Article for the bio/mol/text competition: Many drugs compete with natural molecules for binding to their target. Most of these drugs bind to proteins through weak interactions, but some are able to form strong bonds, “switching off” their target until the end of its “life,” albeit at the cost of their own. Such drugs belong to the class of irreversible covalent inhibitors, figuratively called suicide inhibitors. They will be discussed in our article. How do some of the most effective drugs work and how dangerous are they? Whose disease helped discover aspirin? What do nicotine and grapefruit juice have in common? You will find answers to these and many other questions below.
Description of inhibitors
What is a corrosion inhibitor? Corrosion inhibitors are special substances that stop (delay) the process of chemical and physical reactions. Corrosion inhibitors occupy a special place among such substances.
Inhibitors include agents that form a special protective film on the metal surface, which is obtained during the reaction of an inhibitor solution and corrosion products.
The emergence of compounds that slow down corrosion processes was a breakthrough. At the moment, most methods of protection are protection using inhibitors. The most popular substances in this capacity are amines, nitrogen-containing substances, urea, sulfides, aldehydes, etc.
The effectiveness of protective processes involving inhibitors directly depends on the metal, the characteristics of the external environment, pressure on the material, etc.
It is worth noting that the corrosion inhibitor protection does not work constantly; when the additive gets into the solution, it gradually dissolves, so in the future it is necessary to add it to an aggressive environment in small portions.
3rd generation drugs
The latest generation of ACE inhibitors are characterized by a mild effect and minimal side effects. But at the same time, the result after administration occurs no earlier than 30-60 minutes, which is not always acceptable.
3rd generation drugs include fosinopril and ceronapril. These products are equivalent in their effects and are often prescribed for long-term use. Moreover, the effect after administration lasts for a long time. Medicines are not suitable for emergency situations.
Types and uses of metal corrosion inhibitors
The main types of inhibitors include:
- Cathode – reduce the rate of cathodic interaction.
- Anodic - inhibit the dissolution of the anode.
- Mixed - additives that slow down reactions, both cathodic and anodic.
There is a classification of inhibitors by origin:
- Organic are organic substances that are more versatile because they reduce the rate of cathodic and anodic reactions. These include nitrogen, sulfur, oxygen, and aromatic compounds. The main advantage and difference from inorganic inhibitors is the fact that organic substances are adsorbed only on the surface of the material, without reacting with rust.
- Inorganic corrosion inhibitors, what are they? They contain inorganic substances as part of the inhibitor. The peculiarity of working with inorganic particles in an inhibitor is that if the concentration is incorrectly selected, they may not protect the metal by forming a thin film on it, but rather react with corrosion products and accelerate the destruction process. Includes chromates, sodium and potassium bichromates, calcium bicarbonate, etc.
The principle of action of inhibitors
Classification of inhibitors by mechanism of action
Main types:
- Working in an acidic environment - amines, acetylene alcohols, sulfur-containing compounds, aldehydes. This type of substance is used in the gas and oil production industries; they cover pipelines carrying gas or oil products, as well as products involved in these processes. An acidic environment corrosion inhibitor actively fights cathodic and mixed destruction.
- Inhibitors for neutral environments - phosphates, nitrites, amino acids, chromates, alkylphosphates, sulfonates. They are most widely used in the field of water supply, cooling, and are used on marine vessels. Here, as elsewhere, the inhibitor solution is used as a protective coating for any products of the listed industries, containers, load-bearing structures, and individual elements.
- Occurring in an alkaline environment. The substances are involved in the composition of special detergents. Their action is based on the fact that they reduce the strength of current in its chemical sources. Inhibitors for such purposes are most often used together with cations.
Common Types of Acid Inhibitors
The most economical anti-corrosion inhibitor is acidic. Its consumption in the process of metal etching is minimal, which entails a reduction in the cost of the product and the protection procedure as a whole.
The properties of acid inhibitors can also be supplemented by the fact that when applied to a material, it also cleans it of the formed scales and various oxide films. Also, the additive does not change its properties, is not transformed, and is not destroyed when the ambient temperature increases.
On the domestic market you can most often find such corrosion inhibitors as I-5-V and I-5-VM. These additives are intended for products made of low-carbon, medium-carbon and high-carbon and alloy steels. They are effectively used in industry, and both inhibitors can be combined with each other.
The use of corrosion inhibitors with this composition reduces metal waste, helps clean the surface of the etched material, and also has a beneficial effect on sanitary and hygienic working conditions.
Properties of corrosion inhibitors
All properties of inhibitors are reduced to anti-corrosion protection of metal products. The mechanism of operation is simple: the inhibitor contained in the solution is applied to the surface of the element and protects it from the external influence of aggressive environments. Protection is created by adsorption (increasing the concentration of the inhibitor in the solution and on the surface of the material, respectively) on the metal element. After the appearance of the protective film, its main task is to be completely neutral to external influences, not to change its properties under pressure, temperature, etc., only in this case will the inhibitor be able to fully demonstrate its properties and protect the structure as a whole.
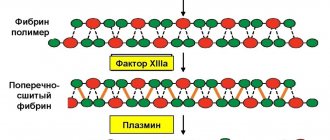
Is there a future for irreversible covalent inhibitors?
For many years, irreversible inhibitors were surrounded by an aura of skepticism, stemming from the toxicity of some members of this group discovered in the 1970s. But the effectiveness and selectivity of many drugs in this series have proven that fears regarding them were not justified. Of course, they have potential danger due to the possibility of nonspecific interactions and allergic reactions, but clinical practice shows their indispensability in the treatment of a number of diseases. Now the number of drugs in this group is growing steadily [6].
The difficulty is that the researcher must pursue several goals at once: increase the selectivity of the drug to the target enzyme and carefully consider the mechanism of its action. But the properties of irreversible covalent inhibitors, such as high selectivity and efficiency, long duration of action, and a reduced risk of developing drug resistance, make this group of drugs an indispensable tool in the arsenal of doctors and drug developers [6].
Cathodic corrosion inhibitor
This type of additive slows down the dissolution of metal during the cathodic process. The system potential moves from its usual neutral state to the negative side, which leads to a decrease in the corrosive current, and an anti-corrosion film is formed on the surface. This film is difficult to dissolve not only under normal conditions, but also for many aggressive environments. It becomes a barrier between the external environment and the metal, maintaining its integrity.
What is cathodic inhibitor corrosion protection? Most often, these are compounds that increase the acidity of the environment, which reduces the possibility of metal dissolution.
Cathode additives are not used in acidic environments, as they are not effective there.
As noted earlier, the inhibitor must be dissolved in some substance before use, the simplest one being water. Experts select the correct concentration of the additive in a given volume of water.
It is worth noting that this type of substance is the least effective in comparison with anodic and mixed inhibitors.
pharmachologic effect
Drugs that inhibit lipase block the activity of the only enzyme that is responsible for the breakdown and absorption of fats - pancreatic lipase, which is part of the pancreatic juice of the pancreas.
Pancreatic lipase breaks down complex fats (triglycerides), which are not absorbed in the intestine due to the large size of the molecule, into simple molecules - fatty acids and monoglycerides.
An enzyme blocked by the drug is not able to perform its function of breaking down and absorbing complex fats. Since fats are not digested, the total intake of calories in the body decreases. This ultimately leads to weight loss for the overweight patient.
Anodic corrosion inhibitors
These substances are passivators - substances that pass from an active state to a passive state and form a film. This film protects the metal element. Corrosion slows down due to a decrease in the rate of transition of metal ions into the solution, and the area of the anodic areas covered with the film decreases due to their separation.
It is the anodic additives, if you overdo them, that may not reduce, but accelerate the corrosive destruction of the material. Carbonates, silicates, phosphates, and sodium nitrite are popular as anodic corrosion inhibitors.
Indications for use
Different generations of proton pump inhibitors are prescribed for the prevention and complex treatment of the following diseases:
- gastritis with high acidity ;
- in combination with the eradication of Helicobacter pylori;
- gastroesophageal reflux disease;
- Zollinger-Ellison syndrome;
- functional dyspepsia;
- structural damage to the gastric mucosa caused by prolonged or uncontrolled use of non-steroidal anti-inflammatory drugs;
- stomach and duodenal ulcers;
- long-term antibiotic therapy.
Drugs from the group of proton pump inhibitors are also actively used during general anesthesia, when it is necessary to avoid Mendelssohn syndrome (reflux of gastric contents into the respiratory tract or reflux disease).
Drugs from this group are available in dosage forms such as tablets, lyophilisate, capsules (including enteric soluble), powders for the preparation of infusion solutions and oral solutions.
Application of inhibitors in the oil and gas industry
Oil and gas are quite aggressive environments, as they contain caustic substances that can corrode materials and adversely affect human health. Based on this, special additives for the protection of metal structures are used at any stage of oil and gas production, transportation, storage and operation. An important aspect is that it is advisable to use the same inhibitor at all these stages.
In addition to protecting the metal, impurities can be added to the petroleum products themselves, which make it a less aggressive environment: anti-corrosion paraffin inhibitors, anti-ignition agents and anti-foaming agents. The most common substance for protecting main oil and gas pipelines is amine-based compounds.
A breakthrough in the treatment of chronic heart failure (results of the PARADIGM-HF study).
Introduction.
Angiotensin-converting enzyme inhibitors (ACE inhibitors) have been the cornerstone of the treatment of heart failure (HF) with reduced ejection fraction for the past 25 years, ever since enalapril was shown in clinical trials to reduce the risk of death from HF. [1]
Long-term enalapril therapy was subsequently shown to reduce the relative risk of death by 16% among patients with mild to moderate symptoms.[2]
Another class of drugs used in the treatment of HF are angiotensin II receptor blockers, however, they are primarily recommended in cases of intolerance to ACE inhibitors (usually due to the development of cough).
Neprilysin is a neutral endopeptidase that destroys several endogenous vasoactive peptides, including natriuretic peptide, bradykinin and adrenomedullin. Inhibition of neprilysin increases levels of the above substances, which counteracts vasoconstriction, sodium retention and remodeling. Combined inhibition of the renin-angiotensin system and neprilysin is superior to either therapeutic strategy alone in experimental studies. However, such treatment is associated with the development of severe angioedema.
The use of a new drug, LCZ696, containing the neprilysin inhibitor sacubitril (AHU377) and the angiotensin II receptor blocker valsartan, according to research results, is accompanied by a minimal risk of developing angioedema.
To prove the benefits of LCZ696 compared to enalapril, a double-blind study was conducted in patients with heart failure and reduced ejection fraction.
Materials and methods.
The study consisted of three phases - a screening period, a period during which all patients received enalapril, a period during which all patients received LCZ696, and a double-blind phase in which patients were divided into 2 groups - one received enalapril, the other received LCZ696.
Inclusion criteria were: age ≥18 years, NYHA class II, III and IV chronic heart failure (CHF) and ejection fraction ≤40%. Patients included in the analysis had to have a serum brain natriuretic peptide (BNP) level ≥ 150 pg/ml or N-terminal pro-BNP (NT-proBNP) ≥ 600 pg/ml or if they were hospitalized for HF in within 12 months before inclusion in the study, then the level of BNP ≥ 100 pg/ml or NT-proBNP ≥ 400 pg/ml.
The exclusion criteria were: symptomatic hypotension, systolic blood pressure less than 100 mm Hg. Art. during the screening period and 95 mm Hg. Art. during the randomization period, glomerular filtration rate (GFR) below 30 ml/min, serum potassium level >5.2 mmol/l, a history of angioedema and intolerance to ACE inhibitors and angiotensin II receptor blockers.
In the blinded phase of the study, patients received LCZ696 at an initial dose of 100 mg twice daily, which was subsequently increased to 200 mg daily. The angiotensin II receptor antagonist component LCZ696 was equivalent to 160 mg of valsartan.
The primary endpoint of the study was death from a cardiovascular cause or first hospitalization for HF. Secondary endpoints were: time to death from any cause, change in Kansas City Cardiomyopathy Questionnaire (KCCQ) score at 8 months, time to an episode of atrial fibrillation, and time to first episode of decline in renal function (GFR decline of at least by more than 50% or more than 30 ml/min compared to the original).
Results.
From December 2009 to November 2012, the study included 10,521 patients from 1,043 medical centers in 47 countries. Of these, 2079 were excluded from the study at the randomization stage and 43 were randomized with errors, as a result of which all of them were excluded from further analysis.
Subsequently, 4187 patients were randomized to receive LCZ696 and 4212 to receive enalapril.
Cardiovascular death or hospitalization for HF was reported in 914 patients (21.8%) in the LCZ696 group and in 1117 (26.5%) in the enalapril group; The HR for the LCZ696 group was 0.80; 95% CI, 0.73-0.87, P
558 deaths (13.3%) in the LCZ696 group and 693 (16.5%) in the enalapril group had a cardiovascular cause (HR = 0.80; 95% CI, 0.71-0.89; P
Over the entire follow-up period, 711 patients (17.0%) died from any cause in the LCZ696 group compared with 835 (19.8%) in the enalapril group (HR=0.84; 95% CI, 0.76-0.93; P
The mean KCCQ score at 8 months decreased by 2.99 points in the LCZ696 group and by 4.63 in the enalapril group (difference between groups, 1.64 points; 95% CI, 0.63 -2.65; P=0.001).
Atrial fibrillation occurred in 84 patients in the LCZ696 group and 83 in the enalapril group (P=0.84). Decreased renal function was diagnosed in 94 and 108 patients, respectively, P=0.28. At the same time, 8 patients taking LCZ696 and 16 enalapril developed end-stage chronic kidney disease during treatment (P=0.11).
Safety.
Patients receiving LCZ696 compared with those receiving enalapril were more likely to experience symptomatic hypotension. However, cough, serum creatinine ≥2.5 mg/dL (221 µmol/L) and potassium >6 mmol/L were more often diagnosed in the enalapril group (P
After 8 months, the average systolic blood pressure was lower in the LCZ696 group by 3.2±0.4 mmHg. Art. compared to the enalapril group (P
Angioedema was detected in 19 patients receiving LCZ696 and 10 enalapril (P=0.13).
Conclusion.
The new drug LCZ696 is associated with a reduced risk of death and hospitalization due to heart failure compared with enalapril.
The combination of an angiotensin II receptor antagonist and neprilysin inhibitor is superior in effectiveness to either component alone in patients with chronic heart failure.
Source: John J. V. McMurray, MD, Milton Packer, MD, Akshay S. Desai, MD, MPH, Jianjian Gong, Ph.D., Martin P. Lefkowitz, MD, Adel R. Rizkala, Pharm.D., Jean L. Rouleau, MD, Victor C. Shi, MD, Scott D. Solomon, MD, Karl Swedberg, MD, Ph.D., and Michael R. Zile, MD Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. August 30, 2014.
Literature.
- The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987;316:1429-1435.
- The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991;325:293-302.
Use of inhibitors at home
Corrosion processes affect not only metal products in industrial and construction industries, but also any metal products in our home, as well as machines and devices.
The simplest home remedy with an inhibitor is a primer. As always, the surface must be prepared and then primed with a spatula or brush. The primer creates a protective layer on the product, separating the metal and the environment.
Some paint coatings also contain inhibitors.
The simplest and most understandable inhibitors for household use are minium (red lead paint) added to the primer, iron or zinc orthophosphates added to the solution, etc.
Red lead paint
The use of inhibitors when processing equipment
Corrosion in technology is the greatest threat to human life. Often, a corroded area in a large member is not as bad as corrosion in a bolted joint or weld.
Most often, of course, car owners are faced with the problem of rust and take their cars to a service station so that the specialists themselves can process the material and apply an anti-corrosion coating, whether using inhibitors or not.
The destruction of equipment is difficult to stop, since areas susceptible to destruction are usually difficult to access, treatment with a corrosion inhibitor, and cleaning the affected area is difficult. In inaccessible places, they try to use liquid compounds for better application and distribution - oils, hot bitumen compounds, soft lubricants, etc.
Side effects
Serious side effects while taking ATP inhibitors occur most often during treatment with first-generation drugs. In most cases, there is dizziness, which can cause fainting. Sometimes nausea, vomiting, stool upset and various other dyspeptic symptoms occur.
If the prescribed dosage is exceeded, there is a sharp decrease in blood pressure below normal, problems with kidney function or hyperkalemia. While taking ACE inhibitors of any generation, a dry cough or allergic reactions may occur. Very rarely, cases of angioedema have been reported with monotherapy. If such symptoms appear, you should immediately consult a doctor.