Peripartum cardiomyopathy (Meadows Cardiomyopathy, Peripartum disease)
Conservative therapy
After confirmation of the diagnosis, patients are necessarily hospitalized in a cardiology hospital.
In case of severe circulatory failure, transfer to the intensive care unit for intra-aortic balloon counterpulsation is indicated. Despite the rapidly progressive course, almost half of the patients experience spontaneous recovery. Non-drug treatment includes limiting fluid intake to 2 liters per day and table salt to 3-4 grams per day. When prescribing drug therapy to pregnant women, it is necessary to take into account the potential undesirable effect of certain medications on the fetus. Recommended:
- Dopamine receptor agonists.
The drugs act on the main pathogenetic link of PPCM. By suppressing the production of prolactin, they reduce the formation of its cardiotoxic fragment (16 kDa). Bromocriptine is used in pregnant women; after childbirth, it is possible to switch to cabergoline or quinagolide. - ACE inhibitors.
Essential medications that slow the progression of heart failure. - Beta blockers.
Prescribed as an alternative to ACE inhibitors for pregnant women, as well as in the presence of tachyarrhythmias. - Vasodilators.
Vasodilator medications are used to relieve vasospasm when systolic pressure is more than 110 mmHg. Art. - Diuretics.
To reduce congestion in the lungs and remove fluid, thiazide and loop diuretics are used. - Cardiac glycosides.
To maintain adequate cardiac output during arterial hypotension, digoxin and levosimendan are indicated. - Anticoagulants.
Since dilatation of the cardiac cavities is accompanied by a high risk of thrombus formation, patients are recommended to take anticoagulants (warfarin, rivaroxaban). Only heparin is allowed for pregnant women.
Surgery
In case of severe CHF (left ventricular ejection fraction reaches less than 40%), which develops in pregnant women, termination of pregnancy by cesarean section is required. Extremely severe peripartum cardiomyopathy, resistant to conservative therapy, is an indication for heart transplantation. For persistent tachyarrhythmias accompanied by severe hemodynamic disturbances (critical drop in blood pressure), implantation of a cardioverter-defibrillator is used.
Introduction
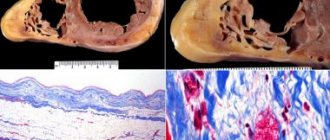
Dilated cardiomyopathy, like myocarditis, is one of the most difficult diseases from a diagnostic point of view, the final verification of which is based on endomyocardial biopsy and magnetic resonance imaging, which are not yet available to conventional cardiology clinics [1, 2], therefore, the accumulated clinical experience in managing such patients seems interesting and may be useful for cardiologists and doctors of other specialties in their practice.
Peripartum cardiomyopathy (PCM), as one of the variants of dilated cardiomyopathy, develops in women in the last months of pregnancy or within 5 months after delivery. Since the emergence of the concept of PCM in cardiology, first described in 1971 by Demakis et al. [3], a fairly large amount of information has accumulated. Diagnostic criteria for PCM were determined [4, 5], results of long-term follow-up of patients were obtained, and recommendations for effective and safe therapy were developed [5]. There are no statistical data on PCM in Russia. According to world statistics, the pathology occurs in less than 0.1% of pregnant women [6], however, the frequency of adverse outcomes with PCM is quite high (5–32%) [6]. The prognosis of the disease is quite difficult to predict - from complete recovery with restoration of myocardial contractility to death due to progressive heart failure [7], information about the etiology and pathogenesis of PCM remains contradictory.
Below is a clinical case of patient P., 37 years old, who has been observed in the cardiology department No. 1 of the Regional Clinical Hospital (RCH) in Saratov for 10 years.
Clinical case
In December 2007, at the 20th week of pregnancy, patient P. (28 years old at that time) noted the appearance of mixed shortness of breath when walking up to 20 m, palpitations, and fatigue. She associated these symptoms with the addition of ARVI (she noted low-grade fever and influenza-like syndrome). She was treated independently (trained as a laboratory technician), mainly with folk remedies. However, subsequently, despite the normalization of body temperature, the symptoms persisted, and by the 34th week of pregnancy, shortness of breath began to bother me at rest, at night and in a horizontal position, intensified with little physical activity, a dry cough, swelling of the lower extremities appeared, and palpitations continued. She was hospitalized at the Saratov perinatal center, where she was delivered by caesarean section. Data from transthoracic echocardiography (EchoCG) performed in the perinatal center are presented in the first column of the table. 13 days after the cesarean section, the patient was transferred for further diagnosis and treatment to the cardiology department No. 1 of the Saratov Regional Clinical Hospital, where she continues to be monitored over time.
Data from an objective examination of the patient: height – 163 cm, weight – 68 kg, body mass index – 26 kg/m2. Body temperature – 36.6°C. There is no swelling in the lower extremities. Mixed shortness of breath when walking 20 meters. Percussion determined a displacement of the left border of the heart 1.5 cm outward from the midclavicular line. On auscultation, the heart sounds are weakened, the systolic murmur at the apex, associated with the 1st sound, decreases, occupies 2/3 of systole, intensifies on exhalation, is carried to the axillary region, the accent of the 2nd tone is over the pulmonary artery. Heart rate – 120 per minute. Blood pressure – 100 and 80 mm Hg.
An assessment of the clinical manifestations and results of EchoCG, as well as the chronology of the development of identified hemodynamic disorders during pregnancy clearly demonstrated that, according to the data obtained, which met the diagnostic criteria for PCM [4, 5], this particular pathology was identified in the patient. The attention of cardiologists was attracted by the high values of some acute-phase indicators (rheumatoid factor - RF, C-reactive protein - CRP), while markers of myocardial necrosis were normal (CPK - 18.3 U/l, CPK-MB - 8.1 U/l , troponins – I, T negative). An increase in CRP and RF indicators raised doubts about the correctness of the diagnosis, because could indicate an infectious or rheumatic process. At the same time, negative markers of myocardial necrosis, an increase in the level of which in most cases accompanies myocarditis, raised doubts regarding the inflammatory nature of myocardial damage and largely indicated the presence of cardiomyopathy. To exclude the rheumatic process, the following indicators were additionally examined: antistreptolysin O, LE cells, antibodies to DNA (IgG class), circulating immune complexes (CIC), the results of which were negative. During a consultation of rheumatologists, the diagnosis of rheumatic heart disease was excluded.
It should be noted that, according to data accumulated in the literature to date, a significant role in the pathogenesis of PCM in patients with this pathology is assigned to the inflammatory component, which is confirmed by an increase in indicators such as tumor necrosis factor α, interferon γ, interleukin-6, apo- 1, SRB [8]. Thus, in our clinical case, an increase in a number of acute phase parameters fit into the clinical picture of PCM.
The relationship between PCM and myocarditis is of particular interest. After ruling out a rheumatic process in the patient, the cardiologists were left with the possibility of infectious myocarditis, which could have developed against the background of acute respiratory viral infection, which was indicated in the patient’s medical history.
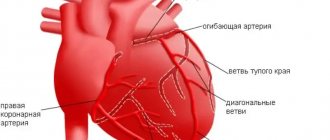
The clinical and pathogenetic relationship between PCM and myocarditis is not completely clear and is the subject of further study. On the one hand, in patients with PCM, a pathomorphological picture of cardiomyocyte necrosis against the background of interstitial and perivascular inflammation has been described [9]. There is evidence that myocarditis often precedes the development of PCM [10] and has a high prevalence among patients with this disease (from 29 to 100%) [11]. Other authors point to a rather rare combination of PCM and myocarditis [12, 13]. In the case of our patient, it is difficult to judge the primary development of myocarditis preceding PCM; this requires highly informative imaging examination methods.
In any case, the observation of patient P. over time demonstrates the persistence of persistent dysfunction of the left ventricular myocardium and the development of chronic heart failure, requiring appropriate symptomatic therapy.
The amount of therapy provided to the patient in the hospital: torasemide 10 mg per day, spironolactone 100 mg per day, perindopril 4 mg per day, carvedilol 12.5 mg per day, ivabradine 10 mg per day. The patient was discharged as an outpatient with recommendations to continue this therapy in full.
After 3 months (March 2008), the patient was admitted to the cardiology department No. 1 again. There was a normalization of all acute phase parameters, which remained within normal limits during subsequent hospitalizations, while myocardial systolic dysfunction persisted according to echocardiography.
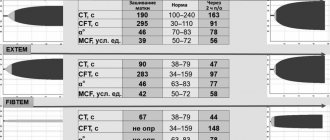
From 2009 to 2021, the patient underwent annual examination and treatment in the cardiology department No. 1 of the Saratov Regional Clinical Hospital. The figure shows an analysis of the patient's complaints and symptoms for some years of hospitalization. We should dwell on the addition since 2008 of interruptions in the work of the heart, which, according to the results of ECG and Holter ECG monitoring, were caused by frequent polymorphic ventricular extrasystoles. The persistence of extrasystoles in dynamics against the background of constant use of β-blockers required an increase in antiarrhythmic therapy; amiodarone was prescribed, the effect of which was positive, but the patient had side effects in the form of photosensitivity, skin rash, and therefore the patient was subsequently prescribed etacizin 100 mg/day. Since 2011, the patient has been diagnosed with dyslipidemia (total cholesterol up to 5.5 mmol/l, low-density lipoproteins 4.0 mmol/l, high-density lipoproteins - 1.8 mmol/l, triglycerides - 0.7 mmol/l, index atherogenicity – 3.4). A low-cholesterol diet was recommended, which the patient tried to adhere to for about 5 years; the issue of prescribing statins is currently being considered. The dynamics of echocardiography data are presented in the table.
The main symptoms and echocardiography data at the time of the last planned hospitalization of patient P. (37 years old) in August 2021 are presented in the table and figure. The patient continues to take angiotensin-converting enzyme inhibitors (perindopril 2.5 mg per day), β-blockers (carvedilol 25 mg per day), spironolactone 50 mg per day, etacizine 100 mg per day. Continues his work activity. Disabled person of the 3rd group. Shortness of breath only bothers me when walking 300–400 meters. Interruptions in heart function are much less common (according to Holter ECG monitoring: rare supraventricular and ventricular extrasystoles). He is hospitalized in the cardiology department once a year. It should be noted that, being a medical professional, the patient demonstrates high adherence to the therapy, which is an important factor in achieving certain positive treatment results.
Discussion
PCM remains a rare but seriously concerning extragenital pathology. Recovery from PC, according to the literature, is defined as restoration of the left ventricular ejection fraction (LVEF), according to echocardiography, to ≥50%, or its increase by more than 20% compared to the initial value [14]. Restoration of myocardial contractility in patients with PCM usually occurs within 3 to 6 months, but can take several years [15]. A number of factors cause a delay in myocardial recovery in PCM: delayed diagnosis, high class of circulatory failure according to NYHA, belonging to the Negroid race, blood clots in the left ventricular cavity, multiparity, concomitant pathology [16–18].
In a two-year cohort study of 123 patients with PCM, LVEF increased from 28 to 46%, about half of the patients reached values >50.5% [19]. The increase in myocardial contractility was greatest at a baseline level of LVEF >30%, and the smallest at LV end-diastolic dimension (LVEDV) >5.6 cm [19]. The worst prognosis in terms of myocardial recovery was observed in patients with an initial increase in troponin and CRP levels [8]. Patients with PCM are not recommended to have a second pregnancy, even with complete recovery of the myocardium, because pathology tends to recur [14].
The initial value of LV EDR, which was 6.54 cm in our patient, a high level of CRP, and shortness of breath at rest at the onset of the disease are associated with a worse prognosis in terms of restoration of the pumping function of the myocardium. Indeed, in the clinical observation we presented, the increase in contractility of the left ventricular myocardium occurred slowly - over 5–8 years (see figure). It is possible that the development of rhythm disturbances – polymorphic ventricular extrasystole and the subsequent addition of hypotension, which led to a decrease in the effective doses of drugs affecting myocardial remodeling, had a certain negative impact on the rate of recovery of the heart muscle.
Patient P. cannot be classified as having fully recovered from PCM, because LVEF did not reach 50%, but its increase over time was 15%. However, despite the predictors of an unfavorable outcome, the development of rhythm disturbances and hypotension, the presented clinical case can be considered an example of successful management of a patient with this pathology. Against the background of drug therapy, stabilization of hemodynamics, a significant increase in LVEF, and a decrease in the size of the heart cavities were achieved.
Conclusion
Currently, non-coronary myocardial diseases continue to attract the attention of researchers and remain one of the most difficult pathologies to diagnose in cardiology. Further study and accumulation of data on PCM should contribute to the development of unified diagnostic and treatment strategies for this disease that are accessible to most clinics and will help save the lives of many patients.
Postpartum (peripartum) cardiomyopathy
Previous | Contents | next Postpartum, or peripartum, cardiomyopathy, according to the definition of a special WHO expert group (1980), means causeless congestive heart failure that developed in the third trimester of pregnancy or in the first 2 months after childbirth in previously healthy women.
The incidence of peripartum cardiomyopathy in Europe and North America is relatively low - 1 case per 1300-4000 births (J. Pierce et al., 1963, etc.), it is much higher in African countries, where it reaches 1 %
(I. Vgos-kington, G. Eolington, 1972).
The pathogenesis of myocardial damage is unknown. As numerous studies have shown, in terms of its morphological substrate and the nature of changes in cardiohemodynamics (according to echo and angiocardiography), postpartum cardiomyopathy is indistinguishable from idiopathic dilated cardiomyopathy. Endomyocardial biopsy can detect signs of healing myocarditis in 33-78 %
such patients (J. San^
Derson et al., 1986; M. Medei et al., 1990, etc.), i.e. more often than with idiopathic dilated cardiomyopathy. A number of women also show serological signs of a previous viral infection and changes in the immunoregulatory subpopulations of T-lymphocytes - an increase in the ratio of helpers to suppressors in the blood (J. Sanderson et al., 1986). There are isolated observations of a noticeable positive effect of immunosuppressive therapy with azathioprine, when signs of chronic inflammation in the myocardium disappeared. Thus, there is reason to assume that some cases of postpartum cardiomyopathy are associated with a subclinical viral infection with the development of myocarditis. At the same time, the increased load on the myocardium caused by pregnancy and childbirth contributes to a deeper damage than would be expected under other circumstances, which probably has an autoimmune pathogenesis. However, it should be noted that in more than half of patients with postpartum cardiomyopathy, it is not possible to detect a connection between the disease and myocarditis, and the nature of the myocardial damaging factor (or factors) remains unclear.
Clinic. Increased risk factors for postpartum cardiomyopathy include Negroid race, age over 30 years, a history of more than 3 births, as well as multiple pregnancies and late gestosis (M. Lampert, R. Lang, 1955, etc.).
The onset of the disease is subacute. The symptoms and signs associated with biventricular congestive heart failure, cardiomegaly, arrhythmias, and thromboembolism are the same as those associated with idiopathic dilated cardiomyopathy. However, the course and prognosis of postpartum cardiomyopathy are more favorable. More than 50% of patients after conventional symptomatic treatment of congestive heart failure after 3-6 months experience persistent clinical improvement and even recovery with normalization of heart size and function (M. Rolfe et al., 1992; G. Cloatre et al., 1996, and etc.). Moreover, subsequent pregnancies are associated with an increased risk of recurrence of the disease, and maternal mortality in such cases reaches 10 %.
If cardiomegaly and signs of heart failure persist for 6 months or more, the prognosis for recovery is unfavorable and is indistinguishable from that for idiopathic dilated cardiomyopathy.
The factors that determine the course of the disease are unknown. Perhaps the mechanisms of myocardial damage and its causes are different, which determines the different prognosis.
Treatment is symptomatic. It is carried out in the same way as for idiopathic dilated cardiomyopathy. If heart failure occurs during pregnancy and is not inferior to drug therapy, termination of pregnancy is necessary. An important means of secondary prevention is the exclusion of repeated pregnancies and childbirths.
Pregnancy in patients with cardiomyopathy/HF: HFA, ESC consensus document
Pregnancy is a condition accompanied by increased stress on a woman’s body. In this connection, it is obvious that its onset can contribute to the decompensation of any chronic diseases, including heart disease. In addition, pregnancy itself can be a factor in one of the forms of dilated cardiomyopathy - periportal cardiomyopathy. In this regard, experts from the Heart Failure Association of the European Society of Cardiology decided on the need to create a consensus document on risk stratification and management of women with cardiomyopathies/heart disease who are planning pregnancy or are pregnant. First of all, the document highlights general approaches to the management of pregnant and lactating patients with heart failure. It is emphasized that, if possible, pregnancy should be prolonged to 32 weeks. The preferred method of delivery, as in healthy pregnant women, is vaginal delivery, accompanied by adequate pain relief. At the same time, it is noted that in most cases, in the presence of heart failure, delivery is possible only by cesarean section. Next, we discuss general issues of managing pregnant patients with both congenital forms of cardiomyopathies (hypertrophic, arrhythmogenic dysplasia of the right ventricle) and acquired ones (periportal, dilated, Takotsubo syndrome). Particularly relevant is the section that discusses some aspects of the management of pregnant women who have previously undergone treatment for cancer and who have developed heart failure during pregnancy. Risk factors for the development of heart failure in such patients have been identified. They are: a decrease in left ventricular ejection fraction <50%, chemotherapy and radiation therapy for cancer, diagnosis of cancer at a very young age <10 years, time from cancer to pregnancy >15 years. In general, it is obvious that any research in this area is almost completely absent. In this regard, the decision on the optimal treatment method should be made by a team of specialists, including obstetricians-gynecologists and cardiologists. In addition, detailed counseling of such patients when planning pregnancy is important.
Source:
Sliwa K, et al. Eur J Heart Fail. 2021. doi: 10.1002/ejhf.2133.
Causes
There are three groups of main causes of the development of primary cardiomyopathy: congenital, mixed, and acquired. Secondary include cardiomyopathies due to any disease.
Congenital heart pathology develops due to a violation of the formation of myocardial tissue during embryogenesis. There are many reasons, ranging from the bad habits of the expectant mother to stress and poor nutrition. Also known are cardiomyopathies of pregnancy and inflammatory cardiomyopathies, which essentially can be called myocarditis.
Secondary forms include the following types:
- Cardiomyopathy is accumulated or infiltrative. It is characterized by the accumulation of pathological inclusions between cells or in cells.
- Toxic cardiomyopathy.
- Endocrine cardiomyopathy (metabolic cardiomyopathy, dismetabolic cardiomyopathy) occurs due to metabolic disorders in the heart muscle.
- Nutritional cardiomyopathy is formed as a result of malnutrition, and in particular during long-term diets with limited meat products or fasting.
The manifestations of HCM are diverse and depend on a number of factors: the degree of myocardial hypertrophy, the presence and magnitude of the pressure gradient, mitral valve insufficiency, which is often found in patients, the degree of reduction of the left ventricular cavity and the severity of the violation of the pumping and diastolic functions of the heart, etc. There are often no complaints.
There are 3 groups of ILC:
Hypertrophic. Dilation (stagnation). Restrictive.
Symptoms of dilated cardiomyopathy:
- Increasing heart failure.
- Shortness of breath on exertion.
- Fast fatiguability.
- Swelling in the legs.
- Pale skin.
- Blueness of fingertips.
Symptoms of hypertrophic cardiomyopathy:
- Dyspnea.
- Chest pain.
- Tendency to fainting, palpitations.
Symptoms of restrictive cardiomyopathy:
- Edema.
- Dyspnea.
The main instrumental method for diagnosing all types of cardiomyopathies is cardiac ultrasound.
Daily Holter monitoring of the electrocardiogram allows you to assess the frequency and severity of cardiac arrhythmias and intracardiac blockades, as well as the effectiveness of the treatment. Laboratory diagnostics are important for monitoring the effectiveness of therapy in assessing the state of water-salt balance, to exclude some side effects of drugs, as well as to identify secondary causes of heart damage.