Folate deficiency anemia belongs to the group of megaloblastic anemia. This means that hematopoiesis stops at the level of large blast progenitor cells and does not reach the production of normal red blood cells.
During metabolism, folic acid salts (folates) are so associated with vitamin B12 that a lack of one substance in the blood leads to a loss of the other. Joint deficiency impairs DNA synthesis in cells. The formed elements of the blood are the most sensitive, therefore they are the first to react with the manifestation of anemia.
How folates are involved in the development of anemia
Like B12, folate comes to humans from foods. They begin to be absorbed in the upper parts of the small intestine. Using a special transport protein, they are delivered to liver hepatocytes and bone marrow. This is the site of active synthesis of blood cells.
The process of division is associated with the formation of new DNA and chromosomes. Biochemical reactions are possible only in the presence of folate and two components of vitamin B12 (methylcobalamin and deoxyadenosylcobalamin). Without them, DNA synthesis is blocked and the nuclear structure of cells is disrupted.
What happens in the blood?
The bone marrow releases erythroblasts into the bloodstream instead of red blood cells. These are large immature cells with an asymmetrically located nucleus, unable to bind and transport oxygen. An important difference from other anemias is the absence of any disturbances in hemoglobin synthesis.
The lifespan of an abnormal erythroblast is two times shorter than that of a normal erythrocyte. The body independently destroys them as unusable. At the same time, the cells of the granulocytic lineage are destroyed, so leukopenia and thrombocytopenia occur in the clinical course of folate deficiency anemia.
Where does folate come from?
Folic acid, or rather its salts, are not synthesized in the body. They come in the required quantities only with food. Foods rich in folic acid:
- lettuce, spinach;
- green vegetables and fruits;
- beans;
- citrus fruits (lemons, oranges);
- liver meat.
Spinach leaves as an additive to salads and meat dishes replace the daily requirement for folate
Serum folate concentrations should be maintained at least 4 ng/ml at all times. The reserves in the liver cells are only enough for a month (rarely up to four months).
The significance of the internal factor
Cyanocobalamin from food products entering the gastrointestinal tract is absorbed using the so-called intrinsic factor (IF)
. Here's how it happens:
- In the stomach, B12 is absorbed with the internal factor, as was previously thought, in no hurry; it finds protein-R and combines with it in order to be sent to the duodenum in the form of a complex “Vit B12 + protein-R” and there, under the influence of proteolytic enzymes, break down;
- In the duodenum, cyanocobalamin is freed from protein-R and, in a free state, meets the internal factor that has arrived there, interacts with it and forms another complex - “Vit B12 + VF”;
- The “Vit B12 + VF” complex is sent to the jejunum, finds receptors intended for internal factor, connects with them and is absorbed;
- After absorption, cyanocobalamin “sits” on the transport protein transcobalamin II, which delivers it to the sites of primary activity or to a depot to create a reserve (bone marrow, liver).
It is obvious why such great importance is given to the internal factor, because if everything is in order with it, then almost all of the cyanocobalamin supplied with food will safely reach its destination. Otherwise (in the absence of VF), only 1% of vitamin B12 will leak through the intestinal wall by diffusion, and then the person will not receive the amount of such an important vitamin he needs.
The body's daily need for cyanocobalamin is from 3 to 5 mcg, and its reserve is from 4 to 5 grams, therefore, it can be calculated that if the supply of vitamin B12 is completely excluded (for example, during a gastrectomy), then the reserves will dry up in 3- 4 years. In general, the supply of vitamin B12 is designed for 4-6 years, while folic acid, in the absence of supply, will disappear in 3-4 months. From this we can conclude that B12 deficiency during pregnancy does not threaten if its level was normal before, but folic acid, if the woman did not consume raw fruits and vegetables, is quite capable of falling below the permissible limit and creating a deficiency state (the development of folate deficiency anemia ).
Vitamin B12 is found in products of animal origin, folic acid is found in almost all food products, however, cyanocobalamin tolerates heat treatment remarkably well for a long time and is preserved for entry into the body, which cannot be said about folic acid - after 15 minutes of boiling, this vitamin does not there will be no trace...
Why is there a shortage?
The causes of folate deficiency anemia have been sufficiently studied.
A shortage is possible due to:
- reducing consumption of foods containing folates;
- impaired absorption in diseases of the stomach and small intestine (a congenital disease detected in children - celiac disease, various gastroenteritis accompanied by diarrhea), lack of pancreatic enzymes;
- destruction of the “depot” in hepatocytes due to liver diseases (hepatitis, cirrhosis, portal hypertension, tumors);
- increased consumption for the needs of building organs and systems of the embryo during pregnancy;
- toxic effects of alcoholic beverages, drugs, medications from the group of anticonvulsants, antivirals, antituberculosis drugs;
- increased removal from the blood during hemodialysis procedures.
Who is at risk of the disease?
The risk group may include:
- people suffering from chronic diseases of the intestines, stomach, liver and pancreas;
- children with signs of poor digestion of food, frequent diarrhea, lag in weight gain;
- vegetarians who do not get enough meat products;
- persons who are fond of fashionable diets for weight loss, bowel cleansing with enemas and laxatives;
- patients with helminthiasis (especially diphyllobothriasis);
- alcoholics and drug addicts;
- pregnant women with a lack of balanced nutrition.
Acute blood loss is always accompanied by a deficiency of vitamins, including folic acid.
Nutritionists point out that artificial vitamin deficiency is caused by the overuse of heat treatment in food preparation using modern kitchen equipment (grill, microwave, steamer). These devices must be used in accordance with the instructions, avoid overcooking, excessively long stewing or cooking.
During pregnancy, folate consumption increases not only for the growing fetus; this condition is characterized by a decrease in the rate of absorption of folic acid. Loss of fluid through vomiting during toxicosis contributes to the development of anemia.
Clinical manifestation
Symptoms of folate deficiency anemia do not differ from the general manifestations of other types of anemia; the patient complains of:
- increased fatigue, a constant feeling of tiredness that does not disappear after rest;
- muscle weakness in the arms and legs;
- headaches and dizziness;
- feeling of rapid heartbeat;
- tendency to diarrhea, frequent loose stools;
- Appetite decreases significantly (to the point of complete anorexia).
Upon inspection, note the following:
- pallor of the skin, possible slight yellowing of the sclera;
- reduced blood pressure;
- dry skin;
- early signs of aging in the form of gray hair, wrinkles.
There may be ulcers in the mouth, a swollen, bright tongue
One of the signs of deficiency in children is the inability to concentrate; schoolchildren have a lag in their studies and memory loss.
Symptoms and signs of folate deficiency anemia
A decrease in the number of red blood cells leads to the inability to adequately supply cells with oxygen: the mucous membranes and skin turn pale, a person quickly gets tired, feels weak, fainting, dizziness, loss of appetite and weight loss are possible. To compensate for the lack of oxygen, the heart beats faster to pump blood faster; breathing accelerates. Palpitations become palpable and shortness of breath appears. Folate deficiency anemia may be accompanied by diarrhea, loss of taste, numbness in the limbs, muscle weakness and depression.
Diagnostics
Folate deficiency anemia does not have typical clinical symptoms, so diagnosis is based first on confirming the presence of anemia using a blood test, and then clarifying the form of anemia.
Laboratory testing in half of the patients allows us to establish the presence of:
- hyperchromic anemia;
- normal or reduced reticulocyte count;
- unchanged number of granulocytes and platelets.
A moderate decrease in hemoglobin and an increase in indirect bilirubin are possible.
The picture of bone marrow examination does not differ from anemia due to vitamin B12 deficiency.
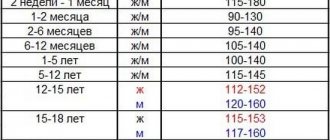
The main and decisive method in diagnosis is the quantitative reaction for determining folic acid in erythrocytes. This is a standard examination of an anemic patient when megaloblasts are detected in the blood. Normal level is 100 – 450 ng/l. With anemia it drops significantly.
In diagnosis, it is important to associate the disease with the cause. Women are referred to a gynecologist for pregnancy testing. The therapist asks the patient about the pills he is taking and finds out whether he is prone to alcoholism.
What are the similarities and what are the differences?
As noted above, B12-deficiency and B12-folate deficiency anemias belong to the group of megaloblastic anemias, which are characterized by a decrease in DNA production, which, in turn, impairs the proliferation of cells capable of rapid division. These are the cells:
- Bone marrow;
- Skin;
- Mucous membranes;
- Gastrointestinal tract.
Among all rapidly proliferating cells, hematopoietic (blood-forming) cells show the greatest tendency to accelerate proliferation, therefore symptoms of anemia are among the first clinical manifestations of these megaloblastic anemias. There are often cases when, in addition to anemia (decrease in the level of red blood cells and hemoglobin), other signs of hematological pathology are observed, for example, a decrease in the number of blood platelets - platelets (thrombocytopenia), neutrophilic leukocytes (neutropenia, agranulocytosis), as well as monocytes and reticulocytes.
blood for B12 deficiency anemia
Why are these types of anemia so interrelated and what is their difference? The fact is that:
- The presence and direct participation of vitamin B12 is very necessary for the formation of the active form of folic acid
, which, in turn, is very necessary for the production of thymidine, an important component of DNA. This biochemical interaction with the participation of all necessary factors allows us to fully ensure the normal formation of blood cells and gastrointestinal tract cells (gastrointestinal tract); - Vitamin B12 is also assigned other tasks - with its participation, individual fatty acids (FAs) are broken down and synthesized. If the content of cyanocobalamin is insufficient, this process is disrupted, and harmful, neuron-killing methylmalonic acid begins to accumulate in the body, and at the same time the production of myelin, a substance that forms the myelin sheath, which has an electrically insulating function for nerve cells, decreases.
As for folic acid, the breakdown of FA does not require its participation, and with its deficiency the nervous system does not suffer. Moreover, if a patient with B12 deficiency is prescribed folic acid as a treatment, then for a short time it will stimulate erythropoiesis, but only until it is in excess. An excessive amount of the drug will force all the B12 present in the body to work, that is, even the one that was intended to ensure the breakdown of fatty acids. Of course, this situation does not lead to good - nervous tissues are even more affected, deep degenerative changes in the spinal cord develop with loss of motor and sensory functions (combined sclerosis, funicular myelosis).
Thus, a deficiency of vitamin B12, along with impaired proliferation of hematopoietic cells and the development of anemia, has a negative effect on the nervous system (NS), while a deficiency of folic acid only affects the division of hematopoietic cells, but does not affect the health of the nervous system.
What diseases are used for differential diagnosis?
For proper treatment, a distinctive diagnosis with B12-deficiency anemia and hemolytic types of anemia is necessary.
A hallmark of B12 deficiency is an increased level of methylmalonic acid in the urine. This test is within the normal range for folate deficiency.
Hemolytic anemia is characterized by a disturbed structure of red blood cells, this is determined by microscopy of a smear
Biochemical tests show the presence of free hemoglobin and hemosiderin in the blood.
Causes of B12 deficiency anemia
A lack of vitamin B12 may be due to the following reasons or a combination of them:
- impaired absorption of the vitamin in the intestine: gastritis associated with Helicobacter pylori infection, Crohn's disease, previous intestinal surgeries, polyposis of the gastric mucosa, cancer, intestinal fistulas, etc.;
- congenital metabolic disorders;
- chronic intoxication with nitric oxide, used as sedation in anesthesiology.
- minor absorption disorders, for example, a decrease in the availability of B12 in food with chronic pancreatitis, moderate atrophic gastritis, taking metformin, medications to reduce the production of hydrochloric acid, etc.;
- poor diet, vegetarianism;
- chronic alcoholism.
One form of B12 deficiency anemia is Addison-Biermer disease. With it, autoantibodies appear against the cells of the gastric mucosa.
It is worth noting that B12 and folate deficiency anemias are closely related. Folic acid (vitamin B9) also takes part in the formation of red blood cells. Even a full supply of these components in food does not guarantee the absence of anemia in cases where there is an increased consumption or there is a violation of utilization in the bone marrow. This may be due to parasitosis, intestinal dysbiosis, pregnancy, liver and blood diseases.
Main objectives in the treatment of folate deficiency anemia
Treatment of folate deficiency anemia is not complete without clarifying and eliminating the causes that caused it. Therapy should include:
- replenishment of vitamin B12 deficiency;
- complete refusal of toxic medications, replacement with other drugs;
- compliance with the conditions of the regime at work and rest;
- mandatory dietary nutrition including foods containing B vitamins and folates;
- replacement therapy with artificial vitamin B9 or folic acid in a daily dosage of 1–5 mg.
The effectiveness of the selected dosage is evidenced by the growth of reticulocytes in the blood test by the fourth day of therapy. The need to take the drug orally has been proven by the much better absorption of folates from synthetic vitamins than by absorption from foods.
To distinguish the degree of influence of B12 deficiency on the severity of anemia, a trial treatment with Cyanocobalamin is carried out intramuscularly, while the level of reticulocytes is monitored.
The course of treatment is most often carried out for 2 months. Recovered patients are not susceptible to relapses of the disease.
Anemia, pathology of hemostasis, oncohematology
Materials are presented from the RUDN textbook
Anemia. Clinic, diagnosis and treatment / Stuklov N.I., Alpidovsky V.K., Ogurtsov P.P. – M.: Medical Information Agency LLC, 2013. – 264 p.
Copying and reproducing materials without indicating the authors is prohibited and is punishable by law.
Megaloblastic anemia is a macrocytic anemia, in which a disorder of hematopoiesis in the bone marrow is determined, in the form of the presence of altered erythrocyte precursors - megaloblasts. Megaloblasts are erythrokaryocytes, characterized by certain features: large, immature in appearance, nuclei are surrounded by relatively more mature cytoplasm. From a biochemical point of view, the primary condition in this condition is a violation of DNA synthesis: cells stop developing in the S-phase of the cell cycle and cannot complete the division process. As a result, there is an accumulation of large cells “waiting” for mitosis and their premature death. Most often, megaloblastosis is caused by a deficiency of vitamin B12 (cobalamin) and folic acid, as well as taking medications that inhibit DNA synthesis.
Vitamin B12 (cobalamin) is produced by microorganisms that live in root vegetables and legumes, and is present in the muscles and parenchymal organs of animals that feed on these plants. A person receives cobalamin from animal food. The total content of cobalamin in the human body is 2 - 5 mg, and since its daily loss is very small (2 - 5 mcg / day), if the intake of the vitamin is completely stopped, its reserves (mainly in the liver) will last for 3 - 5 years .
For the absorption of vitamin B12, intrinsic factor (IF) is required, which is secreted by the parietal cells of the stomach. The cobalamin + VF complex moves to the distal ileum, where cobalamin is absorbed. In the blood, cobalamin binds to transcobalamin II, a transport protein that delivers cobalamin to target tissues. 1% cobalamin is absorbed without forming a complex with intrinsic factor.
Folates (B vitamins) are found in leafy vegetables (legumes, lettuce, spinach), fruits, mushrooms, and animal proteins. Folate reserves in the human body are 5–10 mg, and their daily loss is 100–200 mcg, so a complete cessation of folate intake leads to folate deficiency after 1–2 months. Folates are also absorbed in the distal parts of the small intestine.
Main clinical manifestations and their pathogenesis
All dividing cells are involved in the megaloblastic process, but actively proliferating cellular systems, such as bone marrow and epithelium, are most affected. This determines the prevalence in the clinic of anemia of symptoms associated with impaired hematopoiesis and damage to the gastrointestinal mucosa. In addition, with vitamin B12 deficiency, a number of disorders occur in the central and peripheral nervous system that are never observed with folate deficiency.
In the bone marrow, with a deficiency of vitamin B12 and folates, normoblastic hematopoiesis is replaced by megaloblastic hematopoiesis, which is characterized by impaired proliferation and differentiation of hematopoietic elements and intramedullary death of most nucleated red cells (ineffective erythropoiesis), as well as impaired ripening of granulocytes and megakaryocytes. All this inevitably leads to the development of macrocytic anemia (since some megaloblasts still mature into mature forms - macrocytes), which is accompanied by granulocytopenia and thrombocytopenia.
Since anemia due to vitamin B12 and folic acid deficiency develops gradually over a long period of time, patients are usually well adapted to low hemoglobin levels.
In addition to the general symptoms of anemia, a lemon tint of the skin is possible - a combination of pallor and jaundice, which is on the one hand a consequence of ineffective erythropoiesis (intraosseous breakdown of hemoglobin-containing megaloblasts), on the other hand, indirect bilirubinemia associated with increased hemolysis of large forms of red blood cells (megalocytes) in the spleen. With megaloblastic anemia, there is often increased pigmentation of the nail bed and skin folds. Moderate splenomegaly may develop as a result of extramedullary hematopoiesis.
Impaired division of epithelial cells in megaloblastic anemia leads to the appearance of atypical mitoses and giant cells on the mucous membranes of the tongue, oral cavity, esophagus, stomach and intestines. In this case, the exfoliated epithelial cells are not restored, which leads to inflammatory-atrophic changes in the mucosa, which clinically manifest themselves in the form of glossitis, stomatitis, esophagitis, gastritis and enteritis. A typical picture of Hunter's glossitis is characterized by the appearance of bright red areas of inflammation at the tip and along the edge of the tongue, sometimes covering its entire surface (“scalded tongue”). Aphthous rashes and cracks are often observed on the tongue.
Such changes can spread to the gums, buccal mucosa, soft palate, pharynx and esophagus. Ingestion of food and medications is accompanied by a burning sensation and pain. Sometimes patients complain of a feeling of heaviness in the epigastric region, loss of appetite and upset stool. In addition to impaired bone marrow function and mucosal integrity, deficiency of vitamin B12 (but not folic acid!) causes neurological pathology, which is characterized by patchy demyelination of gray matter in the brain, spinal cord and peripheral nerves. It is assumed that a lack of vitamin B12 leads to inhibition of fatty acid metabolism, resulting in the introduction of abnormal fatty acids into myelin and its destruction.
Neurological symptoms of vitamin B12 deficiency vary depending on the severity of the pathology. Early signs include dysfunction of the dorsal horn of the spinal cord with loss of proprioception and vibration sensation. Patients move with difficulty, spreading their legs widely when walking. Later, they develop damage to the pyramidal, spinocerebellar and spinothalamic tracts, accompanied by muscle weakness, progressive muscle spasticity, hyperreflexia, and a scissor-like gait. Peripheral nerve damage may also occur, with loss of deep tendon reflexes, cranial nerve palsy, and loss of sphincter control. With prolonged deficiency of vitamin B12, dementia and neuropsychiatric diseases occur. Neurological symptoms due to vitamin B12 deficiency can occur without anemia.
Laboratory data
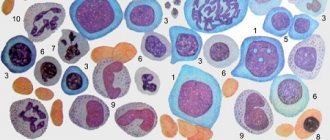
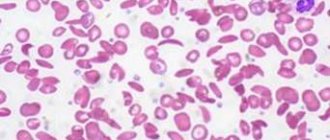
Megaloblastic anemias are macrocytic and normochromic. When viewing blood smears, pronounced macrocytosis is determined with the presence of intensely stained megalocytes, and sometimes megaloblasts (Fig. 29), and poikilocytosis. Often there are schizocytes and erythrocytes with remnants of nuclei in the form of Cabot rings and Havell-Jolly bodies. The number of reticulocytes is reduced. The white blood cell count is usually reduced. Characteristic is a left shift in the leukogram to myelocytes and the presence of giant neutrophils with a hypersegmented nucleus (up to 6–8 segments instead of the usual three). The platelet count, as a rule, does not exceed 100x109/l; sometimes deep thrombocytopenia is observed.
In the bone marrow, which is usually hypercellular, there is a predominance of cells of the erythroid germ (“blue bone marrow”, Fig. 33), megaloblastic erythropoiesis, with the characteristic chromatin structure of erythroblasts (shagreen nuclei), a large number of mitoses (Fig. 34), multinucleated megaloblasts and the presence giant elements of the granulocyte series and atypical megakaryocytes with impaired platelet release.
The lifespan of red blood cells in megaloblastic anemia is shortened to 27–75 days. Increased destruction of circulating megalocytes and megaloblasts in the bone marrow leads to an increase in the plasma content of unconjugated (indirect, unbound) bilirubin. Serum iron levels and bone marrow hemosiderin levels are usually elevated.
Characteristic of megaloblastic anemia is an increase in serum lactate dehydrogenase (LDH) activity, mainly due to the isoenzymes LDH-1 and LDH-2, which is associated with the release of large amounts of lactic acid into the blood and reflects increased destruction of megaloblasts in the bone marrow and megalocytes in the peripheral blood .
Diagnostics
Vitamin B12 deficiency is most reliably established by low serum vitamin B12 levels (less than 160 ng/L). In clinical practice, a reliable diagnostic test for vitamin B12 deficiency is a positive response in the form of reticulocyte crisis to vitamin B12 administration.
The criterion for folate deficiency is a low level of folate (less than 2.5 μg/l) with normal levels of vitamin B12 in the serum and its normal absorption. A diagnostic test for the folic deficiency nature of megaloblastic anemia can consider the development of hematological remission after treatment with folic acid (2 mg/day), which is impossible with vitamin B12 deficiency.
In clinical practice, most often (about 90% of cases) we have to deal with megaloblastic anemia caused by vitamin B12 deficiency and much less often by folate deficiency, but mixed forms also exist (with malabsorption syndrome). Depending on the pathogenetic mechanisms causing the development of vitamin B12 or folate deficiency, all megaloblastic anemias are divided into 4 groups:
1. due to reduced intake from food;
2. due to impaired absorption;
3. due to impaired transport and metabolism;
4. due to increased consumption.
Differential diagnosis
Megaloblastic erythropoiesis is observed in myelodysplastic syndrome. At the same time, in addition to megaloblastic changes, other signs of dyserythropoiesis are observed: binuclear erythroblasts, hemoglobinization disorders and the presence of “bridges” between erythroblasts. In this case, there is no effect from therapy with vitamin B12 and folic acid.
Megaloblastosis and the maturation of oddly shaped red blood cells are characteristic of the picture of bone marrow hematopoiesis in acute erythromyelosis (Di Guglielmo syndrome), however, with this disease there is usually an increased content of blast cells in the myelogram. Vitamin B12 therapy for acute erythromyelosis is also ineffective.
Megaloblastic anemia must be differentiated from other macrocytic anemia, in which normoblastic hematopoiesis is detected. Non-megaloblastic macrocytosis in alcoholism is usually associated with lipid pathology that increases the size of the erythrocyte membrane. The same mechanism is possible in liver diseases and thyrotoxicosis. Alcoholic macrocytosis is observed in all patients who abuse alcohol, and macrocytosis persists throughout the entire life cycle of red blood cells, for a long time after the complete cessation of alcohol intake. An increase in the average volume of red blood cells, detected using a hematology analyzer, is observed in patients with reticulocytosis, since reticulocytes are larger than mature red blood cells. Unlike megaloblastic true macrocytosis, in which the reticulocyte count is reduced, macrocytosis due to reticulocytosis is accompanied by either symptoms of hemolysis or signs of acute blood loss.
Pathogenetic classification of megaloblastic anemia caused by vitamin B12 or folate deficiency
I. Due to reduced intake from food (more often - folate deficiency):
1. nutritional megaloblastic anemia;
2. megaloblastic anemia of alcoholics.
II. Due to impaired absorption:
1. megaloblastic anemia caused by insufficient secretion of internal factor - pernicious anemia and megaloblastic anemia during total and subtotal gastrectomy (B12 deficiency);
2. megaloblastic anemia in diseases of the small intestine (sprue and idiopathic steatorrhea) and its anatomical deficiency (folate deficiency);
3. megaloblastic anemia caused by increased competing consumption: with blind loop syndrome, intestinal diverticula and tapeworm infestation (B12 deficiency).
III. Due to transport and metabolic disorders:
1. megaloblastic anemia when taking anticonvulsants and folic acid antagonists (folate deficiency);
2. megaloblastic anemia in liver diseases (vitamin B12 deficiency).
IV. Due to increased consumption:
1. megaloblastic anemia of pregnancy;
2. megaloblastic changes in hemolytic anemia, tumors, leukemia (folate deficiency).
Megaloblastic anemia due to reduced dietary intake of vitamin B12 or folate (nutritive)
Nutritional megaloblastic anemia, caused by a lack of vitamin B12 in food, is very rare. This type of anemia has been described among people who follow a strictly vegetarian diet, excluding the use of any animal products. In the vast majority of cases, nutritional megaloblastic anemia is caused by a lack of dietary folate, which, although widespread in foods, is easily destroyed by exposure to light and cooking. In addition, the body's reserves of folate are relatively small, and in regular food their content only slightly exceeds the daily requirement.
Nutritional folate deficiency megaloblastic anemia is observed in chronic alcoholics, representatives of the poorest segments of the population, whose diet is devoid of vegetables, meat, eggs and milk; in newborns, often premature, when fed only cow and goat milk and in children receiving a synthetic diet for phenylketonuria.
This type of anemia is also common among residents of some areas of Southeast Asia and Africa, whose diet includes a small amount of meat and vegetables, and whose staple foods (rice and bananas) are subjected to prolonged cooking. Nutritional megaloblastic anemia often occurs with symptoms of glossitis and esophagitis, however, damage to the nervous system is usually absent.
Treatment of nutritional megaloblastic anemia consists of prescribing a diet rich in folates and taking folic acid (1-2 mg/day), as well as vitamins C, B1, B6, B2, PP, etc., since folate deficiency is often combined with deficiencies of other vitamins
Megaloblastic anemia caused by impaired absorption of vitamin B12 or folate
Impaired absorption of vitamin B12 is most often caused by a deficiency of intrinsic factor (IF) in gastric juice, which may be a consequence of impaired secretion of IF from the fundic mucosa of the stomach or its absence during total (subtotal) gastrectomy.
Impaired secretion of VF underlies the so-called pernicious or pernicious anemia (Addison-Biermer disease). This disease, first described by Addison in 1855 and until the 1920s was inevitably fatal (hence the name pernicious anemia), is observed mainly among older people, but can occur in people of any age. The higher incidence of pernicious anemia among residents of Scandinavian countries and African Americans, the high frequency of blood group A in patients and their relatives, and the often familial nature of the disease indicate the possible involvement of genetic factors in the pathogenesis of “malignant” anemia. The overall incidence rate is 25:100,000 people per year among people over 40 years of age.
However, the main role in the development of pernicious anemia undoubtedly belongs to autoimmune mechanisms: in 90% of patients, antibodies against cells of the gastric mucosa are found in the serum and gastric juice, and in 60% of patients, antibodies against VF or the VF-vitamin B12 complex are detected.
The clinical picture of Addison-Birmer disease consists of symptoms associated with anemia, signs of damage to the gastrointestinal mucosa, neurological and mental symptoms; the presence of chronic gastritis with histamine-resistant achlorhydria is pathognomonic.
Diagnosis of pernicious anemia is based on anamnestic data (often a familial nature of the disease), detection of changes in the blood and bone marrow characteristic of megaloblastic anemia, the presence of achlorhydria resistant to histamine stimulation, laboratory and clinical signs of vitamin B12 deficiency (low levels of vitamin B12 in the serum and response for vitamin B12 therapy), positive results of the Schilling test.
The Schilling test is used to assess the intestinal absorption of 57Co-labeled vitamin B12. The patient is given 0.5-2.0 mcg of radioactive vitamin B12 per os, and then 2 hours later 1000 mcg of non-radioactive vitamin B12 is administered intramuscularly to saturate transcobalamin. From the moment of taking labeled vitamin B12, daily urine is collected.
In healthy people, ≥ 7% of the resulting radioactivity is excreted in urine during the day, and in patients with impaired absorption of vitamin B12 - less than 5% (with pernicious anemia, this figure is usually less than 2%).
If VF production is insufficient, its addition to 57Co-labeled vitamin B12 increases the radioactivity of daily urine by at least 5 times. In contrast, the excretion of radioactive vitamin B12 is not significantly altered by impaired absorption of vitamin B12 not associated with VF deficiency.
Although in most cases pernicious anemia can be diagnosed without radioisotope testing, the Schilling test allows one to identify hidden forms of this disease in treated patients and in patients with vitamin B12-deficient neuropathies or psychoses, in whom, after inadequate therapy, megaloblastic changes in the bone marrow may be absent, and neurological violations - persist.
Megaloblastic anemia after total or subtotal gastrectomy usually develops 3-5 years after surgery. With significant similarities to Addison-Birmer disease, agastric megaloblastic anemia is characterized by less severe symptoms of hemolysis and the absence of hypersiderinemia. Often, the decrease in serum iron levels associated with impaired absorption of dietary iron, especially with dumping syndrome, in patients with a resected stomach is so significant that it masks changes in the bone marrow characteristic of megaloblastic anemia, which are detected after therapy with iron supplements.
Treatment of megaloblastic anemia caused by insufficient secretion of VF consists of intramuscular administration of vitamin B12 at a dose of 1000 mcg daily for 2 weeks, and then weekly until the onset of clinical and hematological remission, characterized by the disappearance of all symptoms of the disease, with the exception of histamine-resistant achlorhydria , which persists during the period of remission.
After the administration of vitamin B12, after 24-72 hours, megaloblastic hematopoiesis is transformed into normoblastic, and by 5-6 days the number of red blood cells in the blood increases due to the entry of immature forms into the circulation (reticulocyte crisis). Dyspeptic symptoms and glossitis disappear quite quickly. Neurological lesions are the most resistant to treatment, therefore, in case of funicular myelosis, the administration of 1000 mcg of vitamin B12 should be continued after the hematological parameters are normalized, and then once every 2 weeks for 6 months. To maintain clinical and hematological remissions, patients with pernicious and agastric megaloblastic anemia require lifelong treatment with vitamin B12 (500 mcg 1-2 times a month intramuscularly) or in combination with VF (30 mcg of vitamin B12 and 50 mg VF per os 1 time in Week).
Transfusion of blood or red blood cells is indicated only for pernicious anemic coma, which develops with a very low level of hemoglobin and can threaten the patient’s life due to increasing hypoxemia. Prescribing folic acid for vitamin B12 deficiency is inappropriate. Iron supplements are given only when megaloblastic anemia is combined with iron deficiency.
Megaloblastic anemia due to small intestinal pathology is, as a rule, folate-deficient in nature, since folic acid reserves are limited compared to vitamin B12. Folate deficiency megaloblastic anemia is observed with prolonged enteritis (sprue and idiopathic steatorrhea), with organic lesions of the small intestine (polyposis, lymphomatosis, leukemic infiltration and stricture formation), with resection of a large area of the small intestine, with gastrointestinal fistula.
In addition to impaired absorption of folates, dysbiosis plays a significant role in the development of megaloblastic anenteric anemia, as a result of which the biosynthesis of folates by uric acid bacteria is disrupted. In addition to dysbacteriosis, the development of megaloblastic anemia is facilitated by the appearance in the small intestine of atypical flora (usually Escherichia coli), which competes with the macroorganism in terms of the use of vitamin B12. This mechanism may underlie megaloblastic anemia in blind loop syndrome and small intestinal diverticula.
With a long course of diseases of the small intestine and folate deficiency, vitamin B12 and iron deficiency, protein, mineral and multivitamin deficiency are added.
Treatment of anenteric megaloblastic anemia includes combating the underlying disease and prescribing folic acid in combination with multivitamins.
In addition to diseases that contribute to the appearance of atypical bacterial flora in the small intestine, competition with the macroorganism for vitamin B12 occurs during invasion by the broad tapeworm, which is most often found in the Scandinavian countries, in the Great Lakes region of North America and some lakes on the border of Switzerland and France.
Infection with the broad tapeworm in humans occurs when eating undercooked or raw fish containing prestages of the parasite. The human small intestine sometimes contains up to 3000-4000 tapeworms, some of which can reach 10 meters in length. Megaloblastic anemia as a result of consumption of vitamin B12 by the broad tapeworm develops in only 30% of carriers of this parasite.
The clinical picture of helminthic megaloblastic anemia, depending on the number of parasites in the intestine, varies from slight macrocytosis to full-blown megaloblastic anemia with neurological lesions. Eosinophilia is often detected in the blood.
A long stay of the parasite in the intestines is also accompanied by general exhaustion and the appearance of dyspeptic disorders.
Diagnosis of helminthic megaloblastic anemia is based on the detection of characteristic changes in the blood and bone marrow and tapeworm eggs in the stool.
Treatment of helminthic megaloblastic anemia consists of prescribing vitamin B12, and upon achieving clinical and hematological remission, deworming.
Megaloblastic anemia caused by impaired transport and metabolism of folate and vitamin B12
Megaloblastic hematopoiesis in the bone marrow often develops with long-term use of anticonvulsants (diphenine, phenobarbital) used to treat epilepsy. At the same time, a decrease in folate levels is detected in the blood of patients with a normal concentration of vitamin B12.
Anticonvulsants are thought to interfere with the absorption and transport of folate. It is also possible that anticonvulsants disrupt folate metabolism, acting as direct antagonists. A detailed picture of megaloblastic anemia when taking anticonvulsants is rarely observed and only when combined with an inadequate intake of folate from food.
Prophylactic administration of folic acid during treatment with anticonvulsants usually prevents the development of megaloblastic changes in the bone marrow.
Folate deficiency also develops during treatment with antifolate cytostatics (methotrexate).
Macrocytic anemia is described in chronic liver diseases and is one of the most important indicators of the severity of liver dysfunction. The basis of megaloblastic changes in chronic liver diseases is a violation of the metabolism of vitamin B12, caused by a decrease in its deposition in the liver.
Along with acquired ones, there are hereditary disorders of the transport and metabolism of vitamin B12 and folate.
Immerslund-Gresbeck syndrome is characterized by a genetically determined disorder in the transport of vitamin B12 through the intestinal wall and persistent proteinuria. The secretion of HF and hydrochloric acid is preserved in this disease. There are no antibodies against VF.
Disturbances in the metabolism of vitamin B12 also result from congenital deficiency of transcobalamin II and congenital enzymopathies, in which the conversion of vitamin B12 into coenzyme forms is blocked.
Impaired folate metabolism is observed in hereditary hemocysteine-5-methyltetrahydrofolate methyltransferase deficiency and Lesch-Nahan syndrome (hypoxanthine guanine phosphoribosyl transferase deficiency), characterized by hyperuricemia and increased purine synthesis.
In children with hereditary disorders of folate transport and metabolism, megaloblastic anemia develops already at the age of 3 months. The first symptoms of hereditary vitamin B12 deficiency may appear after 6 months, but they are usually detected by the 3rd year of life.
Megaloblastic anemia caused by increased intake of vitamin B12 or folate
Anemia is one of the most common complications of pregnancy. In most cases, the development of anemia in pregnant women depends on several factors: a significant increase in circulating plasma volume, iron and folate deficiency. Pure megaloblastic anemia due to folate deficiency is quite rare: in 1/5-1/6 pregnant women with a hemoglobin level of 95 g/l.
The development of folate deficiency during pregnancy is due to a 4-10-fold increase in the daily need for folate, especially in the last trimester, with their relatively small reserves in the body.
Multiple pregnancies, malnutrition (often as a result of nausea and vomiting), infections, development of hemolytic anemia, or use of anticonvulsants increase the need for folate. Lactation also significantly aggravates existing folate deficiency in the body. Considering the possible development of folate deficiency during pregnancy, which can be accompanied by complications such as placental abruption, spontaneous miscarriages and bleeding, and patent neural tube of the fetus, it is recommended to prescribe folic acid to all pregnant women at a dose of 5 mg/day.
To treat folate deficiency anemia in pregnant women, large doses of folic acid are required (at least 0.1 g per day).
Vitamin B12 deficiency during pregnancy is extremely rare, since its reserves in the body are quite large. The combination of Addison-Biermer's disease and pregnancy, in which the latter may be accompanied by the development of megaloblastic anemia, is practically excluded, since pernicious anemia mainly affects older people.
Megaloblastic changes in the bone marrow due to increased folate intake can be observed in hemolytic anemias with severe normoblastic hyperplasia, metastatic tumors and leukemia. The level of folate in plasma in these diseases may not change, since their increased consumption is carried out at the bone marrow level by intensively proliferating erythro- and normoblasts, tumor and leukemic cells.
In the presence of megaloblastic changes in the bone marrow, the administration of folate is indicated for patients with hemolytic anemia and patients with neoplasms. Contrary to prevailing beliefs, it has been established that taking folic acid does not worsen the course of chronic myeloid leukemia and acute myeloblastic leukemia.
Folate deficiency is also observed in patients with exfoliative dermatitis, since up to 5-20 mcg of folate per day is lost with the desquamating epithelium. Prophylactic administration of folic acid to patients with exfoliative dermatitis prevents the development of megaloblastic anemia.
Megaloblastic anemia not associated with vitamin B12 or folate
Megaloblastic anemias can sometimes develop in the presence of normal serum vitamin B12 and folate levels. Megaloblastic anemia, caused by a violation of DNA synthesis, which is not associated with a deficiency of vitamin B12 or folate, is observed:
1. during treatment with antimetabolites (6-mercaptopurine, thioguanine, azathioprine, etc.), inhibiting the synthesis of purines, pyrimidines and other nucleotides;
2. with hereditary metabolic disorders (congenital orotic aciduria and hereditary forminotransferase deficiency);
3. in conditions with unknown pathogenesis: pyridoxine - sensitive and refractory sideroblastic anemia and erythremic myelosis or Di Guglielmo syndrome.
Are there any complications?
Possible complications of folic acid deficiency include:
- early aging not only in terms of external signs, but also in terms of the level of lost immunity;
- skin pigmentation;
- development of infertility in men and women;
- progression of heart failure.
Signs of aging appear in the mirror and immune status
Doctors have a special attitude towards signs of deficiency during pregnancy. It has been proven that almost 70% of the development of congenital malformations of the fetus associated with defects of the embryonic neural tube depends on the lack of folic acid in the body of a pregnant woman. To prevent this, you should take vitamin B9 in a timely manner in a daily dose of 800 mcg. The drug reduces the risk of developing autism and diabetes in children.
How to carry out prevention?
The true preventive dose of the drug is determined by the attending physician. Depending on the degree of risk of anemia, from 5 mg per day to 400 mg is prescribed. The maximum dosage should not exceed 1000 mg.
Women who want to carry and give birth to a healthy baby are recommended to start taking folic acid before the period of planned conception and in the first trimester of pregnancy.
Chronic patients who are forced to constantly take anticonvulsants, drugs Metmorphin, Methotrexate, Triamterene, and a group of barbiturates need prophylactic intake of vitamin B9.
You should not determine the dosage of the drug yourself, especially if there is no reason to include yourself in a risk group. It has been proven that unreasonably high levels of the substance in the body contribute to the growth of a malignant tumor. Any vitamins should be taken only as prescribed by a doctor.
Types of anemia
Iron-deficiency anemia
IDA is a syndrome in which the supply of iron in the body is reduced and the synthesis of iron-containing proteins is disrupted. This type of anemia is very common and occurs in both adults and children. Manifested by a decrease in hemoglobin concentration and trophic disorders in tissues. The brain especially suffers from lack of oxygen.
Symptoms in the initial stages should never be ignored!
If suddenly the following symptoms appear for some time, then this is a serious reason to consult a general practitioner:
- constant weakness
- decreased performance
- increased fatigue
- pale dry skin
At first glance, completely harmless symptoms can lead to the development of a serious illness and cause significant damage.
The norm for an adult is up to 5 g of iron.
The state of iron deficiency is provoked by the following conditions:
- chronic and acute blood loss
- diets and fasting
- gastrointestinal diseases
- pregnancy, lactation
- physical activity, sports
If you do not seek help from specialists, then over time the symptoms expand:
- the skin becomes pale with a greenish tint, becomes flabby, dry; hair becomes dry, brittle, quickly turns gray, and begins to fall out;
- nail plates become thin, flat, and can take on a concave shape;
- A characteristic symptom only for IDA is muscle weakness!
Diagnosis of iron deficiency
A complete laboratory diagnosis is required:
- blood test (general and biochemical),
- consultation with a specialist: gynecologist, urologist, proctologist, hematologist
- You may even need a bone marrow puncture.
After diagnostic measures, the therapist prescribes an individual treatment regimen, which is carried out for a long time with iron supplements. Therapeutic dosages are prescribed until hemoglobin levels normalize, then the patient is transferred to prophylactic doses.
Modern studies have shown that following a diet with mild IDA can normalize blood counts and avoid taking iron supplements. You should include foods high in protein and iron in your diet: liver, beef tongue, veal, turkey, red sea fish, eggs, buckwheat, peaches, persimmons, apples. Dairy products and tea should be excluded, because they interfere with iron absorption.
Pay attention to your health, contact experienced specialists, because a correct diagnosis is the key to successful treatment of the disease! Take care of yourself and be healthy!