doctor
A laboratory test aimed at determining the amount of antibodies of three types (IgG, IgA, IgM) in order to assess the potential of a humoral immune response not related to the specificity of antigens. The analysis is prescribed during a comprehensive immunological examination. The results are used in immunology, infectious disease, oncology and surgery. Indications may include immunodeficiency conditions of various origins, long-term and recurrent infections, autoimmune and oncological diseases and postoperative complications. The level of immunoglobulins is determined in venous blood.
You can take a blood test for immunoglobulins at any time convenient for you. Check out the doctors' work schedule and choose a time that is convenient for you. Contact details and directions are on the contact page.
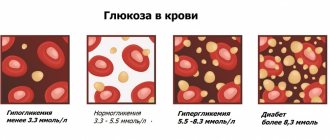
The level of total immunoglobulins IgG, IgA, IgM in the blood reflects the state of humoral immunity. Immunoglobulins are glycoproteins that are produced by B-lymphocytes during infection or the penetration of chemical compounds into the body that are recognized as dangerous. In the human body, there are 5 classes of immunoglobulins, each of which has its own characteristics in structure and function. Immunoglobulins IgG, IgA and IgM are responsible for the formation of the immune response during infection. By interacting with a foreign agent, antibodies neutralize it and enhance the lysis of the damaged cell. Also, during these reactions, the antigen is “memorized”; with repeated infection, antibodies are produced faster.
The analysis for immunoglobulins is a complex study; when interpreting its results, both each indicator separately and the total concentration of antibodies are taken into account. Immunoglobulins G are found in body fluids, make up about 80% of all antibodies, are produced after 5 days during primary infection, are able to “remember” the antigen and more actively protect the body during re-infection. Immunoglobulins A are found on the mucous membranes, protecting the respiratory and genitourinary tracts, and the gastrointestinal tract. They prevent antigens from penetrating deep into tissues. Immunoglobulins M circulate in the blood and lymph fluid, are produced immediately after infection enters the body and trigger an immune response.
A low level of immunoglobulins is determined by insufficient activity of the immune system. High levels may be a sign of hematological diseases and systemic pathologies. To carry out the analysis, blood is taken from a vein.
Detailed description of the study
Serum iron is a microelement that is not produced in the human body independently, but comes only from the outside with food.
Iron is included in:
- Hemoglobin, which is the main carrier of oxygen from the lungs to tissues and organs;
- Myoglobin, which is located in the muscles and is responsible for creating an oxygen reserve.
Iron also participates in cellular metabolism, in the functioning of the immune system, is part of some enzymes, ensuring the normal occurrence of metabolic reactions in them, and also participates in DNA synthesis.
In total, the body contains no more than 5 g of iron. It comes from food from animal and plant sources. This is the so-called heme (found in meat products, as part of hemoglobin) and non-heme (enzymes and proteins of plant foods) iron. Of the total amount of iron that enters the body, only about 10% is absorbed. The absorption process occurs in the duodenum.
In human blood plasma, most of the iron is in the oxidized trivalent state, bound to the protein transferrin. Transferrin transports iron to tissues and organs. Most of the incoming iron goes into the formation of hemoglobin, and the rest is deposited (stored). The main depot of iron in the body is the special protein complexes “ferritin” and “hemosiderin”. From here, iron is consumed if it decreases in the blood serum. The restoration of iron in the blood serum depends on the degree of depot filling. With prolonged iron deficiency, iron reserves are depleted, which can lead to anemia. This regulatory mechanism ensures self-preservation.
Serum iron concentration is an important biochemical indicator, changes in which may indicate possible problems in the metabolism of this microelement.
Iron deficiency is more common than iron excess. As a rule, a person does not notice that he is developing anemia - a pathological decrease in the concentration of hemoglobin in the blood. This usually becomes noticeable when the hemoglobin concentration drops below 100 g/l. Then the person may complain of increased fatigue, dizziness, hair loss, pale skin and brittle nails. In some cases, people report a tingling and burning sensation on the tip of the tongue.
If you do not take any action aimed at restoring the iron depot, shortness of breath, absent-mindedness, and rapid heartbeat (tachycardia) further develop.
Similar conditions are observed in cancer (especially if the intestines are affected), iron deficiency anemia, jaundice, acute and chronic infections, peptic ulcers and ulcerative colitis. Iron deficiency can often be caused by chronic blood loss from hemorrhoids. In case of celiac disease, Crohn's disease and in the postoperative period, anemia is also diagnosed. Iron concentrations in women vary depending on the phase of the menstrual cycle.
There is also the so-called “anemia of pregnancy” - a condition in which the concentration of serum iron in pregnant women decreases. As a rule, in the second trimester, when the fetal iron depot is formed. This condition also requires special monitoring.
Iron overload is less common and is characterized by the following symptoms: abdominal and joint pain, heart rhythm disturbances, and fatigue, similar to anemia.
For the normal functioning of all organs and systems, it is necessary that at least 1 mg of iron be supplied with food per day. Foods rich in this microelement: meat and offal (pork liver), legumes (lentils, beans), greens (spinach, celery), fruits (pomegranate, apples). Ascorbic acid improves the absorption of iron, so it is recommended to use it together with iron-containing foods. Dairy products and caffeine can interfere with iron absorption.
Normal values
Immunoglobulins G penetrate through the placenta to the fetus, so their level in newborns is high, and during the first year it decreases, as maternal immunoglobulins are replaced with their own. The normal limits depend on the age and gender of the patient.
- in the first month of life – 3.97-17.65 g/l for boys, 3.91-17.37 g/l for girls
- from 1 month to one year – 2.05-9.48 g/l for boys, 2.03-9.34 g/l for girls
- from one year to 2 years – 4.75-12.10 g/l for boys, 4.83-12.26 g/l for girls
- from 2 to 80 years – 5.40-18.22 g/l for boys and men, 5.52-16.31 g/l for girls and women
Immunoglobulins A are unable to cross the placental barrier; in newborns, their concentration in the blood is very low. Self-synthesis of antibodies is fully established by the age of 5 years. The normal values for immunoglobulin A are:
- in the first 3 months of life – 0.01-0.34 g/l
- from 3 months to a year – 0.08-0.91 g/l
- from one year to 12 years – 0.21-2.91 g/l for boys, 0.21-2.82 g/l for girls
- from 12 to 60 years – 0.63-4.84 g/l for boys and men, 0.65-4.21 g/l for girls and women
- after 60 years – 1.01-6.45 g/l for men, 0.69-5.17 g/l for women
Immunoglobulins M have a large molecular weight and do not cross the placenta during pregnancy. In children, the concentration of antibodies increases gradually, reaching adult values by the age of 7-12 years:
- in the first 3 months of life – 0.06-0.21 hl
- from 3 months to one year – 0.17-1.43 g/l for boys, 0.17-1.50 g/l for girls
- from one year to 12 years – 0.41-1.83 g/l for boys, 0.47-2.40 g/l for girls
- after 12 years – 0.22-2.40 g/l for boys and men, 0.33-2.93 g/l for girls and women
A physiological decrease in the level of immunoglobulins can be detected during pregnancy, an increase - during intense physical activity or the experience of strong emotions.
Level up
Immunoglobulins are markers of an infectious process in the body; the most common reason for an increase in their level in the blood is respiratory and gastrointestinal infections. An increase in the concentration of IgG and IgA antibodies is characteristic of chronic processes; the number of IgM antibodies increases in both acute and chronic forms of diseases. Other reasons for increased levels of immunoglobulins include autoimmune pathologies, liver damage, as well as multiple myeloma and other monoclonal gammopathies.
Level reduction
The most common reason for a decrease in immunoglobulin levels is acquired deficiency. The concentration of antibodies decreases when their production is disrupted - with neoplasms of the lymphatic system, lymphoproliferative diseases. In addition, the level of immunoglobulins decreases with their increased breakdown and rapid elimination of protein - during irradiation, the use of drugs (for example, cytostatics), enteropathies, nephropathies and burns. Less commonly, the cause of a decrease in immunoglobulin levels is congenital deficiency. It develops with congenital agammaglobulinemia, ataxia-telangiectasia (IgA), Wiskott-Aldrich syndrome (IgG) and selective IgM deficiency.
full list of articles
References
- Federal clinical guidelines for the diagnosis and treatment of iron deficiency anemia, 2015. - 58 p.
- Klochkova-Abelyants, S.A., Surzhikova, G.S. Iron deficiency anemia and anemia of chronic diseases: some aspects of pathogenesis and prospects for differential diagnosis. — Medicine in Kuzbass, 2021. — No. 3. — P.25-28.
- Tiglis, M., Neagu, T., Niculae, A. et al. Incidence of Iron Deficiency and the Role of Intravenous Iron Use in the Perioperative Period, 2021. - Vol. 56(10). - P. 528.
Importance of sample quality control
Quality control (QC) of biospecimens used for scientific research is of fundamental importance to ensure the reliability of the data obtained. Uniformity of biospecimen collection, transport, and storage procedures is critical to ensure the quality of multicenter research studies.
An important and effective tool for such standardization and preservation of a large number of biological samples is the creation of a biobank. A biobank is a structure for the collection, storage and delivery of biological samples and associated data that follows standard operating procedures and provides materials for scientific use [2].
All biospecimens are subject to collection, transportation, sample preparation and storage procedures. Procedures in the biobank are performed strictly according to accepted standards, otherwise the quality of biosamples may decrease. The collection of biomaterial is carried out by medical personnel according to standard methods in accordance with clause 3.2 of GOST 53079.4 - 2008 [3]. Next, the biomaterial, along with the necessary documents, is transported to the biobank, where sample preparation and registration of the resulting biomaterial is carried out (Fig. 1).
Rice. 1. “Life cycle” of biospecimens.
Table 1. QC diagnostic tools
Table 2. Suitability of biological material for research in various fields
Blood sample preparation includes centrifugation and further aliquoting of serum or plasma. In accordance with the standard, it is necessary to perform blood centrifugation no later than 1 hour after collection. Then the aliquoted biomaterial is stored in freezers at a temperature of –80 °C.
QC procedures are designed to evaluate data and biospecimens used in scientific research. QC of data includes control of demographic, clinical information, accuracy of data processing, while QC of biological samples includes analyzes for the authenticity, integrity and identity of samples [1]. QC of biological samples is necessary to ensure accurate characterization and categorization of samples, and to avoid errors in subsequent studies due to inherent heterogeneity within samples.
QC procedures may be carried out by the biobank, end user or subcontracted laboratory and should be performed in compliance with GLP (Good Laboratory Practice) rules or ISO 15189:2012 (Medical laboratories - specific requirements for quality and competence), ISO 17025:2005 or CLIA (Clinical laboratory improvements) [4-6].
Retrospective QC can be applied either to randomly selected samples or to samples whose quality is most in doubt. The first approach allows comparison of different collection sites, while the second allows targeted assessment of samples with the “highest risk” [2].
Suitability of biological material for research in various fields
Blood serum
Hemolysis.
You can check for Hb contamination and ensure that the sample is suitable for proteomic or miRNA-based analysis using ELISA or spectrophotometry. If the Hb concentration is above 50 mg/l, hemolysis has occurred, and the sample is considered contaminated with Hb [9].
Inflammation.
You can check for the presence of an inflammatory marker in a sample by measuring the concentration of C-reactive protein (CRP) using ELISA or nephelometry. If the concentration is above 10 mg/L, the sample indicates the presence of inflammation [9].
Suitability for cardiovascular disease research.
To establish suitability for research, it is necessary to measure:
a) level of BNP, NT-proBNP, using ELISA;
b) ANFPTL3 level using ELISA or electrochemiluminescent immunoassay;
c) level of CK-MB and ET-1 using ELISA;
d) MMP-3 and MMP-9 level using ELISA;
e) troponin I and troponin T levels using ELISA or electrochemiluminescence immunoassay.
These proteins must be detected in order for the sample to be used in cardiovascular disease research [9].
Suitability for liver disease research.
Alanine aminotransferase (ALT) levels should be measured using a fluoroimmunoassay. ALT must be detectable for the sample to be suitable for liver disease testing [9].
Suitability
for the purpose of autoimmunity research.
For research, it is necessary to determine the level of tumor necrosis factor-α (TNF-α) using a sensitive ELISA. TNF-α must be detectable for a sample to meet the criteria for autoimmunity testing [9].
Suitability for research
in endocrinology and diabetes.
Insulin C peptide and insulin growth factor II precursor levels should be measured using ELISA, fluoroimmunoassay, or radioimmunoassay; Aldosterone and somatomedin C levels using ELISA. These proteins must be detected for the sample to be relevant for endocrinology research [9].
Suitable for inflammation
and immunology research.
Complement C levels should be measured using nepholometry or an enzyme immunoassay; ICAM-1 and TNF-α levels by enzymatic immunoassay. These proteins must be detected for the sample to be relevant for inflammation and immunology studies [9].
Suitability
for research purposes in oncology.
To check you need to: use ELISA M65 EpiDeath; measure VCAM-1 levels using ELISA. These proteins must be detectable for the sample to be relevant for oncology research [9].
Suitability for research purposes
in the field of musculoskeletal diseases.
MID-osteocalcin, osteocalcin and calcitonin levels, as well as C-terminal telopeptide and collagen type 1, intact parathyroid hormone (PTH) should be measured using ELISA or electrochemiluminescence immunoassay. These proteins must be detected in order for the sample to be used in research in the field of musculoskeletal diseases [9].
Suitability for
nutrition .
Vitamin B12 levels should be measured using an electrochemiluminescence immunoassay. Vitamin B12 must be detectable for a sample to meet nutritional study criteria [9].
Suitability for research into neurodegenerative diseases.
To establish eligibility for such studies, amyloid Aβ42 levels must be measured by ELISA; neuron-specific enolase levels using the Kryptor immunoassay, ELISA, or electrochemiluminescent immunoassay. These proteins must be detectable for the sample to be relevant for research into neurodegenerative diseases [9].
Blood plasma (EDTA
and
citrate)
Platelet contamination.
You can check for platelets and ensure that the sample is suitable for microparticle or ccfRNA analysis by performing a cell count. If the platelet concentration is below 104/ml, it is considered that there is no significant contamination of the plasma sample with platelets [9].
Platelet activation.
You can also test platelet activation and ensure that the sample is suitable for testing biomarkers affected by platelet activation by measuring the concentration of β-thromboglobulin (βTG) using ELISA. If the concentration is above 200 ng/ml, platelet activation occurs [9].
Hemolysis.
You can check for Hb contamination and ensure that the sample is suitable for proteomic or miRNA analysis by performing ELISA or spectrophotometry. If the Hb concentration is above 20 mg/L, hemolysis has occurred and the sample is considered contaminated with Hb [9].
Inflammation.
You can check the severity of inflammation in a sample by measuring the concentration of CRP using ELISA or nephelometry. Concentrations above 10 mg/l indicate inflammation [9].
Suitability for cardiovascular disease research.
To establish eligibility for research, troponin I and troponin T levels must be measured using ELISA or electrochemiluminescence immunoassay; vasoactive intestinal peptide (VIP) level using ELISA.
These proteins must be detected for the sample to be relevant for cardiovascular disease research [9].
Suitability for studying lipid metabolism.
To test, levels of CETP transporter protein activity can be measured using a fluoroimmunoassay. CETP must be detectable for a sample to qualify for lipid metabolism/lipidomics testing [9].
Suitability for autoimmunity research.
For this study, TNF-α can be measured using a sensitive ELISA. TNF-α must be detectable for a sample to meet the criteria for autoimmunity testing [9].
Suitability for research purposes
in the field of endocrinology, including diabetes.
To assess suitability, glucagon-like peptide 1 levels can be measured using ELISA or radioimmunoassay; adenocorticotropic hormone levels using electrochemiluminescence immunoassay or radioimmunoassay; aldosterone and somatomedin C levels using ELISA.
These proteins must be detected for the sample to be relevant for endocrinology research, including diabetes mellitus [9].
Suitable for inflammation
and immunology research.
To check, you can measure: complement C levels using nepholometry or enzymatic immunoassay; ICAM-1 level by enzymatic immunoassay. These proteins must be detected for the sample to be suitable for inflammation and immunology studies [9].
Suitability for research purposes in musculoskeletal disorders.
C-terminal telopeptide and type I collagen levels should be measured using ELISA or electrochemiluminescent immunoassay. These proteins must be detectable in order for the sample to be used for research in the field of musculoskeletal diseases [9].
Suitability for research into neurodegenerative diseases.
To establish eligibility for research, amyloid Aβ42 levels can be measured using an ELISA. Aβ42 must be detectable for the sample to be suitable for the study of neurodegenerative diseases [9].
Suitability for coagulation studies
(for citrate plasma only). Antifactor Xa and fibrinogen levels can be measured to check; prothrombin fragments 1 and 2 and plasminogen activator inhibitor type 1 activity or antigen using ELISA; analysis of thrombin generation using fluoroimmunoassay; TRA antigen using ELISA. These proteins must be detectable for the sample to be of the required quality to study the coagulation process [9].