Cerebrovascular diseases are among the most pressing problems of modern medicine, since they have a significant impact on such important demographic indicators as morbidity and mortality, and are also one of the main causes of long-term disability. Every year 10-12 thousand people become disabled in the Republic of Belarus. The incidence of stroke in the republic is 2.3-2.5 cases per 100 thousand, the incidence after 55 years doubles with every decade of life, and there is an increase in the proportion of young and working-age people. Organization of care for patients with stroke is a serious medical and social task and needs further improvement.
The World Stroke Federation, the European Stroke Organization - all point to the need to form a unified anti-stroke program based on a systematic approach. At the same time, the main task is to reduce mortality by reducing morbidity with the development of prevention algorithms, as well as reducing mortality by improving medical care for acute stroke using, among other things, high technologies, developing an individual secondary prevention program for each stroke patient, and organizing early and staged neurorehabilitation.
In this regard, the main directions of the strategy for developing the system of medical care for stroke are:
- Primary prevention;
- Adequate evidence-based treatment in the acute period;
- Rehabilitation;
- Secondary prevention;
Logistics of acute stroke
The logistics of acute stroke implies mandatory hospitalization during the “therapeutic window”, rapid transportation of the patient (“time is the brain!”), making a telephone call by the ambulance team to the hospitalization center, preparing the stroke team to admit the patient to a specialized department, round-the-clock operation of MRI ), ultrasound diagnostics of the great vessels of the head and heart, laboratories. “Time from door to CT, MRI” should not exceed 15 minutes, “time from door to needle” should generally not exceed 40 minutes.
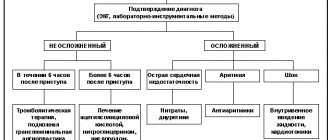
Stages of medical care for patients with acute cerebrovascular accident
Pre-hospital stage
Ambulance and emergency medical aid station team:
- Detects a stroke and determines the period from the onset of the disease, makes an informative call to the hospitalization department;
- Delivers patients with acute stroke in the shortest possible time;
- Provides observation of patients and treatment at the prehospital stage in accordance with the standards of medical care for patients at the prehospital stage, with blood pressure monitoring at the values recommended for maintaining perfusion levels;
- It is possible to use neuroprotectors: glycine 1.0 sublingually, magnesium sulfate 25% 10 ml IV, ethylmethylhydroxypyridine succinate (Mexidol) 200 mg IV, citicoline (Ceraxon) 1000 mg IV;
- It is necessary to avoid the use of aspirin (ASA, CardiASA, thromboASS, aspirinCardio), glucose as a solvent (possible TLT);
- Vasoactive substances and nootropics are not recommended;
- Transport the patient in a horizontal position without lifting the head end to maintain perfusion, but with control of possible aspiration of vomit;
- At the prehospital stage, there are no unconditionally proven effective treatment methods and diagnostic signs that make it possible to absolutely accurately determine the nature of the stroke and carry out early pathogenetic therapy;
- The concept of “time is brain” and the concept of “therapeutic window” mean that stroke care should be urgent while minimizing transport delays.
Hospital stage
A multidisciplinary approach begins already at the diagnostic stage (stroke unit team).
Held:
- Blood pressure measurement;
- ECG (possibly during hospitalization);
- Glucosemetry (if not performed at the emergency stage);
- Installation of a cubital catheter (possible at the prehospital stage);
- Blood collection for laboratory analysis;
- General analysis (platelets!);
- Coagulogram – INR (international normalized ratio), APTT (active partial thromboplastin time);
- Biochemical analysis (urea and creatinine, liver tests) (possible at the prehospital stage).
Organization of the maximum possible implementation of diagnostic procedures at the prehospital level significantly reduces the time “from door to needle,” which determines the greater effectiveness of thrombolytic therapy.
- Analysis of CT, MRI data;
- Ultrasound examination of blood vessels within 3 hours after admission, heart (for cardioembolic stroke) - as indicated.
The neurologist on duty conducts:
- Taking anamnesis;
- Neurological examination (NIHSS);
- Identification of contraindications to thrombolytic therapy (TLT), choice of management tactics.
Consultation with a neurosurgeon is mandatory for hemorrhagic stroke or extensive cerebellar infarction within 60 minutes.
Consultation with a cardiologist is necessary for all patients diagnosed with stroke or suspected TIA.
Introduction
Ischemic stroke (IS) is one of the leading causes of death and disability worldwide.
The wide prevalence and severity of the consequences of this disease make it an acute medical and social problem. In March 2021, during a discussion of the project “Stroke in Focus” by the chief specialist in medical rehabilitation of the Russian Ministry of Health, Professor G.E. Ivanova identified the insufficiently frequent use of thrombolytic therapy (TLT), which in Russia is carried out only in 3.5% of cases, as one of the problems in improving the system of medical care for stroke. The immediate goal is to increase this number to 5%, and in the future, with the advent of new technologies and tools, to 40% [1].
The first experience of using thrombolytic drugs in IS was published in the USA back in 1958 [2]. A study of the effectiveness of TLT using a fibrinolysin-heparin mixture for IS, begun in the Soviet Union in the first half of the 1960s, showed its effectiveness in the first 3-6 hours from the onset of IS. At the same time, the main indications and contraindications for TLT were determined. However, “the widespread clinical implementation of TLT was delayed due to the lack of accurate diagnosis of the nature of cerebral stroke and the significant frequency of hemorrhagic complications” [2]. A new stage in the development of TLT for IS began with the introduction into clinical practice of recombinant tissue plasminogen activator (tPA) in combination with computed tomography and magnetic resonance imaging (CT/MRI diagnostics), which make it possible to accurately determine the nature of the stroke [2]. Currently, the incidence of TLT in Europe and North America is 5–15%. In the Russian Federation, the number of TLT for IS in 2021 exceeded 13,500 procedures, and, according to the Department of Health, in the first 6 months. 2021 in Moscow, 18.7% of patients with IS who were admitted to specialized vascular centers in the first 4.5 hours of the disease received TLT [2].
Researchers evaluate systemic TLT using alteplase as the most effective and safe method of reperfusion in the first 4.5 hours from the onset of IS development [3]. The non-invasive intervention that is intravenous thrombolysis is its main advantage; the disadvantage is the inability to fully control the administration of fibrinolytic [3]. The use of alteplase is called «
the gold standard" TLT (systemic and selective) AI, since this drug has moderate selective activity, has no antigenic properties and an extremely low risk of developing allergic reactions [4].
The mastery of the systemic TLT technique by Sevastopol neurologists began in 2021 and became possible, as was said, thanks to the introduction of CT and MRI into clinical practice, which make it possible to diagnose the nature of the stroke. Creation in 2021 on the basis of the 1st city hospital named after. N.I. Pirogov Regional Vascular Center (RSC) opened up the possibility of wider use of systemic TLT in acute IS, which gradually became common practice in the neurological department for patients with acute cerebrovascular accidents. So, based on the results of 8 months. In 2021, 1226 patients with a diagnosis of IS were admitted to the RSC, of which 217 (16.8%) people were in the therapeutic window. Of these, thrombolysis was performed in 127 (13.6%) patients (with a target rate of 4%). However, both at the development stage and at the present time, questions related to the safety and effectiveness of this method, as well as individual problems of patients that limit its use, remain relevant for us, medical practitioners.
Goal of the work:
Based on personal experience in the use of TLT, try to: 1) systematize the results obtained during thrombolytic procedures, assessing their safety (development/absence of hemorrhagic complications and allergic reactions) and effectiveness, taking into account the gender and age of patients; 2) identify factors limiting the use of TLT.
According to the European Stroke Organization (ESO) Guidelines, 2008
- All patients with suspected TIA or stroke are recommended to undergo CT or MRI examination.
- In patients with TIA, minor stroke and spontaneous regression of symptoms, urgent vascular imaging methods (ultrasound, CT or MR angiography) are recommended.
- In patients with acute stroke and TIA, early clinical evaluation is recommended, including assessment of physiological parameters, as well as routine blood tests.
- A 12-lead ECG is recommended for all patients with TIA and acute stroke.
- For patients with stroke and TIA, Holter ECG monitoring is recommended after the acute period of the disease in the presence of arrhythmia and an unknown type of stroke.
- Echocardiography is recommended only in certain patients
Ischemic stroke of unknown etiology (cryptogenic) requires additional laboratory testing:
- Proteins C and S;
- C-reactive protein;
- Homocysteine;
- Antiphospholipid antibodies.
Confirmation of the diagnosis of stroke and its nature, the choice of treatment tactics is possible only with the availability of neuroimaging methods!
- All patients with suspected transient ischemic attack (TIA) and stroke should be hospitalized.
- Emergency hospitalization should be carried out in a specialized department for acute cerebrovascular accidents in a multidisciplinary hospital (“stroke units”) with specialized multidisciplinary care, neuro- and angiosurgical capabilities, including the provision of high-tech care, which reduces mortality and improves the outcome of the disease.
Antithrombotic treatment for ischemic stroke
A.V. Fonyakin Doctor of Medical Sciences, cardiologist, State Research Institute of Neurology, Russian Academy of Medical Sciences
L.A. Geraskina Candidate of Medical Sciences, neurologist, State Research Institute of Neurology, Russian Academy of Medical Sciences
Stroke is a catastrophic cerebrovascular complication. It is well known that the chance of full functional recovery after a stroke is much lower than after another common cardiovascular disease - myocardial infarction. Experience in the treatment and rehabilitation of patients after a stroke shows that even with perseverance and willpower, recovery is associated with stress, disappointment and exhaustion of the spiritual and physical strength of both the patients themselves and their loved ones. Millions of people take this path every year, although for many patients, full recovery remains a pipe dream. For most of them, the idea of what they previously considered a “normal” life completely changes, and many develop permanent disabilities.
Even without taking into account the funds needed to create and operate full-fledged rehabilitation services, the high number of hospitalizations and the high dependence of many stroke patients on outside care indicate that stroke consumes a very significant share of the health care budget. In addition, patients who have previously suffered ischemic cerebrovascular accident, including transient (transient ischemic attack), and/or minor stroke (with full recovery of impaired functions), are among people at increased risk of recurrent stroke.
One of the central links in the mechanism of ischemic stroke, regardless of the reasons for its development, is a disorder in the blood coagulation system with activation of thrombus formation processes. Thrombosis is the formation of a blood clot (a clot of blood cells and fibrin) and its fixation on the inner surface of a vessel. This interferes with normal blood circulation and leads to ischemia. Thrombosis develops mainly with atherosclerosis of large arteries and damage to small vessels in patients with arterial hypertension. Based on this, antithrombotic therapy for ischemic stroke is recognized as the standard for the prevention of recurrent cerebral ischemic events. To reduce the risk of recurrent stroke and other cardiovascular diseases caused by thrombosis (for example, coronary heart disease), patients with ischemic stroke or transient ischemic attacks are recommended to take long-term use of platelet antiplatelet agents, which block the process of blood clot formation at the very initial stage. They prevent blood cells from sticking together and attaching to the vascular wall. The most common and truly universal remedy from this group is acetylsalicylic acid (ASA, aspirin). If ASA is insufficiently effective, it is advisable to use a combination of ASA and dipyridamole for the same purposes. If ASA is intolerant or if there are special indications, clopidogrel is prescribed.
Quite often, a stroke develops as a result of blockage of a cerebral artery by an embolus (from the Greek embolos - wedge). This is a “broken” fragment of a blood clot that has formed at a distance from the site of brain damage. The source of emboli can be the heart, aorta, and atherosclerotic changes in the large vessels of the head, in particular the carotid arteries. To prevent recurrent cerebrovascular accident, patients at high risk of cardiac embolism should receive anticoagulant therapy.
Long-term treatment with indirect anticoagulants (warfarin, syncumar) is necessary for persistent or paroxysmal atrial fibrillation, acute myocardial infarction complicated by the formation of a left ventricular thrombus, dilated cardiomyopathy, rheumatic damage to the aortic and mitral valve, prosthetic heart valves. However, the use of these anticoagulants may be contraindicated in a number of concomitant diseases (in particular, with peptic ulcer of the stomach and duodenum, the risk of bleeding increases). In these cases, it is safer to prescribe antiplatelet drugs.
However, there are a number of clinical situations when it is necessary to urgently initiate anticoagulant therapy with the least risk of bleeding. First of all, this is an acute stroke with a high risk of recurrence of cerebral and other thromboembolic complications during the first weeks of the disease. In this case, the drugs of choice are direct-acting anticoagulants, which have a direct effect on the activity of coagulation factors circulating in the blood. Direct anticoagulants include heparins: standard (unfractionated) and fractionated (low molecular weight).
Heparin has a long history of use and continues to be widely used in angioneurological practice. However, in a number of patients it is not possible to achieve the desired anticoagulant effect, which is caused by an individual lack of sensitivity to heparin (heparin resistance) due to various reasons. Side effects such as hemorrhages, osteoporosis, skin necrosis, and withdrawal phenomena have also been described.
When standard heparin is depolymerized (under special conditions), its adverse effects are largely eliminated, but the anticoagulant properties are preserved. One of the representatives of low molecular weight heparins is Fraxiparin. Among the most important advantages of this drug are the high bioavailability of small doses, a rapid but predictable anticoagulant effect after subcutaneous administration (1-2 times a day), which eliminates the need for multiple laboratory monitoring during the treatment process. In addition, Fraxiparine has a low incidence of hemorrhagic complications and thrombocytopenia. We especially emphasize that Fraxiparine also exhibits an anticoagulant effect in case of heparin resistance.
Therapy with low molecular weight heparins (Fraxiparin) is indicated in a number of cases listed below. The dosage regimen of Fraxiparine depends on the purpose of its use. For therapeutic purposes, Fraxiparine is administered in a dose of 0.3 ml (2850 IU anti-Xa) subcutaneously twice a day; for the purpose of prevention, a single administration is required.
In patients with cardioembolic stroke and large cerebral infarction or uncontrolled arterial hypertension, oral (indirect) anticoagulants, even if there are indications for their use, should be prescribed no earlier than 2 weeks after the ischemic stroke. This is due to the high risk of complications such as cerebral hemorrhage. Therefore, in the next 2–3 weeks from the onset of stroke, such patients must be prescribed direct anticoagulants, or, more safely, low molecular weight heparins.
The results of various studies suggest that low molecular weight heparins may be effective in preventing subsequent arterial embolism in the setting of dissection (dissection of the inner lining) of the carotid and vertebral arteries, which is currently considered a relatively common cause of stroke, especially among young people. The goal of therapy for dissection and ischemic stroke is to prevent the development of a recurrent stroke and ensure restoration of the damaged vascular wall. Low molecular weight heparin can accelerate the dissolution of mural thrombus, thereby promoting reversal of dissection and restoration of the vessel lumen. The risk of hemorrhagic cerebral complications associated with heparin use is relatively low (<5%).
Another indication for the prescription of direct anticoagulants is stroke with established congenital thrombophilia. One of the variants of the thrombophilic condition is antiphospholipid syndrome, accompanied by venous and arterial thrombosis in various organs, as well as miscarriages. This category of patients should be specifically examined for deep vein thrombosis, which is an indication for short-term and long-term anticoagulant therapy. Long-term anticoagulants should also be considered in patients with a history of recurrent thrombotic episodes.
Thrombotic processes can affect not only the arterial system of the brain, but also the venous system. Thrombosis of the cerebral venous sinuses is an uncommon diagnosis due to the difficulty of diagnosing it, although it is believed that some degree of venous thrombosis is observed in stroke in almost 75% of cases. Magnetic resonance venography can confirm the diagnosis. A small study (20 people) compared the treatment effects of low molecular weight heparin (Fraxiparin) and placebo. The result showed a clear superiority of heparin therapy (p<0.01). 8 out of 10 patients treated with Fraxiparine recovered completely; the remaining 2 patients retained very minor neurological deficits. At the same time, in the placebo group, only 1 patient fully recovered, and 3 died.
Another study conducted more recently, 59 patients with sinus thrombosis were treated with Fraxiparine or placebo, and also demonstrated the benefit of active treatment with a direct anticoagulant. The results of these studies, as well as practice data, indicate that low molecular weight heparins are safe and effective for cerebral sinus thrombosis. Anticoagulant therapy is recommended even if the patient has hemorrhagic venous infarctions. After the acute period of stroke, continuation of anticoagulant therapy with oral anticoagulants for 3–6 months, followed by a transition to antiplatelet agents, is justified. In addition, all bedridden patients with ischemic cerebrovascular accidents are recommended to be prescribed low molecular weight heparin (Fraxiparin) in small doses to prevent thromboembolic complications.
Thus, direct-acting anticoagulants are widely in demand for the treatment of patients with emergency neurological diseases. The effectiveness and safety of this treatment is maximum when using, first of all, low molecular weight heparins. Their timely administration helps prevent thrombotic complications, repeated cerebrovascular accidents and improves the prognosis of patients who have suffered a stroke.
© Magazine “Nerves”, 2006, No. 4
Principles of acute stroke treatment:
- 1. Basic therapy - regardless of the nature of the stroke, is aimed at ensuring an optimal level of functioning of physiological systems for the prevention and treatment of respiratory disorders, relief of central hemodynamic disorders with monitoring and correction of oxygenation levels, maintaining adequate blood pressure, cardiac activity, basic parameters of homeostasis, with monitoring for swallowing, the condition of the bladder, intestines, relief of seizures, nutritional support, skin care, passive gymnastics, massage.
- 2. Treatment of concomitant neurological disorders - cerebral edema, acute occlusive hydrocephalus, hemorrhage in the infarction area, dislocation, vasospasm.
- 3. Special methods of treating different types of strokes - systemic or selective thrombolytic therapy, thrombus extraction, surgical methods.
- 4. Rehabilitation measures (starting from the neuroreanimation unit).
- 5. Prevention and treatment of visceral complications - pulmonary embolism (PE), deep vein thromboembolism of the lower extremities.
- 6. Individual secondary prevention of vascular events – medication and surgery.
Neuroprotective therapy for acute stroke should be carried out in the first 3 hours from the onset of stroke, which may determine its effectiveness.
Neuroprotective therapy has certain goals:
- Reducing the size of cerebral infarction;
- Extending the “therapeutic window” period, expanding the possibilities for thrombolytic therapy;
- Protection against reperfusion injury.
Pathogenetic therapy is divided into primary and secondary neuroprotection.
Primary neuroprotection
It is aimed at interrupting the fast mechanisms of the glutamate-calcium cascade in order to correct the imbalance of the excitatory and inhibitory systems and activate natural inhibitory processes. Primary neuroprotection begins from the first minutes of ischemia. This type of therapy includes glutamate receptor antagonists. An effective non-competitive antagonist of NMDA receptors is magnesium sulfate, which regulates calcium current through voltage-sensitive and agonist-dependent channels. The advantage of the drug is its safety and the absence of significant side effects. It is recommended to administer a 25% solution at a dose of up to 30 ml/day.
Glycine, which has neurotransmitter and general metabolic effects, is a natural activator of inhibitory neurotransmitter systems. It provides anti-ischemic protection of the brain in patients with different locations of vascular damage and varying degrees of severity of the condition. The recommended effective dose of the drug is 20 mg/kg body weight (on average 1-2 g/day) sublingually in the first days of a stroke.
The concept of “calcium cell death” determines the interest in a group of drugs – antagonists of voltage-gated calcium channels. Currently, among the drugs in this group, nimodipine (nimotop) is used in the treatment of stroke, which, penetrating the blood-brain barrier, selectively binds to specific dihydropyridine receptors. These receptors are localized in the central nervous system both on neuronal and glial membranes and in the vascular wall, which determines the presence of a double effect in nimotope - neurotropic and vasotropic action. The effectiveness of the drug in reducing the risk of developing constrictive-stenotic arteriopathy in subarachnoid hemorrhage has been reliably proven. According to international studies, in the treatment of ischemic stroke the drug is effective during the first 12 hours; in a later period, a worsening prognosis of stroke was noted. The drug can be included in the complex therapy of stroke only in patients with high blood pressure (above 220/120 mm Hg), as it has a vasodilating effect and causes arterial hypotension, as a result of which perfusion pressure in the brain decreases.
According to the results of the meta-analysis, only citicoline (Ceraxon) was proven to be effective.
Citicoline (ceraxon) is a natural endogenous compound that is an intermediate in the reactions of phosphatidylcholine synthesis in cell membranes. The mechanisms of action of citicoline are to weaken the accumulation of free fatty acids in areas of stroke-induced nerve damage, restore the neuronal membrane by enhancing the synthesis of phosphatidylcholine, and restore damaged neurons by intensifying the production of acetylcholine. As a result of these processes, cell protection from damage, restoration of functional activity of neurons, and improvement of motor functions are achieved.
Recommended doses of Ceraxon:
- IV or IM 500-1000 mg 1-2 times a day depending on the severity of the condition; the effect of reducing the ischemic focus is shown at a dosage of 2000 mg per day;
- the maximum daily dose for parenteral administration is 2000 mg, for oral administration – 1000 mg;
In addition, it was found that the use of citicoline (Ceraxon) is safe in the acute phase of intracerebral hemorrhage. This result allows the drug to be used for acute stroke at the prehospital stage and when it is impossible to clarify the nature of the stroke.
Treatment and prevention of cerebral edema
The most severe stroke occurs when cerebral edema develops. Cerebral edema usually develops in the first 24-48 hours from the onset of an ischemic stroke, reaches its peak on the 3rd – 5th day and begins to slowly regress on the 7-8th day. There is a direct relationship between the size of the infarction and the degree of cerebral edema. In some patients with an almost complete infarction in the middle cerebral artery (malignant infarction), cerebral edema and intracranial hypertension can lead to herniation and death. About 80% of patients with malignant infarction of the middle cerebral artery die due to severe cerebral edema, which leads to brain dislocation, compression of vital structures of the brainstem, which is accompanied by increasing depression of consciousness. The more severe the cerebral edema, the more severe the stroke.
To prevent the development of cerebral edema, the patient's head and upper torso must be elevated by 20-30 degrees. It is necessary to normalize body temperature, control blood pressure, relieve pain, strive for normovolemia, and avoid intravenous administration of glucose-containing and hypotonic solutions. The main methods with which the treatment of cerebral edema begins are osmotherapy and hyperventilation. The goal of osmotherapy is to increase plasma osmolarity to 300-320 mOsm/L. Among osmodiuretics, glycerol, mannitol, and Hyperhaes are used.
Dehydration
Dehydration is carried out to combat cerebral edema and increased intracranial pressure
.
Indications for prescribing drugs:
1. signs of cerebral edema identified by CT scan,
2. rapidly increasing neurological symptoms, indicating incipient dislocation and signs of brain herniation.
For dehydration, osmotic diuretics, saluretics, corticosteroid hormones, and mechanical ventilation in the mode of moderate hyperventilation are used. In addition, in the acute stage of a stroke, in the initial phase of the formation of cerebral edema, normalization of breathing, hemodynamics, stimulation of venous outflow from the cranial cavity plays no less a role than the prescription of dehydrating agents.
Osmodiuretics
- Glycerin
is the most preferable, has a longer effect (with intravenous administration - 10 hours), does not cause a significant rebound phenomenon, sudden hypervolemia and a rise in blood pressure. Prescribed IV drip - 10% solution per saline. solution at the rate of 1-2 ml/kg for 2 hours. There is a rapid regression of cerebral symptoms and a decrease in platelet aggregation. Glycerin can be administered into the stomach through a tube at the rate of 1 g/kg 1-2 times a day. A decrease in maximum liquor pressure in ischemic stroke by 72%, and in hemorrhagic stroke by 85–90% has been shown (Misyuk N.S., Kurgaev V.I., 1981). The action of glycerol is shorter than that of mannitol. - Mannitol –
increases the osmotic pressure in the tubules and interferes with the reabsorption of water, which leads to water retention in the tubules and an increase in urine volume. Administer intravenously in a stream or drip in the form of a 10-20% solution at a dose of 0.5-1.5 g/kg body weight, followed by 0.5 g/kg every 3-6 hours. If necessary, the administration of mannitol in this mode can last 3-4 days. Long-term use of mannitol, as well as exceeding the osmolarity level above 320 mOsm/L, can lead to changes in water and electrolyte balance, renal pathology, and can also cause rebound intracerebral hypertension. To prevent rebound syndrome, furosemide can be added at a dose of 1 mg/kg intravenously.
Saluretics
- Furosemide (Lasix) and uregit
increase diuresis by inhibiting the resorption of potassium and chlorine ions in the renal tubules and reduce the production of cerebrospinal fluid. - Their use is advisable only with sufficient central nervous system; they complement the action of osmotic diuretics. They themselves cannot quickly and effectively reduce ICP, but they reduce the production of cerebrospinal fluid.
- Furosemide (Lasix
) is administered intravenously and intramuscularly at a daily dose of 40 – 100 mg (1 amp. – 2 ml of 1% solution contains 20 mg of the drug). - Uregit
is administered intravenously 50 mg in 50 ml of isotonic solution; it is less effective
When prescribing any dehydrating agents, it is necessary to monitor osmolarity ( N = 295-300 mmol/kg) and the concentration of sodium, glucose, urea in the blood serum, diuresis (normally 100 ml per hour or 1500 - 2000 ml per day).
Corticosteroid hormones
(dexamethasone, prednisolone) have a predominantly membrane-stabilizing effect and contribute to the normalization of the BBB.
- Their use has no evidence base
Dexamethasone
administered at a dose of 32 mg per day 2 to 4 times a day, depending on the severity of the stroke, lasting 3-4 days.
Dexamethasone and other corticosteroids have not proven effective as a treatment for cerebral edema in strokes, and their use even increases mortality due to the development of infectious and hemorrhagic complications.
can be used to quickly and effectively reduce intracranial pressure .
It lasts for about 2-3 hours and may be useful as a maintenance measure before surgery. As with osmotherapy, if normal ventilation is resumed too quickly, the effects of increasing intracranial pressure may occur.
If the above methods are ineffective, hypothermia can be used to treat cerebral edema .
Moderate hypothermia (33-36°C) significantly reduces mortality in patients with malignant infarctions of the middle cerebral artery, as evidenced by data from studies. Side effects of hypothermia include thrombocytopenia, bradycardia, and pneumonia.
In case of ineffectiveness of drug treatment, hyperventilation and hypothermia, increasing cerebral edema (usually in patients with malignant middle cerebral artery infarction), it is necessary to consider decompression surgery
. The purpose of the decompression method is to prevent the spread of cerebral edema into the lateral ventricles, diencephalon, midbrain, reduce intracranial pressure, increase perfusion pressure, and preserve cerebral blood flow by preventing compression of collateral vessels.
Surgical treatment of cerebral edema ( hemicraniectomy
) for malignant infarctions of the middle cerebral artery can reduce mortality from 80 to 40%. Early use of this method (within the first day after the onset of stroke), according to data, can further significantly reduce mortality. Decompression of the posterior fossa for cerebellar infarctions is the first choice method and can reduce mortality from 80% with conservative treatment to 30%. Currently, several multicenter studies have been conducted that will allow us to draw a conclusion about the effectiveness of this treatment in selected groups of patients.
Neurological complications, in addition to cerebral edema, include seizures
, which may occur in 4-7% of patients. As a rule, they occur on the first day after the onset of stroke in patients with large infarctions involving the cerebral cortex, as well as in ischemic strokes caused by embolism.
Special methods for the treatment of acute stroke. Reperfusion therapy
Basic reperfusion methods:
- restoration and maintenance of systemic hemodynamics (maintaining blood pressure at a sufficient perfusion level, which is reflected in Basic therapy);
- Thrombolysis;
- hemangiocorrection (anticoagulants and antiplatelet agents).
Reperfusion should be active and short-term with a reperfusion period of no more than 3-6 hours.
Thrombolytic therapy (TLT)
- the only method with a high degree of evidence leading to recanalization, provides complete physical independence in 1 out of 10 treated patients.
Types of TLT:
- 1. Drug TLT: systemic intravenous thrombolysis (IV TLT);
- selective (intraarterial) thrombolysis (V/A TLT);
- combined thrombolysis (V/A + mechanical);
- staged thrombolysis (V/V+V/A or mechanical) – rt-PA bridging.
- Ultrasound destruction of thrombus
When a patient is admitted with a stroke clinic during the “therapeutic window” period, it is necessary to immediately resolve the issue of indications and contraindications for intravenous thrombolysis.
According to the European Stroke Organization Guidelines (2009)
Intravenous administration of rt-PA is recommended in the first 3 hours from the onset of the first signs of cerebral infarction at a rate of 0.9 mg/kg administered as a 10% bolus followed by infusion over 60 minutes. IV TLT has also been shown to be successful when administered between 3-4.5 hours after the first symptoms of stroke appear.
Before TLT, it is recommended to correct blood pressure if it increases to 185/100 mmHg or higher.
IA TLT is recommended as an additional treatment for acute MCA occlusion within a 6-hour “therapeutic window.”
IA TLT is performed for acute basilar occlusion in selected patients. IV TLT in case of basilar artery occlusion is a valid alternative even after 3 hours.
IV TLT for ischemic stroke should be carried out in an intensive care ward (unit) of a multidisciplinary hospital with the mandatory availability of round-the-clock neuroimaging services and clinical laboratory diagnostics. Potential risks and benefits should be discussed with the patient and family.
When deciding to conduct TLT, it is necessary to monitor blood pressure, heart rate, respiratory rate, body temperature, oxygen saturation, and control of biochemical blood parameters for at least 48 hours. Blood pressure levels and biochemical blood parameters cannot be an absolute obstacle to TLT; provided they are corrected during the permissible “therapeutic window,” thrombolysis is possible.
As a thrombolytic, today (based on evidence) it is possible to use a single drug: recombinant tissue plasminogen activator (rt-PA) - ALTEPLASE, ACTILYSE.
Based on the results of an analysis of foreign experience (more than 10 years of use of IV rt-PA, for example, 10 years ago in a regular vascular center in Germany or Spain an average of 15 TLTs were performed per year, now - 70-80) it became obvious that using a “one size fits all” approach is ineffective. The use of IV TLT is not successful in all cases of acute stroke, since not all strokes are the same. For small lesions, TLT is not justified economically, because A small stroke recovers well with conventional treatment. On the other hand, large strokes caused by occlusion of medium and large vessels are a difficult aspect of intravenous thrombolysis, in which the rate of achieving a therapeutic effect is low. In case of tandem occlusions of the ICA and MCA, the use of IV TLT is usually ineffective. However, the use of IV TLT remains the standard treatment for all patients with large artery occlusion when there are no contraindications.
Acute MCA occlusion is the most common type of occlusion in stroke patients and is often caused by cardiogenic embolism or large artery disease. The natural course of the disease in untreated acute occlusion of the MCA has an unfavorable prognosis, including such outcomes as persistent disability (more than 70%) and death (20%). It has been established that IV TLT is effective only in 1/3 of cases when complete recovery occurs. However, it is not just recanalization that is important. Only timely, rapid recanalization achieved within the “therapeutic window” is effective, i.e. before the development of irreversible changes in the brain. Late recanalization, not accompanied by clinically significant improvement, is ineffective. However, it is better than no therapy.
Thrombolytic therapy (thrombolysis)
Thrombolytic therapy is a highly effective treatment for ischemic stroke, which allows you to restore blood flow in the affected vessel and prevent irreversible changes in brain tissue.
Currently, for thrombolysis in ischemic stroke, preference is given to alteplase (Actilyse) - the drug has undergone clinical trials and has proven itself well in randomized trials. How it works: Recombinant tissue plasminogen activator (Actilyse) directly activates the conversion of plasminogen to plasmin. After intravenous administration, alteplase remains relatively inactive in the circulation. It is activated by binding to fibrin, which causes the conversion of plasminogen to plasmin and leads to the dissolution of the fibrin clot (the main component of the blood clot).
Thrombolysis is performed in patients with stroke in the first 3-4.5 hours from the onset of neurological symptoms. Only in a hospital, after determining the criteria for indications/contraindications and conducting a number of necessary studies.
Today, VTT is a standard method of treating patients in the most acute period of IS in the absence of contraindications. The method is applicable in most neurological hospitals and does not require lengthy or complex preparation. To make a decision on starting VTT, a relatively small amount of clinical, instrumental and laboratory studies is required. At the same time, due to a significant number of contraindications, only about 5–10% of patients with acute ischemic cerebrovascular accident (ACVA) can potentially be selected for this type of treatment, and the narrow “therapeutic window” (4.5 hours) presents high requirements for the speed of transportation and examination of the patient. The effectiveness of the drug of choice, recombinant tissue plasminogen activator, depends on the level of serum plasminogen, the volume and duration of the thrombus.
However, there are contraindications:
- Bleeding of various localizations. During TLT, all blood clots are dissolved in the vessels, and those that form as a result of bleeding are not excluded.
- Possible aortic dissection.
- Arterial hypertension.
- Intracranial tumors.
- Hemorrhagic stroke (hemorrhage caused by rupture of the walls of cerebral vessels).
- Liver diseases.
- Pregnancy.
- Brain surgeries.
Thrombolytic therapy for ischemic stroke should be carried out in an intensive care unit. According to international recommendations, the time from the patient’s admission to the hospital to the start of thrombolytic therapy should not exceed 60 minutes (door-to-needle time). During this time, it is necessary to determine the indications and exclude contraindications to thrombolytic therapy. Required: 1. Examination by a neurologist and collection of anamnesis, assessment of vital functions and neurological status. An examination using the NIHSS stroke scale is necessary. Thrombolytic therapy is indicated for NIHSS scores between 5 and 25. 2. Immediate CT scan of the brain. 3. Changes in blood pressure levels in both arms. 4. Installation of a cubital peripheral venous catheter. 5. Measuring serum glucose levels. 6. Taking blood and performing the following laboratory tests: a) platelet count; b) APTT; c) INR. 7. Provide monitoring for at least 24 hours: 1) blood pressure levels; 2) heart rate; 3) frequency of respiratory movements; 4) body temperature; 5) oxygen saturation.
Thrombolysis can be:
- Systemic;
- Local.
Methods of thrombolytic therapy
The first method is advantageous in that the medicine can be injected into a vein without having any idea where the blood clot is hidden. With the bloodstream, the drug is carried throughout the entire blood circulation, where on its way it encounters an obstacle in the form of a blood clot and dissolves it. But systemic thrombolysis has a significant drawback: an increased dose of medication is required, and this is an additional burden on the entire circulatory system.
INDICATIONS FOR THROMBOLYSIS IN ACUTE ISCHEMIC STROKE: • severe neurological deficit associated with acute ischemic stroke and, apparently, caused by occlusion of a large artery (basilar, vertebral, internal carotid): a form of movement disorder, speech disorder, facial paresis, level of consciousness disorder. Using special scales (NIHS scale), a neurologist assesses the level of neurological deficit. • absence of hemorrhage according to computed tomography • development time from the beginning of the clinic to 3 hours (up to 6 hours with selective thrombolysis, up to 12 hours with infarction in the main artery basin) THROMBOLYSIS CONTRAINDICATION: ABSOLUTE CONTRAINDICATIONS: 1) minor and rapidly regressing neurological deficit 2) hemorrhage, clearly visible extensive acute cerebral infarction or other CT data that are contraindications (tumor, abscess, etc.) 3) convincing evidence of the presence of a vascular malformation or tumor of the central nervous system in the patient 4) bacterial endocarditis RELATIVE CONTRAINDICATIONS: 1) severe trauma or stroke in within the past 3 months 2) history of intracranial bleeding or suspected diagnosis of subarachnoid hemorrhage 3) major surgery within the past 2 weeks 4) minor surgery within the past 14 days, including liver or kidney biopsy, thoracentesis and lumbar puncture 5) arterial puncture within the past 2 weeks 6) pregnancy (ten days after birth) and breastfeeding 7) acute gastrointestinal bleeding, urological or pulmonary bleeding in the last three weeks, history of hemorrhagic diathesis (including renal and liver failure) 9) peritoneal or hemodialysis 10) changes in coagulogram ( PTT more than 40 seconds, prothrombin time more than 15 (INR more than 1.7), platelets less than 100,000) 11) convulsive seizure as the onset of a stroke (careful differential diagnosis is required) 12) changes in blood glucose levels (hypo or hyperglycemia) ADMINISTRATION OF THE DRUG: Non-selective thrombolysis is more often carried out. To carry it out, after a minimal examination of the patient (examination by a neurologist, computed tomography to exclude hemorrhage), a general blood test with platelet levels, blood biochemistry (glucose level), coagulogram, if possible), 100 mg of the drug akilyse is administered intravenously: 10 mg is administered as a bolus, the rest 90 mg – intravenous drip on physical therapy. solution 0.9% 400.0 for 1 hour. COMPLICATIONS OF THROMBOLYSIS: The main complications are the risk of bleeding (nasal, gastrointestinal, renal) and the risk of transformation of the ischemic focus into hemorrhage in the brain. Thrombolytic therapy makes it possible to witness a truly dramatic improvement in the patient’s condition, when severe neurological disorders literally disappear “on the needle”, and he not only survives, but also recovers, which was previously almost impossible.
Using special scales (NIHS scale), a neurologist assesses the level of neurological deficit. • absence of hemorrhage according to computed tomography • development time from the beginning of the clinic to 3 hours (up to 6 hours with selective thrombolysis, up to 12 hours with infarction in the main artery basin) THROMBOLYSIS CONTRAINDICATION: ABSOLUTE CONTRAINDICATIONS: 1) minor and rapidly regressing neurological deficit 2) hemorrhage, clearly visible extensive acute cerebral infarction or other CT data that are contraindications (tumor, abscess, etc.) 3) convincing evidence of the presence of a vascular malformation or tumor of the central nervous system in the patient 4) bacterial endocarditis RELATIVE CONTRAINDICATIONS: 1) severe trauma or stroke in within the past 3 months 2) history of intracranial bleeding or suspected diagnosis of subarachnoid hemorrhage 3) major surgery within the past 2 weeks 4) minor surgery within the past 14 days, including liver or kidney biopsy, thoracentesis and lumbar puncture 5) arterial puncture within the past 2 weeks 6) pregnancy (ten days after birth) and breastfeeding 7) acute gastrointestinal bleeding, urological or pulmonary bleeding in the last three weeks, history of hemorrhagic diathesis (including renal and liver failure) 9) peritoneal or hemodialysis 10) changes in coagulogram ( PTT more than 40 seconds, prothrombin time more than 15 (INR more than 1.7), platelets less than 100,000) 11) convulsive seizure as the onset of a stroke (careful differential diagnosis is required) 12) changes in blood glucose levels (hypo or hyperglycemia) ADMINISTRATION OF THE DRUG: Non-selective thrombolysis is more often carried out. To carry it out, after a minimal examination of the patient (examination by a neurologist, computed tomography to exclude hemorrhage), a general blood test with platelet levels, blood biochemistry (glucose level), coagulogram, if possible), 100 mg of the drug akilyse is administered intravenously: 10 mg is administered as a bolus, the rest 90 mg – intravenous drip on physical therapy. solution 0.9% 400.0 for 1 hour. COMPLICATIONS OF THROMBOLYSIS: The main complications are the risk of bleeding (nasal, gastrointestinal, renal) and the risk of transformation of the ischemic focus into hemorrhage in the brain. Thrombolytic therapy makes it possible to witness a truly dramatic improvement in the patient’s condition, when severe neurological disorders literally disappear “on the needle”, and he not only survives, but also recovers, which was previously almost impossible.
Local thrombolysis: During local thrombolysis, the drug is injected directly into the location of the thrombus. The drug is supplied through a catheter, which is why the method is called catheter thrombolysis. However, this method is more difficult to implement than the first and is associated with a certain danger. During the procedure, the doctor monitors the movement of the catheter using x-rays. The advantage of this method is its low invasiveness. It is used even if the patient has a large number of chronic diseases.
In any case, it is better to treat than not to treat!
IA TLT is indicated for patients with occlusion of the proximal segments of intracerebral arteries. Its use requires the patient to remain in a high-level stroke center with 24-hour access to cerebral angiography. IA TLT is the method of choice in patients with severe ischemic stroke lasting up to 6 hours, for stroke in the VBB - up to 12 hours. During endovascular intervention, it is possible to administer a thrombolytic intravenously (only on the basis of an accepted and approved internal protocol due to the lack of an authorization document for the use of thrombolysis outside the 4.5 hour “therapeutic window” and the use of mechanical recanalization methods.
It is important that the possibility of endovascular (I/A) intervention should not be a reason for refusing IV TLT in accordance with the indications.
The following patients may be candidates for endovascular technologies:
- 1. IV TLT beyond the time frame (up to 6 hours in patients with cerebral infarction in the carotid system);
- 2. With an unknown time - “night” stroke (up to 8 hours) with occlusion of the main artery, because inaction is fatal!
- 3. With occlusion of the distal parts of the ICA, with T-occlusion, with occlusion of the M1 and M2 segments of the MCA, with basilar occlusion, after ineffective IV TLT. At the same time, verification of recanalization is not required for transfer to the angiosurgical operating room. Endovascular interventions are performed under multicomponent drug anesthesia in the department of X-ray surgical methods of diagnosis and treatment (complete head immobility during manipulations).
Own results
Below is data describing the experience of one doctor. The counting of thrombolytic procedures used began in June 2021 (Fig. 1). In total, from June to December 2021, 44 TLT procedures were performed, from January to July 2021 - 37, thus, in total, during the period under review, this procedure was performed 81 times.
An analysis of the possibilities of use and effectiveness of TLT was carried out on the basis of data obtained for the period from January to July 2021.
Note that we were able to use TLT to help patients with IS only in 30% of cases. What factors have limited the use of TLT?
According to researchers of this problem [3], such factors are:
time of initiation of therapy;
the presence and size of a brain region with potentially reversible changes;
features of systemic and local hemodynamics;
hemostasis factors;
sensitivity of the brain substance to ischemia;
the degree of damage to the blood-brain barrier [3].
The time factor, which comes first in this list, is determined by the presence/absence of a window of therapeutic opportunity (4.5 hours from the onset of stroke). Our observations confirm its significance among all the obstacles limiting the use of TLT: out of 103 patients hospitalized by us with a diagnosis of IS, 52 (50.5%) were outside the therapeutic window (Table 1). However, in most cases, even with a window of therapeutic opportunity, we had to act under deadline conditions, since in real conditions we are often faced with the simultaneous admission of several patients requiring immediate examination and diagnostic procedures. The objective difficulties associated with this lead to inevitable losses of already strictly limited time. The speed and coherence of the actions of the medical staff involved in the TLT procedure and the uninterrupted operation of diagnostic equipment are of great, often decisive importance. Table 1 provides data illustrating the relationship between the time factor and other factors that impede TLT. Thus, the impossibility of performing thrombolysis in 17.5% of cases was associated with the presence of such contraindications in patients as uncontrolled arterial hypertension, trophic disorders in joints and limbs, and blood clotting problems. In approximately 2% of cases, we encountered patients refusing TLT.
In addition to the time factor, the presence of contraindications, the consent of the patient and his relatives when performing TLT, it is important to take into account the age of the patients. According to the instructions for use of the drug alteplase, the recommendations of the European Stroke Organization, and the Russian clinical guidelines for thrombolytic therapy for ischemic stroke [5], patients under 18 years of age should not undergo TLT, and patients over 80 years of age —
carry out with extreme caution. Most researchers agree that TLT in people over 80 years of age is not associated with an increase in complications, such as hemorrhagic transformation of the brain lesion, and, therefore, does not lead to an increase in mortality [6]. A good degree of recovery of impaired neurological functions (26–30%) serves as evidence that thrombolysis in patients aged 80–89 years and 90–99 years is equally safe and effective [6].
Regarding young patients (up to 45 years), data are provided indicating a more favorable outcome of the disease with a low incidence of symptomatic hemorrhagic transformation and a better degree of restoration of impaired neurological functions [6]. On this basis, it is concluded that TLT is effective and safe in all age groups. However, the older the patients, the higher the number of functionally unfavorable outcomes and deaths [6].
Further in our work, we tried to trace the relationship between the effectiveness and safety of TLT and the gender and age of patients. The results of TLT were assessed as follows:
positive dynamics—regression of neurological deficit with successful recanalization and reperfusion;
lack of dynamics - no changes are observed due to the lack of recanalization and/or the development of irreversible damage to the brain substance in the lesion;
negative dynamics - clinical deterioration (manifested by the development of complications, primarily hemorrhagic, reocclusion or re-embolism, or an increase in cerebral edema in case of insignificant effect of TLT).
Table 2 presents data that allows us to trace the relationship between the effectiveness of TLT and the gender and age of patients. We divided our patients into 6 age groups: 1st (20–49 years), 2nd (50–59 years), 3rd (60–69 years), 4th (70–79 years); 5th (80–89 years), 6th (90 or more years). The effectiveness and safety of TLT (positive dynamics, no dynamics, negative dynamics, absence/presence of allergic reactions) was assessed based on the results of daily observation. According to Russian clinical guidelines for thrombolytic therapy for ischemic stroke, it is necessary to monitor the dynamics of the neurological status during the day; Positive dynamics during the first 24 hours after TLT, as a rule, predict subsequent good recovery [5].
Summarizing our observations, we can provide the following data.
The total number of patients who underwent the TLT procedure was 37. There were no cases of an allergic reaction.
The number of men and women among our patients turned out to be almost equal (19 and 18 people, respectively).
The oldest age group (90–99 years) is represented by a single patient - a 92-year-old woman, who, as a result of TLT, showed positive dynamics (restoration of facial expression and speech disorders).
The largest group (10 people) are patients aged 60–69 years, the number of men and women is the same. The results of TLT in each of the subgroups were the same: in 4 men and 4 women, complete or partial restoration of impaired functions was noted (positive dynamics); in 1 man and 1 woman there was no dynamics (no changes occurred); the TLT effectiveness rate in this group is the highest - 82%; No negative dynamics were observed among representatives of this group.
According to the effectiveness of the thrombolytic procedure, age groups can be ranked as follows:
60–69 years old - 82%;
20–49 years old - 80%;
50–59 years old - 75%;
70–79 years old - 43%;
80–89 years old - 33%.
(The group of 90–99 years old, consisting of 1 person, is not included in the rating.)
Cases of negative dynamics with a fatal outcome were noted in 2 groups (70–79 years old and 80–89 years old): a 79-year-old woman and an 84-year-old man. However, in both cases, according to the pathological and anatomical study, a direct connection between the death and the TLT procedure was not established. In the age group of 80–89 years, there was 1 case of hemorrhagic transformation (in an 88-year-old man).
The total number of women who experienced complete or partial recovery of impaired functions after TLT (14 people, 58%) exceeds the number of men with positive dynamics (10 people, 42%).
The overall effectiveness of TLT is quite high and amounts to 64.9% (24 people out of 37) against 8.1% (3 people) of cases of negative effect and 27% (10 people) of the absence of any effect of this procedure.
Factors influencing stroke outcome during TLT
- 1. Time to start therapy: the sooner the better!
- 2. Clinical signs that worsen the outcome of TLT: age over 85 years
- severe stroke (more than 25 points on the NIHSS scale)
- Blood pressure above 180/110 mmHg
- hypodensity of more than 1/3 of the MCA basin
Thus, the question of whether IV TLT is the best treatment method for patients with ischemic stroke remains relevant. After years of continued improvement in endovascular techniques and devices, endovascular neurosurgeons have now achieved unprecedented rates of revascularization for MCA occlusion, with decreased procedure times, very low rates of intracerebral hemorrhage, and favorable outcomes. After more than a decade of attempts at IA TLT using all available drugs, doses, and combination techniques with only modest results, we are entering the era of mechanical thrombectomy. Without a doubt, modern endovascular revascularization techniques have changed the course of the disease in MCA occlusion much more than IV TLT. This is convincing evidence that the endovascular treatment method, if in capable, experienced hands, should be considered the treatment of choice for MCA occlusion!