainage veins should be performed after complete isolation of the AVM. The principle of gradual switching off of the arterial inflow as the tangle is released, and the venous inflow upon completion of AVM dissection, is well known. When venous drainage is reduced in conditions of continued arterial inflow, the AVM node becomes tense and bleeding. In certain cases, when there is an imbalance in blood flow, spontaneous ruptures of the thin vessels of the malformation occur, leading to intense bleeding that is extremely difficult to stop. The only way out in such a situation is the rapid isolation of the AVM with the afferents turned off. Since the walls of the malformation arteries, unlike normal vessels, have a thinned muscle layer, the vessels must be coagulated carefully and extensively. Significant intraoperative complications include air embolism. In the postoperative period, deterioration in neurological status was noted in 30% of patients, with a significant regression in the severity of symptoms in most cases. Postoperative mortality was 1.3%. Postoperative visual disorders are quite persistent and practically do not regress. With postoperative speech disorders, in the vast majority of cases, it is possible to achieve a significant regression of symptoms, and in many patients they will completely get rid of the speech defect.