Depression is a common illness, affecting approximately 20% of people in developed countries. Her therapy includes an integrated approach, including the use of medications. The new generation tranquilizer Grandaxin for depression is prescribed in case of moderately severe symptoms. It has a gentle effect on the patient’s psycho-emotional background, while exhibiting a minimum of side effects.
Pharmacological group
Grandaxin is classified as an anxiolytic (anti-anxiety) drug or tranquilizer. Most representatives of this group, in addition to anti-anxiety effects, also exhibit others, including:
- sedative – decreased mental and motor activity;
- hypnotic;
- anticonvulsant;
- muscle relaxant - relaxing.
Such effects make it impossible to take such drugs during the day while driving.
Grandaxin is a fundamentally new tranquilizer with a pronounced anxiolytic effect. Its active ingredient is tofisopam, an atypical benzodiazepine derivative. It is synthesized by transforming the molecular structure of diazepam. Thanks to this feature, it is not capable of causing addiction and withdrawal symptoms.
The action of benzodiazepines is based on increasing the sensitivity of receptors to GABA - gamma-aminobutyric acid. This is the main neurotransmitter that causes inhibition in the brain and thereby stops excitement and has a calming effect. However, its excess provokes the opposite reaction - hyperactivity, anxiety, trembling in the arms and legs.
All benzodiazepines are divided into 3 classes, taking into account the predominant effect:
- with anxiolytic;
- with sleeping pills;
- with anticonvulsant effect.
Most representatives have all of the listed properties with one pronounced one. Grandaxin has an anti-anxiety effect, and other manifestations are practically absent.
Considering that the drug does not cause a feeling of drowsiness, relaxation and lethargy, and does not interfere with concentration, it is called a daytime tranquilizer and it is allowed to combine its use with driving, but only after consulting a doctor.
How to recognize depression
As already mentioned, the drug helps get rid of depression, but not all of its forms. Grandaxin is taken only for mild to moderate disease with moderately severe symptoms.
During the course of the disease, several syndromes are distinguished:
- Emotional disorders: low mood, melancholy, anxiety and internal tension, pessimistic attitude; self-flagellation, lack of interest in the world around us, inability to have fun.
- Behavioral reactions: solitude, reduced activity; bad habits;
- Cognitive functions: inability to concentrate, slow thinking, decreased memory and perception; pessimistic thoughts, thoughts of suicide, low self-esteem.
- Autonomic disorders: poor sleep, appetite distortion, weakness and malaise, intestinal dysfunction; discomfort in the heart and other organs, headaches.
A specific indication for the use of the drug is reactive depression. It develops in response to the action of some traumatic factor. The condition is characterized by a consistently reduced emotional background and fixation on the problem.
The patient reproaches and blames himself for what happened, his conversations are aimed only at the problem. And, constantly analyzing it, he seeks support from those around him.
A person is worried about insomnia. And he is fully aware of his condition.
Attacks of despair and demonstrative hysterics are possible. Excitement reaches a state called “melancholic raptus.” At the same time, the person rushes about aimlessly, sobs, screams, and suicide attempts are not ruled out.
This state can be replaced by a depressive stupor, when the patient “freezes” in one position, not reacting to what is happening.
Impact of Grandaxin
The main effect of the drug is manifested in the anxiolytic effect. It helps eliminate feelings of anxiety and fear, reduce emotional stress, anxiety and agitation, both mental and motor.
Tofizopam exhibits vegetative stabilizing properties to a high degree. With him it is expressed to the greatest extent compared to other tranquilizers:
- normalizes sleep - eliminates insomnia, promotes rapid falling asleep;
- restores blood pressure;
- stabilizes heart rate;
- reduces sweating;
- improves the functioning of the gastrointestinal tract, eliminates constipation.
The vegetative stabilizing effect is achieved by establishing a balance between the sympathetic and parasympathetic systems.
The stress-protective effect of Grandaxin protects the central nervous system from the effects of a destructive factor. It gives the patient a state of psychological comfort and provides an adequate assessment of what is happening. Protects against the development of unwanted vegetative-vascular reactions: tachycardia, vascular spasms, destruction of the gastrointestinal mucosa. Eliminates excitement.
Tofizopam has a coronary effect to some extent:
- improves coronary circulation;
- reduces the need for oxygen in the heart muscle;
- normalizes metabolism in cardiac tissues;
- increases the amount of oxygen delivered to the heart.
This ability of Tofizopam is widely used in the treatment of coronary heart disease. The drug stabilizes the functioning of both parts of the autonomic nervous system, and thereby improves the electrical conductivity of the heart muscle.
The psychostimulating effect of the drug helps restore the patient’s mental and physical activity. Helps improve cognitive functions, restores thinking abilities, improves mood. Strengthens the ability to resist the effects of unwanted environmental factors.
Grandaxin is recommended to be included in complex therapy as:
- enhancing the effect of antidepressants;
- to eliminate anxiety and panic attacks that can be triggered by starting to take antidepressant medications;
- in cases where the dosage of antidepressants is insufficient, and increasing it is impossible due to the risk of adverse reactions.
The use of the drug as monotherapy is undesirable.
Bisoprolol in the treatment of complex heart rhythm disorders during pregnancy
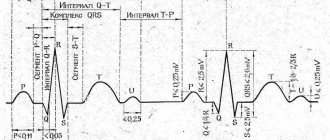
Heart rhythm disturbances during pregnancy, according to various authors, occur in 20-40% of women (1). The main causes of arrhythmias can be divided into extracardiac, cardiac and arrhythmias, the etiology of which has not been established (idiopathic, primary electrical heart disease).
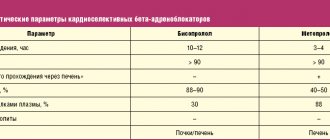
Table. Blood pressure level and Holter ECG monitoring indicators in pregnant women during treatment with bisoprolol
Extracardiac factors include functional and organic lesions of the central nervous system, dysfunction of the autonomic nervous system with a predominance of the tone of the sympathetic department or a decrease in the tone of the parasympathetic department, endocrine diseases, primarily diseases of the thyroid gland, electrolyte imbalance, mechanical and electrical injuries, hypo- and hyperthermia, excessive physical activity, intoxication with alcohol, nicotine, coffee, medications. Medicines such as sympathomimetics, cardiac glycosides, diuretics, most psychotropic drugs, some antiarrhythmic drugs and antibiotics can cause rhythm and conduction disturbances.
Cardiac factors that can cause cardiac arrhythmias are coronary heart disease, congenital and acquired heart defects, including valve prolapse, arterial hypertension, inflammatory and non-inflammatory myocardial lesions, heart failure, diagnostic procedures and operations on the heart and coronary vessels, some congenital diseases of the cardiovascular system.
Pregnancy, even in practically healthy women, can be a factor provoking the development of heart rhythm disturbances. This is facilitated by gestational changes in the woman’s body relating to hemodynamic, electrophysiological and neurohumoral parameters. In particular, hormones of the sympathetic-adrenal system (SAS) have a proarrhythmogenic effect, the activity of which increases significantly during pregnancy, reflecting the adaptation of the woman’s body to new conditions for the functioning of the “mother-placenta-fetus” system (2, 3). Changes leading to an increase in the activity of the SAS can occur at the level of the central nervous system, which is manifested by a decrease in central inhibitory influences and a decrease in the sensitivity of baroreceptors, an increase in the impulse activity of sympathetic nerves, facilitation of ganglion impulse transmission, increased release of norepinephrine into the synoptic fissure and disruption of its metabolism in this area. cracks. Along with this, the peripheral link of the SAS also changes - the density and/or sensitivity of adrenergic receptors, the interaction of receptor-contractile proteins. The indirect effect of SAS on the level of blood pressure and heart rate occurs through stimulation of the synthesis of renin, vasopressin, the occurrence of insulin resistance, disruption of the functional state of the endothelium and other mechanisms (4,5). All these processes can lead to various heart rhythm disturbances, the treatment of which during pregnancy is a complex and responsible task, because the issue of not only improving the clinical condition and quality of life of a woman, but also the condition of the fetus and newborn is being resolved.
One of the most common heart rhythm disturbances during pregnancy is extrasystole, which in almost half of patients occurs without any organic changes in the cardiovascular system (CVS), gastrointestinal tract, or endocrine system. The main causes of extrasystole during pregnancy are considered physiological changes in hemodynamics, emotional arousal, drinking alcohol, coffee, strong tea, smoking, overeating, and abuse of spicy foods. Of great importance is the electrolyte imbalance towards hypomagnesemia and hypokalemia, the use of drugs - sympathomimetics, caffeine, as well as neurocirculatory dystonia and organic damage to the cardiovascular system, in particular, previous myocarditis, heart defects, cardiomyopathies, etc.
The treatment strategy for cardiac arrhythmias is determined by the basic rule: the prescription of antiarrhythmic drugs should be avoided if the arrhythmia does not pose a threat to the patient’s life. If medication is necessary, treatment approaches are the same as for non-pregnant women. At the same time, the possible effect of the drug on the physiological course of pregnancy, childbirth, and the condition of the fetus and newborn should be taken into account. In Russia there is no classification of drugs according to safety criteria for the fetus, and therefore it is possible to use the American classification of drugs and food products by the Food and Drug Administration (FDA) (6). According to these criteria, there are 5 categories of medicines.
- Controlled studies have shown no risk to the fetus.
- Lack of evidence of risk to the fetus: a risk to the fetus has been found in animals, but not in humans, or there is no risk in the experiment, but there is insufficient research in humans.
- A risk to the fetus cannot be excluded: side effects have been identified in animals, but there is insufficient research in humans. The expected therapeutic effect of the drug may justify its use despite its potential risk to the fetus.
- Strong evidence of risk: There is a proven risk to the fetus in humans, but the expected benefit to the expectant mother may outweigh the potential risk to the fetus.
- Use during pregnancy cannot be justified: a drug that is dangerous to the fetus when the negative effect on the fetus exceeds the potential benefit from this drug in the expectant mother.
The drugs of choice for the treatment of extrasystole during pregnancy are verapamil and beta-blockers.
Beta-blockers (BAB) according to the classification of E. Vaughan-Williams (1971) belong to class II antiarrhythmic drugs. The principal mechanism of the inhibitory effect of beta blockers on adrenoreactive structures is to weaken or eliminate the effects associated with the excitation of β1-adrenergic receptors by catecholamines, which cause increased heart rate, increased automaticity of the atrioventricular node and myocardial excitability, increased impulse conduction speed, increased myocardial contractility, decreased refractory period , activation of lipolysis. Excitation of β2-adrenergic receptors by catecholamines leads to dilation of arterioles, decreased tone of smooth muscles of the bronchi, bladder, uterine tone during pregnancy, tremor of skeletal muscles, inhibition of the release of histamine, leukotrienes in mast cells during allergic reactions of type I, hypokalemia, increased hepatic glycogenolysis (7) . Cardioselective beta blockers contribute to a lesser extent to increasing the tone of peripheral arteries, which is very important during pregnancy, when peripheral vascular resistance is physiologically reduced, including in the “mother-placenta-fetus” system. The degree of cardioselectivity (effect on β1/β2 receptors) for metoprolol is 1:35, for bisoprolol - 1:75, while for non-cardioselective propranolol the cardioselectivity index was 1.8:1. The later any drug, including beta blockers, is prescribed during pregnancy, the lower the risk of its negative impact on the course of pregnancy and the condition of the fetus. There are reports of a slowdown in intrauterine development of the fetus in women who took propranolol during pregnancy and atenolol in the first trimester of pregnancy, while the use of this drug, like other beta blockers, from the second trimester is considered safe for the fetus and newborn (8). Data from a few randomized studies on the use of beta blockers in the treatment of hypertension during pregnancy have shown their greater clinical efficacy, safety for the fetus and newborn, and the absence of a negative effect on the physiological course of pregnancy and childbirth (9-11).
Cardioselective beta blockers without intrinsic sympathomimetic activity include bisoprolol, which in experimental studies on the reproductive characteristics of animals did not have a teratogenic effect at a dose of 375 and 77 times the MRL, depending on weight and body surface area, respectively, but its fetotoxicity was noted in these supramaximal doses (increased number of late fetal resorptions). The drug has established itself as an active antihypertensive and antiarrhythmic drug in patients with hypertension and arrhythmias of organic origin.
The purpose of the study was to study the clinical effectiveness and safety of bisoprolol in pregnant women with frequent ventricular extrasystole.
Patients and research methods
Under observation in the specialized cardiology department for pregnant women with diseases of the cardiovascular system of City Clinical Hospital No. 67 were 32 patients in the second trimester of pregnancy aged 19-47 years (average age 27.3±3.8 years), of which 30 women were multipregnant and 2 – primigravidas. All patients gave informed consent to participate in the examination and treatment. A comprehensive clinical and laboratory examination included, along with the routine, a blood test for electrolytes - potassium and sodium, thyroid hormones T3, T4, TSH using the radioimmune method, ECG in dynamics, Echo-CG with Dopplerography in continuous and pulsed modes using standard methods on the device “Acuson 128 XP/10” (USA), 24-hour Holter ECG monitoring was performed on the “Medilog Prima” device on days 1-2 of hospital stay and after 3 weeks of treatment with bisoprolol. All pregnant women were observed by an obstetrician. The activity of the sympathetic-adrenal system was assessed by the value of β-adrenoreception of erythrocyte membranes (β-ARM) using the author’s method, based on changes in the osmoresistance of erythrocytes in the presence of a beta-blocker using a set of reagents “ARM-AGAT” (Agat-Med LLC, Moscow) (12) . Bisoprolol (Concor, Nycomed) was used as an antiarrhythmic drug; treatment began with 2.5 mg under the control of blood pressure, heart rate, ECG and subjective state; if the dose was ineffective, it was doubled, and subsequently, if necessary, increased to 10 mg per day. The majority of patients (28 people) received 5 mg of bisoprolol and 4 people. – 10 mg of the drug per day. The course of treatment in hospital averaged 3 weeks. The effectiveness of treatment was assessed by subjective sensations and the results of Holter ECG monitoring.
Statistical processing of the study results was carried out using the “Biostatistics, Version 4.03” software package using standard methods of variation statistics and Student’s test to assess differences in paired measurements of indicators. The difference was considered significant at p
Results and discussion
During the initial examination, according to Holter ECG monitoring, frequent ventricular extrasystoles were registered in all examined patients; the number of extrasystoles per day ranged from 8 thousand to 50 thousand, in some (6 people) with couplets (from 13 to 80 per day) and triplets (in 4 people, the number of triplets – from 3 to 150 per day), ventricular tachycardia runs were registered in 5 women (from 1 to 5 per day) with a heart rate from 156 to 229 per minute. These rhythm disturbances corresponded to class III-IV according to the classification of B.Lawn and N.Wolff (1971). Arrhythmia in all patients was manifested by pronounced subjective sensations - a feeling of interruptions and fading of the heart, palpitations, sometimes accompanied by fear, sweating, and weakness. It should be noted that in the majority of subjects (26 people, 81.3%), arrhythmia appeared during pregnancy, in the remaining 6 people. (18.7%) it was present before pregnancy, but with increasing gestational age, subjective tolerability of arrhythmia became worse.
According to the anamnesis and comprehensive clinical and laboratory examination, in half of the patients the cause of arrhythmia was organic or functional changes in the cardiovascular system: corrected congenital heart disease (4 people), dilated cardiomyopathy (2 people), hypertrophic cardiomyopathy without obstruction of the left ventricular outflow tract (1 person .), mitral valve prolapse with mitral regurgitation of II (6 people) and III degree (2 people), post-myocardial cardiosclerosis (1 person). In 16 women, no changes in the cardiovascular system, thyroid gland or biochemical parameters were detected, and these arrhythmias were regarded as idiopathic. As mentioned above, pregnancy causes pronounced hemodynamic changes in a woman’s body due to an increase in body weight due to the growth of the placenta and the increasing weight of the fetus, increased metabolism, the development of physiological hypervolemia, and the formation of uteroplacental blood flow. During gestation, physiological myocardial hypertrophy develops - myocardial mass increases by 10-31% by the end of the third trimester and after childbirth, myocardial mass quickly returns to its original level. In pregnant women, cardiac output increases by 15-50% and stroke volume by 13-29%, blood flow speed increases by 50-83%, heart rate is 15-20 beats per minute higher than pre-pregnancy heart rate, general peripheral vascular resistance decreases by an average of 12-34% (13-15). These hemodynamic factors can act as proarrhythmogenic mechanisms contributing to the development of various cardiac arrhythmias.
The proarrhythmogenic effect is also enhanced by physiological hypersympathicotonia, identified during examination in patients with arrhythmia. Analysis of β-ARM values showed significant fluctuations in this indicator among the examined people - from 25 to 85.5 conventional units. units (on average 39.1±2.8 conventional units). According to the conditions of the method, β-ARM values exceeding 20 arb. units, indicate desensitization of adrenergic receptors under the influence of increased concentrations of endogenous catecholamines in individuals with high SAS activity (11). The high values of β-ARM that we discovered are consistent with the results of other authors who showed that the gestational period is characterized by high functional activity of the SAS (2, 3). It should be noted that in some patients this indicator significantly exceeded the average physiological norms, and in 3 women it reached 70-80.5 conventional units. units Such β-ARM values were recorded in the group of patients with idiopathic extrasystole, which gave us grounds to consider severe hypersympathicotonia as the main proarrhythmogenic factor in this category of patients.
According to the results of treatment, 26 women showed a significant improvement in their clinical condition, which was manifested by a significant decrease in the number of extrasystoles per day according to Holter ECG monitoring (Table 1). It is important to emphasize that paired extrasystoles - doublets and triplets - completely disappeared in 4 patients or decreased by an average of 57% in 3 patients compared to the baseline; no episodes of ventricular tachycardia were recorded. We did not detect a significant decrease in systolic (BPs) and diastolic (BPd) blood pressure.
Treatment with bisoprolol was ineffective in 6 patients (3 of them with organic CVS pathology). One patient had hypertrophic cardiomyopathy, the second had mitral valve prolapse with grade III mitral regurgitation, and the third woman was diagnosed with idiopathic arrhythmia. It should be emphasized that the analysis of the adrenoreactivity indicator revealed a significant increase in all three patients with idiopathic arrhythmia - their β-ARM values exceeded 70 conventional units, while the average for the group β-ARM was 39.1±2. 8 conventional units Such high levels of β-ARM indicate pronounced desensitization of adrenergic receptors, as a result of which there are no “points of application” for beta blockers on the cell membrane. Similar data on adrenergic receptor desensitization under the influence of excessively high concentrations of endogenous catecholamines were obtained in experimental studies in our work, which showed the absence of the hypotensive effect of betaxolol in hypertensive patients with high β-ARM values (16). After inpatient treatment, all 26 patients were recommended to continue taking bisoprolol in an individually selected clinically effective dose, and they continued to be observed by us in the consultative and diagnostic center of City Clinical Hospital No. 67.
An analysis of perinatal outcomes was assessed in 13 of 26 patients observed by us at the consultative and diagnostic center and who gave birth in the specialized maternity hospital of City Clinical Hospital No. 67. All women gave birth independently at 39-40 weeks of pregnancy with a full-term fetus with an Apgar score at the 5th minute 8-9 points. The weight of newborns ranged from 2300 g (in a woman with dilated cardiomyopathy) to 3200-4300 g in all other women. At the same time, researchers note the possibility of symptoms of β-blockade in the form of fetal distress, bradycardia, hypoglycemia and intrauterine growth retardation in children whose mothers took beta blockers (17). In our study, no such or any other complications from the fetus or newborn were noted.
Thus, bisoprolol in pregnant women with complex heart rhythm disorders (class III-IV according to B.Lawn and N.Wolff) is an effective antiarrhythmic drug, does not affect the physiological course of pregnancy and childbirth and does not have a negative effect on the condition of the fetus and newborn.
conclusions
- Bisoprolol is an effective antiarrhythmic drug in the treatment of complex cardiac arrhythmias (class III-IV according to B.Lawn and N.Wolff) in pregnant women with organic diseases of the cardiovascular system and idiopathic arrhythmia.
- In patients with an adrenoreactivity index (β-ARM) of 70 arb. units and more, bisoprolol is ineffective due to pronounced desensitization of adrenergic receptors under conditions of high activity of the sympathetic-adrenal system.
- Treatment of pregnant women with complex heart rhythm disorders with bisoprolol does not affect the physiological course of pregnancy and childbirth and does not have a negative effect on the condition of the fetus and newborn.
- The study of the adrenoreactivity indicator based on the β-ARM value can be a prognostic criterion for the individual sensitivity of patients to β-blockers.
Grandaxin and other tranquilizers
Due to its mild and selective effect, the drug has a minimum of side effects, unlike other anxiolytics. It does not cause the negative consequences inherent in most representatives of this group. The main ones are:
- "Behavioral Toxicity." Manifested by increased sleepiness during the day, weakness and malaise. Characterized by decreased reaction speed and inability to concentrate. Coordination of movements is impaired, and confusion may occur.
- Addiction. A common problem when taking tranquilizers. There is a possibility that it will worsen even to substance abuse. But more often, addiction develops with long-term treatment with such drugs, with an unauthorized increase in dose or concurrent use of alcohol.
- Withdrawal syndrome occurs when you suddenly stop using a tranquilizer. In this case, the symptoms of the disease return to the patient in a more pronounced or even complicated form.
Grandaxin does not exhibit such complications. It is low-toxic and resistance to it is not developed upon repeated use. Therefore, it is taken during the daytime.
The drug does not reduce physical and mental activity and does not lead to drowsiness. It does not suppress the efficiency of perception, the speed of reactions, and does not interfere with professional activities.
Concor® AM
For amlodipine:
Amlodipine can be safely used for the treatment of arterial hypertension together with thiazide diuretics, alpha-blockers, beta-blockers or ACE inhibitors. In patients with stable angina, amlodipine can be combined with other antianginal agents, for example, long- or short-acting nitrates, beta-blockers.
Unlike other BMCCs, no clinically significant interaction with amlodipine (III generation BMCCs) was detected when used together with non-steroidal anti-inflammatory drugs (NSAIDs)
, including with
indomethacin
.
It is possible to enhance the antianginal and hypotensive effects of BMCC when used together with thiazide and loop diuretics, ACE inhibitors, beta-blockers and nitrates
, as well as enhancing their hypotensive effect when used together with alpha1-blockers and neuroleptics. Although negative inotropic effects have generally not been observed in amlodipine studies, some CBMCs may enhance the negative inotropic effects of antiarrhythmic drugs that cause QT prolongation (eg, amiodarone and quinidine).
Amlodipine can also be safely used concomitantly with antibiotics and oral hypoglycemic agents.
Single dose of 100 mg sildenafil
in patients with essential hypertension does not affect the pharmacokinetic parameters of amlodipine.
Repeated use of amlodipine 10 mg and atorvastatin
at a dose of 80 mg is not accompanied by significant changes in the pharmacokinetics of atorvastatin.
Simvastatin
: Simultaneous repeated use of amlodipine at a dose of 10 mg and simvastatin at a dose of 80 mg leads to an increase in simvastatin exposure by 77%. In such cases, the dose of simvastatin should be limited to 20 mg.
Ethanol (beverages containing alcohol):
amlodipine with single and repeated use at a dose of 10 mg does not affect the pharmacokinetics of ethanol.
Antivirals (ritonavir):
increases plasma concentrations of BMCC, including amlodipine.
Neuroleptics and isoflurane
: increased hypotensive effect of dihydropyridine derivatives.
Calcium preparations
may reduce the effect of BMCC.
When combined with BMCC and lithium preparations
(no data available for amlodipine), possibly increasing the manifestation of their neurotoxicity (nausea, vomiting, diarrhea, ataxia, tremor, tinnitus).
Studies of concomitant use of amlodipine and cyclosporine
in healthy volunteers and all groups of patients, with the exception of patients after kidney transplantation, were not carried out. Various studies of the interaction of amlodipine with cyclosporine in patients after kidney transplantation show that the use of this combination may not lead to any effect, or increase the minimum concentration of cyclosporine to varying degrees, up to 40%. These data should be taken into account and cyclosporine concentrations should be monitored in this group of patients when cyclosporine and amlodipine are co-administered.
Does not affect serum digoxin
and its renal clearance.
Does not significantly affect the action of warfarin
(prothrombin time).
Cimetidine
does not affect the pharmacokinetics of amlodipine.
in vitro studies
amlodipine does not affect the binding of digoxin, phenytoin, warfarin and indomethacin to plasma proteins.
Grapefruit juice:
A simultaneous single dose of 240 mg of grapefruit juice and 10 mg of amlodipine orally is not accompanied by a significant change in the pharmacokinetics of amlodipine. However, it is not recommended to use grapefruit juice and amlodipine at the same time, since genetic polymorphism of the CYP3A4 isoenzyme may increase the bioavailability of amlodipine and, as a result, enhance the hypotensive effect.
Aluminum or magnesium containing antacids:
their single dose does not have a significant effect on the pharmacokinetics of amlodipine.
CYP3A inhibitors4:
with simultaneous use of diltiazem at a dose of 180 mg and amlodipine at a dose of 5 mg in patients from 69 to 87 years of age with arterial hypertension, an increase in the systemic exposure of amlodipine by 57% was observed.
The simultaneous use of amlodipine and erythromycin in healthy volunteers (from 18 to 43) does not lead to significant changes in the exposure of amlodipine (an increase in the area under the curve of “slow” calcium channels (SCCC) such as verapamil and, to a lesser extent, diltiazem, when used simultaneously with bisoprolol may lead to a decrease in myocardial contractility, a pronounced decrease in blood pressure and impaired AV conduction. In particular, intravenous administration of verapamil to patients taking beta-blockers can lead to severe arterial hypotension and AV blockade.
Centrally acting antihypertensives (such as clonidine, methyldopa, moxonidine , rilmenidine)
when used simultaneously with bisoprolol, they can lead to a decrease in heart rate and a decrease in cardiac output, as well as vasodilation due to a decrease in central sympathetic tone. Abrupt withdrawal, especially before discontinuation of beta-blockers, may increase the risk of developing rebound hypertension.
Combinations requiring caution
BMCA dihydropyridine derivatives (for example, nifedipine)
when used simultaneously with bisoprolol, they may increase the risk of developing arterial hypotension. In patients with CHF, the risk of subsequent deterioration in cardiac contractility cannot be excluded.
Class I antiarrhythmics (eg, quinidine, disopyramide, lidocaine, phenytoin, flecainide, propafenone)
when used simultaneously with bisoprolol, they can reduce AV conductivity and myocardial contractility.
Class III antiarrhythmics (eg, amiodarone)
may increase AV conduction disturbances.
Parasympathomimetics
when used simultaneously with bisoprolol, they may increase AV conduction disturbances and increase the risk of developing bradycardia.
The effect of beta-blockers for topical use
(for example, eye drops for the treatment of glaucoma)
may enhance the systemic effects of bisoprolol (lowering blood pressure, lowering heart rate).
Hypoglycemic effect of insulin or oral hypoglycemic agents
may intensify. Beta blockade may mask signs of hypoglycemia, particularly tachycardia. Such interactions are more likely when using non-selective beta-blockers.
Means for general anesthesia
may weaken reflex tachycardia and increase the risk of developing arterial hypotension (see section "Special Instructions").
Cardiac glycosides
when used simultaneously with bisoprolol, they can lead to an increase in AV conduction time and the development of bradycardia.
Nonsteroidal anti-inflammatory drugs
(NSAIDs)
may reduce the antihypertensive effect of bisoprolol.
Concomitant use of bisoprolol with beta-agonists (for example, isoprenaline, dobutamine)
may lead to a decrease in the effect of both drugs.
The combination of bisoprolol with adrenergic agonists
that affect beta and alpha adrenergic receptors (for example, norepinephrine, epinephrine)
may enhance the vasoconstrictor effects of these drugs that occur with the participation of alpha adrenergic receptors, leading to an increase in blood pressure. Such interactions are more likely when using non-selective beta-blockers.
Antihypertensives, as well as other drugs with possible antihypertensive effects (eg tricyclic antidepressants, barbiturates, phenothiazines)
, may enhance the antihypertensive effect of bisoprolol.
Combinations to consider
Mefloquine
when used simultaneously with bisoprolol, it may increase the risk of bradycardia.
MAO inhibitors
(with the exception of MAO-B inhibitors) may enhance the antihypertensive effect of beta-blockers. Concomitant use may also lead to the development of a hypertensive crisis.
Rifampicin
slightly shortens the half-life (T1/2) of bisoprolol. As a rule, no dose adjustment is required.
Ergotamine derivatives
when used simultaneously with bisoprolol, they increase the risk of developing peripheral circulatory disorders.
Limitations and adverse reactions
Contraindications to the use of the drug are:
- severe forms of depression - with them there is a high probability of suicidal thoughts and actions;
- phobias, obsessions - possible increase in aggression;
- allergic reactions;
- uncompensated respiratory failure.
The drug is prescribed with caution to elderly people due to their increased susceptibility to it. It is not recommended to use it at this age for too long a time.
The drug is contraindicated for children under 18 years of age. It can be taken only in rare cases after carefully weighing the need and risks. In this case, therapy should be short-term.
Taking the drug in the 1st trimester of pregnancy is contraindicated. It easily crosses the placental barrier and can cause developmental defects in the child. Grandaxin can pass into breast milk, so you should avoid breastfeeding while treating with the drug.
Otherwise, when it enters the child’s body, it accumulates and causes a sedative effect. The newborn's sucking reflex decreases, he cannot suck out milk fully, and loses weight.
Despite the mild effect of the drug, it still exhibits some side effects:
- headaches, agitation and aggressiveness;
- sleep problems;
- confusion;
- increased frequency of seizures in epileptic patients;
- breathing disorders;
- poor appetite, thirst, digestive problems;
- jaundice;
- muscle hypertonicity, muscle pain;
- allergic manifestations.
In such cases, taking the drug is canceled.
How to take it correctly
Grandaxin is a small, round, disc-shaped tablet with the GRANDAX logo. They are white or gray in color and have virtually no odor. Located on a blister of 10 pieces.
The dosage of 1 tablet is 50 mg. The package contains 20 or 60 tablets.
Once in the digestive tract, Grandaxin is quickly absorbed into it. Undergoing transformation in the liver, it enters the blood. Here it reaches its maximum concentration 2 hours after administration. It is excreted by the kidneys and does not accumulate in the body.
The dose of the drug depends on the severity of the condition. It is calculated and prescribed only by a doctor. Self-medication is unacceptable. Usually the scheme is as follows: 1–2 tablets. from 1 to 3 times a day.
The maximum dose per day is 300 mg, in children – 200 mg.
Due to the fact that the drug accumulates in the liver and is evacuated through the kidneys, the drug is prescribed to patients with renal and liver failure with caution. In such patients, the severity and duration of the effect may change, as well as the development of side effects. In this regard, their dose is reduced by half. The same scheme is used for elderly people.
In case of an overdose of the drug, complications such as vomiting, changes in consciousness, coma, seizures and impaired respiratory function are possible.
Grandaxin is not used in parallel with cyclosporine and immunosuppressants such as tacrolimus, sirolimus.
Tablets can intensify the effect of drugs that depress the activity of the central nervous system: analgesics, sedatives and hypnotics, etc.
The use of Grandaxin along with alcohol, smoking, barbiturates, along with antiepileptic therapy reduces its activity.
It is strongly recommended to take Grandaxin before 17.00 to avoid insomnia.
The drug is available with a doctor's prescription. The average cost of 20 tablets is 400 rubles, 60 tablets - 900 rubles.
Concor, 30 pcs., 10 mg, film-coated tablets
The effectiveness and tolerability of bisoprolol may be affected by concomitant use of other medications. This interaction can also occur when two drugs are taken within a short period of time.
The doctor must be informed about taking other medications, even if taken without a doctor's prescription (i.e., over-the-counter drugs).
Combinations not recommended
Treatment of chronic heart failure
Class I antiarrhythmic drugs (for example, quinidine, disopyramide, lidocaine, phenytoin; flecainide, propafenone), when used simultaneously with bisoprolol, can reduce AV conduction and cardiac contractility.
All indications for use of the drug Concor®
Blockers of “slow” calcium channels (SCBC) such as verapamil and, to a lesser extent, diltiazem, when used simultaneously with bisoprolol, can lead to a decrease in myocardial contractility and impaired AV conduction. In particular, intravenous administration of verapamil to patients taking beta-blockers can lead to severe arterial hypotension and AV block.
Centrally acting antihypertensives (such as clonidine, methyldopa, moxonidine, rilmenidine) can lead to a decrease in heart rate and cardiac output, as well as vasodilation due to a decrease in central sympathetic tone. Abrupt withdrawal, especially before discontinuation of beta-blockers, may increase the risk of developing “rebound” arterial hypertension.
Combinations requiring special caution
Treatment of arterial hypertension and angina pectoris
Class I antiarrhythmic drugs (for example, quinidine, disopyramide, lidocaine, phenytoin; flecainide, propafenone), when used simultaneously with bisoprolol, can reduce AV conduction and myocardial contractility.
All indications for use of the drug Concor®
BMCC dihydropyridine derivatives (for example, nifedipine, felodipine, amlodipine) when used simultaneously with bisoprolol may increase the risk of arterial hypotension. In patients with CHF, the risk of subsequent deterioration in cardiac contractility cannot be excluded.
Class III antiarrhythmic drugs (eg, amiodarone) may worsen AV conduction disturbances.
The effect of beta-blockers for topical use (for example, eye drops for the treatment of glaucoma) may enhance the systemic effects of bisoprolol (lowering blood pressure, lowering heart rate).
Parasympathomimetics, when used simultaneously with bisoprolol, may enhance AV conduction disturbances and increase the risk of developing bradycardia.
The hypoglycemic effect of insulin or oral hypoglycemic agents may be enhanced. Signs of hypoglycemia - in particular tachycardia - may be masked or suppressed. Such interactions are more likely when using non-selective beta-blockers.
General anesthesia agents may increase the risk of cardiodepressive effects, leading to arterial hypotension (see section "Special Instructions").
Cardiac glycosides, when used simultaneously with bisoprolol, can lead to an increase in impulse conduction time, and thus to the development of bradycardia.
Nonsteroidal anti-inflammatory drugs (NSAIDs) may reduce the hypotensive effect of bisoprolol.
The simultaneous use of Concor® with beta-agonists (for example, isoprenaline, dobutamine) may lead to a decrease in the effect of both drugs. The combination of bisoprolol with adrenergic agonists that affect beta and alpha adrenergic receptors (for example, norepinephrine, epinephrine) may enhance the vasoconstrictor effects of these drugs that occur with the participation of alpha adrenergic receptors, leading to an increase in blood pressure. Such interactions are more likely when using non-selective beta-blockers.
Antihypertensive drugs, as well as other drugs with a possible antihypertensive effect (for example, tricyclic antidepressants, barbiturates, phenothiazines) may enhance the hypotensive effect of bisoprolol.
Mefloquine, when used simultaneously with bisoprolol, may increase the risk of bradycardia.
MAO inhibitors (except MAO B inhibitors) may enhance the hypotensive effect of beta-blockers. Concomitant use may also lead to the development of a hypertensive crisis.