| Alkalosis, , blood pH, body pH, urine pH, urine pH measurement, saliva pH, acidification of the body, acidity of the body, acidosis |
Maintaining acid-base balance in the body is very important for the normal functioning of all internal human systems. The pH level determines the optimal activity of all enzymes involved in metabolism.
When the balance of acids and alkalis is disturbed, the activity of enzymes decreases, metabolism is disrupted, which is why toxins begin to accumulate in the body. Therefore, the first stage of cleansing the body of toxins is restoring the pH balance.
The lifestyle of a modern person often leads to disturbances in the acid-base balance in the body. Most often, people suffer from increased levels of acidity - acidosis . This is caused by modern lifestyle.
An increase in the acidity of the body is caused by a lack of diet and a decrease in physical activity, stress, strict diets, alcohol abuse and smoking.
Acidosis is now much more common than excess alkali - alkalosis.
What it is?
Stomach acidity is an indicator that characterizes the amount of hydrogen ions in the lumen of the organ. Its values are most influenced by hydrochloric acid, which is produced by the parietal cells of the mucous membrane. In addition, gastric juice contains bicarbonates, water, and various enzymes. They, as well as food or drink, can change the acidity of the stomach.
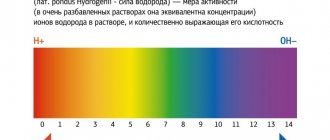
Help The unit of measurement throughout the world is the hydrogen index (pH). The acidity of pure water at a temperature of 25°C was chosen as the standard, which is 7.0. When it increases, the indicator drops to zero, and when it decreases, it increases to 15.
Acidity plays an important biological role. Due to the high concentration of hydrochloric acid, pathogenic bacteria that enter the digestive tract along with food are killed. Also, under conditions of high acidity, pepsin is activated , an enzyme that takes part in the breakdown of proteins. In addition, its fluctuations affect the functional activity of the pancreas and the motility of the digestive tract.
What to do if the pH is lower than normal?
We can regulate our acid-base state using three main mechanisms:
- physical activity and proper breathing;
- choosing certain foods;
- the use of various biologically active components.
If the pH deviates towards the acidic side, it is necessary to increase the content of alkaline foods in the diet. The daily diet of a healthy person should include at least 75-85% of alkalizing foods, and in the diet of a person suffering from acidification, their share should be increased to 90%.
Alkalinizing foods include vegetables and fruits. Freshly prepared vegetable or fruit juices alkalize the blood more effectively. And if you add 1-2 spoons of Eureka BALANCE , you will get a powerful alkalization of the body, and much softer and safer than what happens when you take soda internally.
Norm
The acidity level differs in different parts of the stomach in a healthy person. This is due to the fact that parietal cells, which produce hydrochloric acid, are located mainly in the area of the body and bottom of the organ. And most of the secretory cells that produce bicarbonates and mucus are located immediately before the entrance to the duodenum.
There are also age differences. In newborns, acidity significantly reduced , which is due to the nature of their nutrition and the low activity of parietal cells. Starting from the first days of life, it gradually increases, and already at 3-4 months it reaches adult levels.
Standard indicators of acidity in various parts of the stomach are collected in the following table:
| Index | Norm, pH |
| Gastric lumen on an empty stomach in adults (basal acidity) | 1,5-2 |
| Antrum | 1,6-7,3 |
| Vault | 1,0-4,6 |
| Back wall | 1-1,8 |
| Front wall | 0,9-1,4 |
| Deep in the epithelial layer | 5,8-7,7 |
| The lumen of the body of the stomach on an empty stomach in newborns | 4,2-6,6 |
Deviation from the norm has many negative consequences for the patient:
- increased risk of developing an inflammatory process or a defect in the mucous membrane of the stomach or duodenum;
- disruption of the functioning of stomach enzymes (pepsin, lipase), which slows down the digestion process;
- increased or decreased motility of the digestive tract;
- increased risk of developing intestinal infections and food poisoning;
- changes in the functioning of the pancreas.
Rules for measuring urine pH:
- The first one froze. We do not measure the first morning urine because it contains more acid than other urine collections. It contains all the acids filtered and stored by the kidneys overnight. The first test is done during the second urination in the morning.
- The second measurement is taken before lunch.
- The third one is before dinner. It is important to test before meals because the pH changes quickly depending on the foods consumed.
- We record events that could affect the pH in the note For example, too much lunch, dinner at a restaurant, drinking alcohol, working overtime, playing sports, severe stress and other overloads.
- We take the average for 4 days. For example: (6 + 7 + 7 + 6.5 + 6.5 + 6.5 + 7 + 6.5 + 6.5 + 6 + 6.5 + 6.5):12= 6.54
| Measurement day | 1 | 2 | 3 | 4 | |
| Morning | index | 6 | 7 | 7 | 6,5 |
| note | dinner, feast | ||||
| Day | index | 6,5 | 6,5 | 7 | 6,5 |
| note | Stress at work | ||||
| Evening | index | 6,5 | 6 | 6,5 | 6,5 |
| note | workout |
- Results.
pH below 7 (pH acidic)
Urine is oxidized. The internal environment of the body is also oxidized. The lower the pH, the higher the oxidation of the environment. For example, at a urine pH of 6 to 6.5, the internal environment is slightly oxidized, and at a pH of 5 to 4.5, it is highly oxidized.
The acidic environment of the body is the cause of all diseases caused by oxidation. We advise you to immediately take measures to deoxidize the body.
pH between 7 and 7.5 (pH neutral)
We are talking about the normal pH value for a person in good health. We need to strive for this value. This is true, but with one condition: if the first morning urine is oxidized (the one we did not measure). If the first collection is also neutral, this is not acceptable for a healthy person. The first urine collection in the morning removes the acids filtered out overnight and must be acidic.
If this is not the case, then the kidneys do not remove acids well, and the pH remains unchanged all day. Acids that are not removed from the body remain inside, and the internal environment becomes oxidized.
pH above 7.5 (pH alkaline)
- The internal environment of the body is in acid-base balance or slightly alkaline. This most often occurs when the diet consists only of alkaline foods. This may occur in vegetarians who eat few grains and dairy products. Also, people who daily consume a complex of mineral substances, which they either do not need, or the need for them is not great, can have an alkaline pH. But these are special cases; alkaline pH is not a serious disorder or disease.
- People who consistently have a urine pH above 7.5 have poor glands (the adrenal glands or parathyroid glands) or other rare diseases. Usually these people are aware of their illnesses, know that they are caused by such an imbalance, and are under the supervision of doctors.
- The third group is the most common. These are people whose urine contains a lot of alkali, and the internal environment of the body, on the contrary, is oxidized. The alkaline pH of the urine in these people is not caused by excessive consumption of bases in food (which the body would try to get rid of, as is the case with excess acids), but by too much withdrawal of bases from organic tissues to neutralize the highly oxidized internal environment of the body.
There are three options:
This often occurs in people suffering from acid metabolism disorders. Insufficiently oxidized acids do not leave the body through the respiratory tract. The kidneys come to the aid of the body; they take on double duty. But if the kidneys are weak, acids accumulate in quantities dangerous to the body.
It is very important to pay attention to changes in pH levels in time and, if necessary, take urgent measures.
What affects the pH of gastric juice?
The following factors can increase it:
- irregular or unbalanced diet;
- smoking;
- gastritis;
- presence of Helicobacter pylori infection;
- gastrinoma (benign tumor of the pancreas);
- frequent stressful situations ;
- excessive activation of the parasympathetic nervous system;
- long-term use of certain groups of medications (non-steroidal anti-inflammatory drugs, glucocorticoids);
- severe concomitant diseases or injuries that require hospitalization in the intensive care unit.
A decrease in acidity occurs due to the following factors:
- autoimmune damage to parietal cells;
- long-term malnutrition (cachexia);
- gastritis;
- presence of Helicobacter pylori infection;
- radiation therapy for malignant tumors;
- decreased thyroid function (hypothyroidism);
- cardiovascular pathologies, atherosclerotic changes in the arteries of the stomach;
- portal hypertension (increased pressure in the portal vein system due to chronic liver diseases);
- long-term use of antibiotics , cytostatics, hormonal anti-inflammatory drugs.
Symptoms
An increase or decrease in acidity is accompanied by various symptoms of digestive disorders. Their severity is more pronounced in childhood and young age. In elderly patients, detecting them is quite problematic:
| Symptoms of increased acidity | Symptoms of low acidity |
|
|
How to check PH?
There are various methods for determining pH levels. Some of them are widely used in clinical practice and have become the diagnostic standard for gastric diseases, others have experimental value or low information content.
Gastroscopy (FGDS)
FGDS is considered the gold standard for diagnosing gastric pathologies. To carry it out, it is necessary to insert a special probe (endoscope) through the mouth or nose . The procedure is done under local anesthesia of the posterior wall of the oropharynx, sedation or general anesthesia.
At the end of the endoscope there is a sensor that measures the acidity of the environment in real time. The received data is transmitted to the screen.
Please note: FGDS makes it possible to measure not only
the acidity of gastric juice in various areas of the stomach , but also in the esophagus , as well as the acidity of intestinal juice in the duodenum .
The study is carried out on an empty stomach, so basal acidity is measured. In addition, the doctor has the opportunity to visually assess the condition of the gastric mucosa, and, if necessary, conduct a biopsy followed by cytological examination in the laboratory.
Help The price for determining acidity is on average 300 rubles. and is an additional analysis to gastroscopy. In order not to swallow the probe twice and to choose the most effective treatment for gastritis, we recommend that you definitely add this analysis when paying for an FGDS.
Acidotest
This research technique allows you to approximately estimate the acidity of the stomach without inserting a probe. It is used in practice if there are contraindications to FGDS (for example, recent myocardial infarction). But its accuracy is quite low, which significantly reduces the value of the Acidotest results.
The patient drinks 2 tablets of caffeine on an empty stomach, which is a stimulant of gastric secretion. Then he gives the first portion of urine, after which he is given 3 tablets of the drug to drink. They contain a resin that, when reacted with hydrochloric acid, forms a dye. It gets into the urine and turns it scarlet. Its intensity is compared with a special color scale , which allows you to assess the acidity of the environment in the stomach.
Acidotest
Blood analysis
Assessing the concentration of gastrin and pepsinogen in the blood allows us to approximately assess the severity of gastric secretion. Their amount in the blood is directly dependent on the level of acidity. The norm for gastrin 17 is 3-25 pmol/l, and for pepsinogen – 30-150 µg/l.
The disadvantage of the analysis is its lack of accuracy , which is often not enough to determine treatment tactics. However, laboratory blood tests to assess gastric acidity remain a promising area that many research centers are working on.
Litmus test strip
An indirect method for assessing acidity by measuring it on the tongue. There is evidence that the pH values of the stomach and oral cavity are interrelated. In the oral cavity, it should normally remain in the range of 6.6-7.4 , which corresponds to a neutral environment.
Before the procedure, it is advisable not to eat for 1.5 hours . To evaluate, take 1 litmus strip and place it on the upper surface of the tongue for a few seconds. With increased acidity, the litmus color will appear orange, and with low acidity, it will turn green or blue.
Important The accuracy of the study is low. Therefore, doctors do not recommend using it in diagnosis.
Determination at home
There are several other methods for roughly assessing stomach acidity at home. However, their information content remains quite low.
How to find out your body's pH?
Using pH test strips, you can easily, quickly and accurately determine your pH level.
To make a conclusion about the state of the internal environment of the body, one measurement is not enough. The pH value can change throughout the day depending on the activity of the body, food taken, physical activity, stress, etc. For the readings to be objective, you need to take them several times a day for 4-5 days in a row.
Enter the results obtained into a table, and then a complete picture of urine pH will appear.
Self-diagnosis test
Using a short questionnaire, you can preliminarily determine the type of gastric acid disorder at home.
The first group of questions includes:
- Do you experience burning pain in your stomach or chest after eating?
- Do you experience heartburn after fatty, spicy or sour foods?
- Are you prone to constipation?
- Do you feel heaviness in your upper abdomen after eating?
- Is burping sour?
If the patient says “yes” to most of the answers, then he most likely has increased acidity.
The second group also consists of 5 questions:
- Do you feel quickly full while eating?
- Does burping have an unpleasant or putrid odor?
- Do you experience periodic aching abdominal pain, regardless of food intake?
- Are you prone to diarrhea or flatulence?
- Can nausea occur on an empty stomach?
If the answers to most of these questions are positive, the patient most likely has low acidity.
What is the normal pH of water
Due to the rapid pace of modern life, poor nutrition, and poor eating and drinking habits, the pH level in the human body drops. Thus, the acid-base balance shifts towards increased acidity (pH up to 7 implies an acidic environment, and up to 14 - alkaline, respectively, the lower this level, the higher the acidity), which can lead to serious diseases. This problem can be solved by daily drinking mineral water with an optimal level of hydrogen ion activity. That is why it is important to know what pH value is normal for the water you regularly drink.
Take the test
So, what should the pH of water be? Professionals argue that this value should approximately correspond to the normal pH of human blood (7.5). That is why the pH standard for drinking water is calculated from 7 to 7.5. Thanks to clean drinking water with a normal level of hydrogen ion activity, metabolic processes in the body are improved, overall life expectancy increases and oxygen exchange is optimized. Conversely, sugary, carbonated and colored drinks reduce the pH of human blood, which can be immediately noticed by an unpleasant dry mouth.
Therefore, it is best to give preference to water with the “correct” pH value. You can always find this information on the label of any bottle. No filter with fillers and absorbents can replace real natural water with an optimal pH level. Some try to lower the pH of the water and add beneficial properties to the liquid by adding lemon or cucumber juice, however, this does not always have the desired effect. Another well-known method of changing the pH of water is electrolysis, which allows you to obtain alkaline and acidic water in two containers. Alkaline water with a high pH is considered “living”, it is used for treatment, and acidic water is “dead”, which is most often used for washing.
However, such methods are not suitable for daily use. In this situation, there is only one rational solution - to give preference to low-mineral natural water with the acidity level necessary for health.
Read the material on the topic: Is it possible to drink tap water?
First aid
To quickly reduce stomach acidity, the patient is advised to drink a glass of still alkaline mineral water . As an alternative, you can use antacids (Almagel, Maalox, Rennie, Phosphalugel). These drugs neutralize hydrochloric acid in the lumen of the stomach, which quickly normalizes acidity and relieves the patient of unpleasant symptoms. You can check out our rating of antacids.
Popular antacids
For effective therapy it is necessary to establish the cause of the pathology . If Helicobacter pylori infection , then antibacterial therapy with 2-3 drugs is required. In other cases, the use of proton pump inhibitors (omeprazole, pantoprazole, rabeprazole) is sufficient. They allow you to reduce the secretion of hydrochloric acid for 18-24 hours after administration.
In case of reduced acidity, replacement therapy with hydrochloric acid preparations, as well as pepsinogen, shows effectiveness. antispasmodics helps relieve pain and discomfort . But at the same time, it is necessary to carry out a thorough diagnosis and treatment of the causes in the near future, since such patients have an increased risk of developing stomach cancer.