Norilsk Interdistrict Children's Hospital
A.I. Lobanov, O.G. Lobanova
The article discusses the problems of hemorrhagic disease of newborns with late onset. Currently, in maternity hospitals, the prevention of hemorrhagic disease is not carried out for all newborns. A clinical analysis of 9 cases of the disease was carried out. A dangerous feature of the late manifestation of hemorrhagic disease (after a month of life) is the development of massive intracranial hemorrhages against the background of increased bleeding. The late onset of hemorrhage due to vitamin K deficiency is characterized by a combination of three factors: lack of prevention of hemorrhagic disease, breastfeeding and transient cholestasis.
In the practice of a pediatric resuscitator, non-traumatic intracranial hemorrhages in children in the first months of life are quite rare. It is not always possible to determine the true cause of hemorrhage. At the same time, it is known that one of the reasons for the development of intracranial hemorrhages in children in the first months of life is late hemorrhagic disease of the newborn (LDH). It is impossible to predict the development of late onset of the disease, so prevention of tension-type headache at the maternity hospital stage is extremely important.
The development of HDN in newborns, especially those who are breastfed, is preceded by vitamin K deficiency: gamma-carboxylation of vitamin K-dependent blood coagulation factors is impaired in the hepatocyte. As a result, factors that have not undergone carboxylation lose their ability to participate in the blood clotting process. Immunologically, they are found in the blood in normal quantities, in the form of non-carboxylated and non-functioning molecules, denoted in the literature by the abbreviation PIVKA (Protein-Induced by Vitamin K Absence) [1].
These defective coagulation factors are not able to qualitatively influence blood coagulation processes, which leads to the development of tension-type headache. The most dangerous manifestation of this pathology is intracranial hemorrhage, which occurs against the background of general bleeding (skin hemorrhages, gastrointestinal bleeding).
For the period from 2004 to 2008. 9 children with non-traumatic intracranial hemorrhages were hospitalized in the intensive care unit of City Clinical Hospital No. 4 in Izhevsk. The age of the patients ranged from 1 to 2.5 months of life. Six children were hospitalized from home and three from city hospitals.
Anamnesis
When collecting anamnesis, it was found that all children were from an almost normal pregnancy, term birth, and were breastfed. In the maternity hospital, all newborns were vaccinated against hepatitis B and BCG. Prophylactic administration of sodium menadione (Vikasol) to newborns has not been carried out. On days 5-7, all children were discharged home. Subsequently, 2 children were vaccinated against hepatitis B twice.
Debut Clinic
The onset of the disease was almost the same in all children. 1-2 days before the occurrence of intracranial hemorrhage, hemorrhagic elements appeared on the skin or oral mucosa (Table 1).
Single deep ecchymoses with a diameter of 5 to 20 mm were found by parents more often on the extremities, less often on the torso. Small multiple hemorrhages on the oral mucosa were detected already during examination in the intensive care unit. One sick child had blood in the stool and prolonged bleeding from the injection site during repeated vaccination against hepatitis B. Upon consultation with a surgeon, no pathology was identified.
In all patients, intracranial hemorrhage manifested itself with sudden, painful, but short-lived crying. 8 children immediately developed constant and persistent vomiting, in two cases with blood. Vomiting was not observed in only one child. At first, the children showed periodic restlessness, began to moan, refused to feed, then became apathetic and indifferent to their surroundings. 7 children had tonic convulsions. The skin became increasingly pale. Short-term low-grade fever gave way to hypothermia.
Almost all children were hospitalized very late: the time spent at home from the moment of hemorrhage to hospitalization ranged from one day or more. Upon admission to the intensive care unit, 8 children were in extremely serious condition. All of them had decompensation of pulmonary ventilation, systemic circulatory disorders, focal and cerebral neurological symptoms, and disorders of coagulation hemostasis. In 6 cases, those admitted had rare, arrhythmic breathing or its complete absence. In 2 cases, respiratory disturbances were only in the form of severe tachypnea (respiratory rate - within 100/min).
All patients had severe pallor of the skin and mucous membranes with a cyanotic tint, as well as bleeding from the injection sites. In 2 children there were signs of mild pulmonary and gastric bleeding, in the form of hemorrhagic discharge from the endotracheal tube and gastric tube. Eight children had a slight jaundiced skin tone upon admission. The deficit in circulating blood volume ranged from 30 to 39% of the norm (the norm of bcc in children in the first months of life is 85 ml/kg; bccfact = body weight/weight part of bcc according to hematocrit in the table). Blood pressure ranged from 80/40 to 110/72 mmHg. Art. In 7 cases, children had tachycardia with dull heart sounds. In the neurological status of 6 children, disturbances in the central regulation of breathing were observed. Pathological forms of breathing in the form of severe bradypnea or apnea in these children developed even before hospitalization and continued to progress until the complete loss of automatic breathing. Brainstem reflexes from mucous membranes (cornea, pharynx, trachea) were not evoked. There was severe dryness and swelling of the oral mucosa and sclera. The oculocephalic reflex was absent. Fixed, bilateral, paralytic mydriasis and diffuse muscle atony were detected.
In all children, the large fontanel was bulging, dense to the touch, with no pulsation. Severe hypothermia of the scalp was noted. 5 children experienced attacks of hormetonia (periodic tonic tension of the muscles of the limbs and trunk, occurring against the background of muscle atonia spontaneously or under the influence of irritants, lasting no more than 10 s). In this case, the appearance of hormetonia is associated with damage to the trunk at the level of the midbrain and pons due to transtentorial herniation, when functional separation of the trunk and cerebral hemispheres occurs. As the coma deepened, the attacks of hormetonia stopped. Thus, all 6 patients were diagnosed with a 3rd degree coma (extraordinary). All patients admitted to the intensive care unit in a state of extreme coma died.
Two children were diagnosed with grade 1-2 coma upon admission to the intensive care unit. Tonic convulsions, transient symptoms of damage to the III, VI, VII pairs of cranial nerves, and tachypnea were noted. The large fontanelle was dense, bulging, but retained its pulsation. The reaction of the pupils to light, brainstem reflexes from the mucous membranes (cornea, pharynx, trachea), and oculocephalic reflex remained intact. One patient had signs of moderate intracranial hypertension in his neurological status.
Laboratory examination
In peripheral blood tests, most patients showed a significant decrease in hemoglobin levels (Table 2).
In all patients, blood clotting time (Morawitz) was sharply prolonged. The prothrombin index was determined in only one patient and was reduced. The platelet count was normal or elevated and tended to increase further as the day progressed. Normal fibrinogen levels were observed in 4 patients; in the rest, fibrinogen was not determined. Direct bilirubin remained elevated in 8 children, liver enzymes were slightly elevated in 1. Diagnosis
All children had subarachnoid hemorrhages. In 8 cases the hemorrhages were massive. Diagnosis of intracranial hemorrhages was carried out on the basis of anamnesis, clinical data and ultrasound examinations. Carrying out neuroimaging research methods (CT, MRI of the brain) in the vast majority of patients was impossible due to the extremely serious condition of the patients. In 3 patients, after the bleeding subsided, when intracranial hypertension (irreversible cerebral edema) was not extremely pronounced, the hemorrhage was confirmed by lumbar puncture (one day later). Liquor often flowed out under low or normal pressure and was red-brown in color. When centrifuged, sharp xanthochromia was revealed; microscopy revealed large numbers of altered (hemolyzed) red blood cells. A biochemical study of the cerebrospinal fluid determined low levels of glucose and high levels of protein and lactic acid (lactate). Therapy
8 children were on artificial ventilation. All patients underwent correction of blood volume, hemostatic and metabolic disorders, as well as anticonvulsant and neuroprotective therapy. Relief of bleeding began with the introduction of 10 mg of Vikasol through a tube, a single transfusion of fresh frozen plasma in a volume of 15 ml/kg. With the cessation of bleeding (usually after 10-12 hours), further administration of sodium menadione (intramuscular) was continued to create a depot of 5 mg/day for 2-3 days. Eight patients received red blood cell transfusion due to the development of posthemorrhagic anemia. Subsequently, none of the patients had any bleeding. Autopsy results
At autopsy, massive subarachnoid hemorrhages were discovered in the dead children; in 2 cases with the presence of blood also in the subdural space and in 1 case - in the ventricular system of the brain. In 2 cases the brain was in a state of necrosis. In addition, all the dead children had changes in the liver, most often in the form of nonspecific reactive hepatitis with extracellular cholestasis and initial fibrosis of the portal tracts.
Discussion
During clinical observation, it was noted that all children admitted to the intensive care unit with generalized bleeding were united by a number of common factors and clinical features characteristic of the late onset of HDN. The main factor is the lack of prevention of tension-type headache. The incidence of late onset tension-type headache without vitamin K prophylaxis, according to some data, is 5-20 cases per 100 thousand newborns [2]. None of the 9 children admitted to the intensive care unit with generalized bleeding were treated in the maternity hospital with the administration of sodium menadione.
The second factor is breastfeeding. All observed children were full-term and were exclusively breastfed. Under physiological conditions, vitamin K (K± or phylloquinone) enters the child’s body with food (breast milk) and is additionally synthesized in the intestine in the form of vitamin K2 or menaquinone. But the synthesis of vitamin K2 in the intestines occurs mainly by Bacteroides fragilis and some Escherichia coli - flora that is colonized during artificial feeding.
During natural feeding, the intestines are populated by Bifidobacterium, Lactobacillus and Clostridium - flora that are practically incapable of synthesizing vitamin K2 [3]. The third factor is the phenomenon of transient cholestasis. They were noted in 8 cases. Natural vitamin K is absorbed in the small intestine in the presence of bile and fat. A decrease in bile flow leads to malabsorption of fats and fat-soluble vitamins, increasing even greater vitamin K deficiency. Newborns, due to the immaturity of the excretory function of the liver, are especially prone to cholestasis [4].
In 8 out of 9 children admitted to intensive care after the age of 1 month of life, the level of direct bilirubin still remained elevated (from 12 to 27 mmol/l), which indicates transient, functional and excretory liver failure. Of the clinical features characteristic of the late onset of HDN, it should be noted: firstly, the development of massive intracranial hemorrhages in combination with skin hemorrhages and bleeding from the gastrointestinal tract. Secondly, the observed children did not have severe pathology at the onset of the disease with a high risk of developing DIC syndrome.
Almost on the eve of the development of late TTH, all children were examined by local pediatricians. Thirdly, late hospitalization of these children in the intensive care unit. The reason for the late hospitalization of the observed children was the fact that the increase in intracranial pressure in children in the first months of life occurs more slowly due to the fact that the child’s skull (before the fusion of the cranial sutures and the closure of the fontanelles) remains temporarily pliable and adapts to the emerging conditions. When blood enters the intrathecal space of the brain, the threatened symptoms are delayed for some time, so the parents sought medical help too late.
Fourthly, changes in peripheral blood and cerebrospinal fluid. These changes are adaptive reactions of a sanogenetic nature. They are caused by blood poured into the intrathecal space and its breakdown products. Therefore, the observed children experienced a reactive increase in body temperature, changes in peripheral blood were noted in the form of leukocytosis and a shift in the leukocyte formula to the left. The entry of the blood itself and its breakdown products into the cerebrospinal fluid space caused reactively expressed pleocytosis and hyperproteinrachia. These changes are also protective (sanogenetic) in nature and disappear as the cerebrospinal fluid is cleared of blood by the end of 2-3 weeks.
Blood breakdown products have pronounced toxicity (oxyhemoglobin, serotonin, bilirubin, etc.) and additionally cause severe cerebral ischemia, leading to cerebral infarction. And finally, another feature of late HDN is the hypocoagulation direction of the results of available tests for assessing hemostasis and their stabilization during the administration of vitamin K (Vikasol).
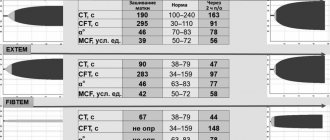
The basis of laboratory diagnosis of tension-type headache is the determination of prothrombin time and index, which reflect the total level of (three out of four) vitamin K-dependent coagulation factors (II, VII, X) (Table 3).
In TTH, the platelet count and thrombin time should be normal [5]. In 8 observed children, the prothrombin index was not determined, and in 1 it was reduced. None of the observations revealed a decrease in the number of platelets; on the contrary, in response to prolonged bleeding, compensatory thrombocytosis developed in 7 cases. This circumstance excludes the involvement of platelets in pathological consumption reactions associated with DIC syndrome.
In the absence of clinical signs of DIC, fibrinogen was not detected upon admission in more than half of the patients. This is due to a number of reasons, such as a decrease in the protein-synthetic function of the liver during the development of a critical condition, the influence of hypovolemia, hypoxia, and acidosis. In the classic form of HDN, which occurs in newborns in the maternity hospital, in contrast to its later form, intracranial hemorrhages, such severity and decompensation of vital functions do not occur.
Conclusion
Based on the clinical observation, it can be concluded that full-term newborns are susceptible to the occurrence of late tension-type headache, in whom a combination of factors such as the lack of prophylactic administration of sodium menadione, breastfeeding and transient cholestasis became possible. In newborns who are expected to breastfeed, prevention of tension-type headache is especially important.
REFERENCES 1. Barkagan 3. S. Introduction to clinical hemostasiology. - M.: Newdiamed-AO, 1998. - P. 21-23. 2. Dolgov V.V., Svirin P.V. Laboratory diagnosis of hemostasis disorders. - M.: Triada, 2005. - 213 p. 3. Gusel V. A., Markova I. V. Pediatrician’s Handbook of Clinical Pharmacology. - Leningrad, 1989. - pp. 161-163. 4. Shabalov N. P. Neonatology. Volume 2. - St. Petersburg, 1996. - P. 95. 5. Barkagan 3. S. Hemorrhagic diseases and syndromes. - M.: Medicine, 1988. - 498 p.
Read the article on the website www.evrika.ru
General information
Hemorrhagic syndrome or a tendency to hemorrhage (bleeding) is bleeding of the skin and mucous membranes caused by pathological changes in one or more components of hemostasis .
They usually consist of damage to the vascular walls, disturbances in the structure, functional abilities and number of platelets, and deviations in coagulation blood stasis. In addition to superficial hemorrhages, this pathological condition is characterized by nasal, gastrointestinal and uterine bleeding, as well as bleeding gums. In addition, hemorrhages can occur in internal organs, including brain tissue, retina and joint cavities.
When determining the causes of hemorrhagic syndrome, it is important to consider that acquired and hereditary types of pathology may occur a little more often. Acquired disorders are usually caused by secondary thrombocytopenia and thrombocytopathy , disseminated intravascular , prothrombin factor deficiency, and hemorrhagic vasculitis . Hereditary pathological changes are usually represented by hemophilia A and B , von Willebrand disease and vascular form of telangiectasia .
Classification
There are 5 types of hemorrhagic syndrome:
- Hematoma - caused by a deficiency of blood coagulation factors VIII, IX and XI, usually found in hemophilia A or B, manifesting itself as painful intense hemorrhages into the thickness of soft tissues (petechial rashes) and joints, which leads to gradual functional disorders of the musculoskeletal system, characterized by the appearance of delayed bleeding - a couple of hours after injuries, even hemorrhages in the brain tissue are possible - most often due to thrombocytopathies.
- Petechial-spotted, capillary, microcirculatory - the so-called bruise variety, which occurs with deficiency of blood coagulation factors II, V and X, is usually caused by thrombocytopenia , thrombocytopathy , such disorders of the coagulation system as hypofibrinogenemia , dysfibrinogenemia and hereditary deficiency of blood clotting factors.
- The mixed, microcirculatory-hematoma form is usually initiated by severe deficiency of factors of the prothrombin complex and factor XII-XIII, von Willebrand disease, disseminated intravascular coagulation syndrome, overdose of thrombolytics and anticoagulants, the appearance of immune inhibitors of factor VIII and IX in the bloodstream, externally manifested by a combination of petechial-spotted skin hemorrhages and individual large hematomas in the retroperitoneal space and intestinal wall; in extremely rare cases, hemorrhages occur in the joint cavity, and the resulting bruises are widespread and painful.
- The vasculitic purpuric form is caused by infectious or immune vasculitis and can easily transform into DIC syndrome; it is characterized by hemorrhagic rashes and the development of erythema with an inflammatory process, the addition of nephritis and the development of intestinal bleeding are possible.
- The angiomatous type of hemorrhagic syndrome can occur in the area of telangiectasia, angioma or arteriovenous shunt in the form of persistent local hemorrhages in areas of vascular pathologies.
Pathogenesis
There are several pathogenetic mechanisms for the development of hemorrhagic syndrome:
- Along the path of coagulopathy - in individuals with hereditary or secondary (acquired) deficiency of plasma blood clotting factors - procoagulants , as a result of their insufficient activation, synthase disorders, and also, on the contrary, excessive activation of anticoagulants .
- Loss of platelet link - a pathological condition occurs as a result of insufficient number of platelets (with immune conflicts, lack of vitamin B12 , aplastic anemia , tumor processes in the bone marrow and mechanical destruction as a result of splenomegaly ) or in case of a violation of their adhesive-aggregation properties in various thrombocytopathies.
- Hyperfibrinolytic conditions are associated with excessive fibrinolysis.
- Caused by pathologies of the vascular wall or its increased permeability.
Causes
Hemorrhagic syndrome is usually caused by various clotting factor deficiencies and thrombocytopathy, including:
- hemophilia A or B;
- injuries, hypothermia and overheating;
- ARVI and acute bacterial infections;
- preventive vaccination;
- acute radiation injuries;
- von Willebrand disease;
- systemic lupus erythematosus;
- thrombocytopenic purpura or Werlhof's disease ;
- DIC syndrome;
- HIV infection;
- severe liver damage;
- infectious and immune vasculitis;
- hypo- or dysfibrinogenemia.
The psychogenic form of hemorrhagic syndrome is usually represented by neurotic bleeding or can be caused by Munchausen syndrome .
It must be remembered that due to the general availability of medications, hemostasis disorders - the development of hemorrhagic syndrome can be provoked by taking medications - non-steroidal anti-inflammatory drugs, glucocorticoids, anticoagulants and antiplatelet agents, which interfere with blood clotting and the ability of platelets to aggregate.
Symptoms
In the complex of symptoms of hemorrhagic syndrome there are various manifestations, the most pronounced are externally noticeable changes, as well as:
- petechiae - a pinpoint rash that occurs when there are changes in vascular-platelet hemostasis, consists of multiple elements no larger than 3 mm in size, does not rise above the skin and is not palpable, most often violet, purple or red;
- bruising and hematoma formations - can be subcutaneous, muscular, etc. most often caused by coagulation disorders, characterized by pain and a gradual increase in size;
- dissecting hematoma is usually caused by changes in coagulation hemostasis and is a false channel filled with blood of varying length, located in the media of the aorta;
- the presence of many superficial skin hemorrhages ( ecchymoses ) of small size - no more than 3 mm, which can also be observed on the mucous membranes;
- “delayed bleeding” - occurring several hours late;
- hemarthrosis - hemorrhages in the joint cavities, which cause an increase in the volume of the joint, local hyperemia and increased temperature of the skin, as well as a pronounced pain syndrome ;
- bleeding even with minor injuries - cuts or scratches.
Diet for hemorrhagic syndrome
Diet Table No. 1
- Efficacy: therapeutic effect after 3 weeks
- Terms: 2 months or more
- Cost of products: 1500 - 1600 rubles. in Week
Most often, when hematological disorders are detected, dietary Table No. 1 , which is characterized by a fairly high calorie content and fractional nutrition. The menu usually consists of such dishes and ingredients as:
- vegetable soups with breadcrumbs;
- steamed poultry breast;
- boiled fish;
- eggs;
- semi-viscous porridge with milk;
- berries and fruits – ground in jelly and compotes;
- There are practically no restrictions on the types of oil, the main thing is that it is natural, unsalted and in moderation.
Excessively salty, peppery, fried, fresh dough, fatty meat, canned food, snacks, carbonated drinks and coffee should be excluded from the diet. “Specific prohibitions” include the need to avoid white cabbage, mushrooms, sorrel, spinach, onions and cucumbers.
Tests and diagnostics
The basis for suspecting a violation of the vascular-platelet and coagulation processes of hemostasis are skin hemorrhagic manifestations, their nature and appearance. Differential diagnosis is carried out taking into account the number of peripheral blood platelets and the obtained data from simple coagulation tests, including the Rumpel-Leede-Konchalovsky test, blood coagulation rate, bleeding time, etc.
A fairly important aspect of the examination is determining the root cause of the pathological condition, that is, the disease that caused the hemorrhagic syndrome.
Mallory-Weiss syndrome and differences from gastrointestinal bleeding
The most important task of the diagnostician is the ability to timely recognize Mallory-Weiss syndrome , which is manifested by vomiting blood, causing mild gastrointestinal bleeding and melena . Factors contributing to the development of this condition include repeated vomiting as in bulimia , alcoholism , also a hiatal hernia, chronic inflammatory diseases of the upper part of the digestive tube, severe cough, portal hypertension , etc.
With gastroesophageal rupture-hemorrhagic syndrome, both rupture of only the lining integumentary structures and more deeply lying submucosal and muscular structures can occur with complete rupture of the esophagus and the risk of developing peritonitis , mediastonitis and pneumothorax . In this case, bleeding in the mildest form of the pathology can resolve spontaneously after a few days; therapy usually includes the use of cold and antacids ; in more severe cases, endoscopic treatment or gastrotomy may be necessary.