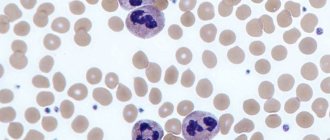
Normoblasts (normocytes) are the last, still nuclear, stage of red blood cells (erythrocytes) on the way to an adult, full-fledged state.
At this stage, normoblasts have a nucleus, so that, having lost it, they can turn into a young, nuclear-free cell containing hemoglobin and already capable of performing the main task of red blood cells (participation in respiration).
Before becoming normoblasts, future red blood cells go through a certain path.
As is known, all blood elements originate from a stem cell - it is the ancestor of future leukocytes, platelets, erythrocytes, etc., since it gives rise to several sprouts, among which is the erythrocyte (from it will come the cells of the erythroid series, including, and those of interest to us are normoblasts).
The youngest, morphologically distinguishable cell of the red row is the erythroblast,
which was formerly called proerythroblast. This is a fairly large cell (14 - 20 microns), containing an equally large nucleus, but does not even have signs of what an adult red blood cell is so valued for - it does not contain hemoglobin.
Normal blood level is zero
Normally, these cells are not found in the blood,
therefore, there can be no talk of increased values of normoblasts when studying preparations (norm – 0).
They may or may not exist, and then it’s time to count if they can be detected. The exception to these rules is newborn children.
In a child in the first days of life, their presence in a general blood test should not be surprising: the increased content in the bone marrow and the appearance of normoblasts in the blood is explained by the increased production of erythropoietin during this period, which leads to an increase in red blood cells and hemoglobin. A few days later, when erythropoietin production decreases, the values of these indicators also drop down.
After some time from birth, more precisely, between 2 and 3 months of life, the child again has an increased level of
normoblasts and reticulocytes and again due to increased synthesis of erythropoietin, which does not cause concern for pediatricians, because this process is physiological.
After this surge, the child’s norms for these indicators will begin to approach the adult norms more and more every month, therefore, normoblasts will also not be found in children’s blood products, but will begin to be detected, as they should be, only in the myelogram.
normoblasts released from the bone marrow into the blood
Their absence in the blood, however, does not exclude their diagnostic significance, because they do not appear just like that, but due to some pathological changes, one way or another, affecting the main hematopoietic organ - the bone marrow.
Since the word “blast” means “sprout,” all descendants of blast can no longer be considered sprouts, so it would be more correct not to apply this name to further forms, but to attach the ending “cyt” to them. In this regard, the outdated word “normoblasts” is present in the vocabulary of specialists with extensive experience only out of habit, while young doctors already call this cell a normocyte.
Hypochromic anemia - symptoms and treatment
Treatment of iron deficiency anemia
IDA therapy includes:
- treatment of the disease that caused the anemia;
- diet therapy;
- prescribing iron supplements: oral (in the form of tablets) or parenteral (in the form of intravenous or intramuscular injections);
- blood transfusion - transfusion of red blood cells.
Goals of treatment for IDA:
- relieve signs of anemia (applies to acute anemia);
- normalize hemoglobin levels;
- restore iron reserves in the body and maintain their normal level.
The course of therapy is prescribed individually by the attending physician [2].
Diet therapy
It is impossible to cure anemia with diet alone, but a diet high in animal protein and iron will still be helpful to maintain hemoglobin levels [37].
For normal body function, adult men require 65–117 g of protein per day, women – 58–87 g per day. Proteins are found in plant foods (legumes, grains, vegetables and fruits) and in animal products (milk, dairy products, eggs, meat, fish and seafood). Protein and iron are better absorbed from animal products [35]:
- 15–35% of iron is absorbed from meat and fish;
- from plant products - 1–5% [24].
Apples, pomegranates, red juice (beets or tomatoes) cannot compensate for the lack of iron.
Meals should be 4-6 times a day with a small amount of food at one time. For normal digestion, food must be at room temperature: too cold or too hot food irritates the gastric mucosa, which prevents the absorption of nutrients. It is also recommended to give up alcohol and smoking [20].
Treatment with oral iron supplements
Two groups of drugs are used:
- containing the divalent form of iron (II): iron sulfate (Sorbifer Durules, Tardiferon, Aktiferrin, Fenyuls, Maxifer), iron fumarate (Ferretab, Ranferon-12), iron gluconate (Totema);
- containing the trivalent form of iron (III): iron hydroxide polymaltosate (Maltofer, Ferrum Lek), iron hydroxide sucrose complex (Venofer), iron hydroxide dextran (Cosmofer, Ferkail).
Difference between groups of iron preparations
| Main characteristics | Ferrous form of iron | Ferric form of iron |
| Route of administration | Orally (by mouth) | Orally (by mouth) Parenterally (injection) |
| Side effects | Occurs frequently with long-term use | Rarely occur |
| Effect of food on absorption | Dairy products, tea, coffee impair iron absorption. Products with vitamin C improve absorption | Does not affect |
| Suction effect | Well absorbed | Absorbed worse |
Iron supplements are not retained in the body and are quickly excreted by the kidneys, so it is not possible to quickly replenish iron deficiency. To replenish iron stores, the dose should be 100 to 300 mg of ferrous iron per day in tablet form. It makes no sense to increase the dosage, since iron absorption does not increase; the excess will be excreted in the feces.
On the 3rd–5th day from the start of treatment, the number of reticulocytes (immature red blood cells) usually increases in the CBC, which indicates the effectiveness of therapy. If the patient regularly takes iron supplements in a therapeutic dosage, the hemoglobin level normalizes after about 3-4 weeks from the start of therapy. Treatment should not be stopped at this stage. Maintenance therapy must be continued to replenish the iron depot. Maintenance therapy continues for about 6 months at a dose of 100 mg per day.
Possible side effects. All preparations containing iron salts can cause irritation of the gastric mucosa. Therefore, side effects such as vomiting, diarrhea or heartburn sometimes occur. To get rid of them, you need to:
- reduce the dosage of the drug;
- reduce treatment time;
- take the drug before bedtime;
- Do not combine with incompatible products.
If these measures do not help, the drug is discontinued [13].
What affects iron absorption? Vitamin C helps iron to be better absorbed: the acid prevents the oxidation of iron and maintains it in its divalent form. Therefore, it is recommended to add foods containing this vitamin to your diet. Additional vitamin C supplementation is not required [36].
Pregnant women are recommended to take iron supplements together with folic acid (vitamin B9), as it is involved in the process of hematopoiesis [28].
The presence of phytic acid in food (found in cereals, legumes, seeds, nuts, etc.), caffeine and tannin (in tea, coffee), phosphates, oxalates (in plant products) impairs iron absorption by 4–6 times, since they form insoluble complexes with ferric iron and are excreted in feces [23]. It is recommended to limit their consumption or consume them 6 hours before taking iron supplements.
Calcium, iodine, magnesium, zinc, chromium and selenium found in foods and supplements also interfere with iron absorption, so should not be taken together.
Treatment with parenteral iron supplements
Indications for parenteral administration of iron supplements:
- Intolerance to iron supplements in tablet form.
- Impaired absorption of iron in malabsorption syndrome, resection of the small intestine, enteritis, celiac disease and other gastrointestinal diseases.
- Exacerbation of chronic gastrointestinal diseases: gastric and duodenal ulcers, Crohn's disease, ulcerative colitis.
- Severe anemia (hemoglobin 70 g/l or less).
Preparations for parenteral administration: Ferinject, Ferractin, Ferrum Lek, Ferbitol, Ectofer. Drugs are prescribed only by the attending physician after an examination and clarification of the type of anemia. An excess of iron in some cases is even more dangerous than its deficiency: the enamel deteriorates, severe toxic hepatitis can develop before transforming into cirrhosis, etc.
Blood transfusion (transfusion of red blood cells)
Red blood cell transfusion may be required according to individual indications determined by the attending physician. Indications may be:
- Severe IDA;
- concomitant cardiovascular pathologies (for example, coronary heart disease), if there is a risk of decompensation of the condition due to anemia [38].
Treatment of thalassemia
Treatment tactics for different forms of the disease differ. β-thalassemia minor does not require treatment. For β-thalassemia major, treatment must begin in the first months of life: blood transfusion and chelating drugs that remove excess iron from the body (Deferasirox).
For all forms of thalassemia, taking B vitamins is indicated. With the development of hypersplenism, in which the spleen enlarges and actively destroys blood cells, it is necessary to remove the spleen.
One of the treatment methods for thalassemia is bone marrow transplantation [24].
From birth to great achievements
However, by focusing on the names, we have deviated somewhat from the topic. So, the events take place in the bone marrow:
Stage 1: erythroblast
Erythroblast
- the first cell that can be identified under a microscope in a bone marrow preparation. A rounded nucleus, a delicate mesh structure of chromatin, several small nucleoli (usually 2 - 4), no clearing around the nucleus yet - this is the morphology of the ancestor of cells that will later become erythrocytes. In a general analysis of the blood of a healthy person, you don’t even need to look for it, since it simply cannot be there, because it has only just arisen and, before it goes “out into the world,” it must acquire new features and qualities in order to become workable in the peripheral blood, and, therefore, useful.
Stage 2: pronormocyte
Having passed the erythroblast stage, a very young cell slightly reduces its size (10 - 15 microns) and begins to change the structure of the nucleus, so that later it is easier to get rid of it (the nucleus becomes smaller and coarser, the nucleoli disappear, a slight perinuclear clearing appears around the nucleus) - this is no longer erythroblast. The new cell is called a pronormocyte
, although some continue to call it in the old way -
pronormoblast
. At this stage, the cell of the erythroid series is very poorly differentiated in the myelogram, because it has not yet completely lost the features of its predecessor, and has not yet acquired new ones.
Stage 3: normoblast (normocyte)
However, very little time passes before the “hero of our story” appears from an unrecognizable cellular structure - the normoblast
or
normocyte
.
It begins to be saturated with hemoglobin, which first concentrates around the nucleus ( basophilic
normocyte), and then spreads to the entire cytoplasm, turning the cell into
a polychromatophilic
normoblast, that is, the cell is clearly preparing to perform its responsible function.
As normoblasts accumulate complex chromoprotein (Hb), the need for the nucleus disappears; it only prevents the accumulation of hemoglobin by its presence. Having received a sufficient amount of Hb, the normocyte becomes oxyphilic
: the cytoplasm spreads over almost the entire territory, the nucleus loses its significance, therefore it becomes very small (pyknotic), coarsened with a structure changed beyond recognition, reminiscent of a cherry pit.
Stage 4: birth of a red blood cell
A normoblast, which is about to get rid of a nucleus that is no longer needed, remains a normoblast for some time, but in small numbers. Having finally pushed out the nucleus, the cell turns into a “newborn” polychromatophilic erythrocyte
, preserving a small amount of hereditary information (RNA), which will finally leave the cell within 24 hours, although it is difficult to call the “newly formed” form a cell (more likely, also out of habit).
Young red blood cells, saturated with hemoglobin and having lost their last connection with their “homeland,” are called reticulocytes
, which very soon, after they arrive in the bloodstream (up to 48 hours), will lose the last thing that emphasizes their young age - the reticulum, and turn into full-fledged adult blood cells -
erythrocytes
. A special stain helps detect reticulocytes in the blood. The entire path traveled by an erythrocyte from an erythroblast to a cell that has lost its nucleus takes at least 100 hours.
It is obvious that normally red cells at the normoblast level (before it becomes a reticulocyte) do not appear in the blood of a healthy person of all ages.
Is normoblastosis a sign of pathology?
The appearance of normoblasts in a general blood test (the word “increased” somehow does not fit - this was mentioned earlier) is a clear sign of pathology in the body. These cells enter the peripheral blood under the following circumstances:
- Anemia of various etiologies (thalassemia), some forms of acute and chronic leukemia. In these cases, it can reach normoblastosis, that is, their values are not just increased - there are a huge number of normoblasts; In addition, these pathological conditions in adults can cause the formation of foci of extramedullary hematopoiesis (liver, spleen), which will also produce their portion of normocytes.
- Massive blood loss - in order to save the body, the bone marrow has no choice but to begin active regeneration of cellular elements;
- In an effort to somehow compensate for the deficiency in the bloodstream, normoblasts leave the bone marrow prematurely with increased destruction of red blood cells (hemolysis) without inhibiting the functioning of the hematopoietic system;
- Acute erythroleukemia
(o. erythromyelosis, Di Guillemo's disease) is a rare but malignant disease. The main characteristic feature of acute erythromyelosis is the appearance in the peripheral blood of a large number of erythroid cells that have not lost their nucleus; - When metastases
of malignant tumors spread to the skeletal system, increased concentrations of these cells will be observed in the bone marrow, then they will begin to leave it and enter the peripheral blood. It should be noted that in such cases there is often no direct relationship between normoblastosis and the degree of anemia (significantly increased numbers of normoblasts can be seen in mild anemia);
The sudden appearance of representatives of the young erythroid population in the blood is called a blood crisis, which is characteristic of pernicious anemia.
In this case, the appearance of such a sign, on the contrary, is somewhat encouraging, since it is a harbinger of an impending remission. But calm blood in this pathology makes one suspect the low regenerative ability of the hematopoietic organs (aplastic anemia) and expect an unfavorable prognosis.
Sometimes erythroid cells that have not completely passed the normoblast stage leave the bone marrow prematurely due to the person’s serious condition, which is not caused by a pathology of the hematopoietic system. For example, this can happen during various pathological processes occurring with circulatory failure.
Clinical significance of a blood test (excerpt from a lecture by Prof. E.B. Vladimirskaya)
Hematology:
12.03.2009
Doctor of Medical Sciences, Prof. E.B. Vladimirskaya Research Institute of Pediatric Hematology, Ministry of Health of Russia
Blood, being the internal environment of the body, carries within itself the stigmata of the vital functions of various organs and systems, the study of which is of undoubted clinical significance and is necessary for the diagnosis, prognosis of the course and monitoring of therapy of almost all internal human diseases.
The most accessible is the study of the morphological composition of blood; its results are included in the diagnostic algorithm for most pathological processes.
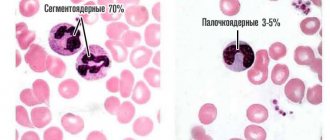
Since the first studies of blood under a microscope without the use of staining (the middle of the last century), blood cells have been divided into red - erythrocytes (based on the color of hemoglobin) and white - leukocytes. Leukocytes, in turn, are divided into cells containing specific granularity in the cytoplasm, and according to the ratio of this granularity to color, they are divided into (neutrophils, eosinophils and basophils) and those without - lymphocytes and monocytes. Based on the shape of the nucleus, the former are often called polymorphonuclear cells, and the latter are called mononuclear cells.
In recent years, clinical blood tests have increasingly been performed on automatic counters, which significantly increases the accuracy of calculations, but, however, does not negate the value of data obtained “manually” using light-optical microscopy. A comparison of the results of these two methods, together with the reference values of the indicators used, is presented in Table 1.
Table 1
| Automatic counting | Units _ | Normal limits | Manual counting |
| hgb -hemoglobin | g/liter | M: 132 - 173 F: 117 - 155 | Hb |
| rbc- red blood cells | 1012 /liter | M: 4.3 – 5.7 F: 3.8 – 5.1 | er. |
| hct-hematocrit | % | M: 39 – 49 F: 35 – 45 | ht |
| mcv - average volume red blood cell | 1 µm3 = 1 femtoliter | 80,0 – 95,0 | Spherical index (3,2-3,4) |
| mch - average hemoglobin content in an erythrocyte | picograms 1 g = 1012 picograms | 27,0 – 31,0 | Color index (0,85 – 1,0) Color.= Nb (g/l) x 3_____ Er (first three digits) |
| mchc – average concentration of HB in 1 erythrocyte | g/dl | 32,0 — 36,0 | |
| rdwwidth of distribution of red blood cells by volume | width histograms | 11,5 – 14,5 | No analogue |
| plt – platelets | *109 /l | 150 — 400 | Platelets |
| wbc- leukocytes | *109 /l | 4,5 –11,0 | Leukocytes |
| neu - neutrophils | *109 /l % | 1,8 – 5,5 47,0 – 72,0 | Neutrophils |
| lym – lymphocytes | *109 /l % | 1,2 – 3,0 19,0 – 37,0 | Lymphocytes |
| mon – monocytes | *109 /l % | 0,1 – 0,9 3,0 – 11,0 | Monocytes |
| eos – eosinophils | *109 /l % | 0,02 – 0,3 0,5 – 5,0 | Eosinophils |
| bas – basophils | *109 /l % | 0,0 – 0,07 0,0 –1,0 | Basophils |
When commenting on the data presented in the table, it is necessary to note:
- the vast majority of automatic counters do not detect young forms of leukocytes, normoblasts and reticulocytes - this data can only be obtained “manually”;
- normative values are never expressed in one figure; there is a limit of permissible fluctuations (it is presented in the table for all indicators), which fits 99.9% of the norm.
Let's analyze the clinical significance of individual blood test indicators.
Red blood counts.
Anemia is a decrease in hemoglobin below 120 g/l: mild - 110-120 g/l; average degree – 90-110 g/l; severe degree – below 90 g/l.
Based on the ratio of red blood indicators, 3 types of anemia are distinguished, which is the starting point for further diagnosis.
Microcytic-hypochromic anemia:
MCV < 80 Color.p. <0.85
MCH < 27; MCHC < 32
- Iron-deficiency anemia
- Anemia due to chronic inflammation
- Congenital spherocytic hemolytic anemia.
- Thalassemia.
Normocytic-normochromic anemias:
MCV 80 – 95
MCH = 27-31; MCHC = 32-36 Color.p. = 0.85-1.0
Acute blood loss.
- Anemia in chronic renal failure
- Anemia due to endocrine pathology.
- Anemia in cancer.
- Hemolytic anemias, immune and non-immune.
- Aplastic anemia.
- Myelodysplastic syndrome.
Hyperchromic macrocytic anemia.
MCV > 95
MCH>31; MCHC = 32-36 Color points > 1.0
- Megaloblastic B-12 is a deficiency (pernicious) anemia.
- Megaloblastic folate deficiency anemia.
- Autoimmune hemolytic anemia.
Thus, when detecting a decrease in hemoglobin, one should first determine the nature of anemia: normo-, hypo- or hyperchromic.
Let us dwell in more detail on iron deficiency anemia and anemia due to chronic inflammation, the diagnosis and treatment of which are the prerogative of general practitioners and do not require special hematological studies.
Iron deficiency anemia (IDA) is detected in 50% of women and 40% of men, representing one of the most common human diseases. The most common cause of IDA is a latent form of bleeding: in men – from the gastrointestinal tract and bronchopulmonary system, in women – menopause and metrorrhagia. Pregnancy is also one of the factors in the development of iron deficiency in women. Insufficient dietary intake of meat products is another significant and at the same time socially determined cause of the development of iron deficiency. In children born with a reserve of iron received from the mother in the last month of intrauterine development, further intake of iron occurs only from complementary foods. Thus, the reasons for the development of iron deficiency in young children may be prematurity, multiple pregnancies, late introduction of complementary foods, infections (increased iron consumption by microbial flora and malabsorption).
The clinical picture of IDA consists of the following clinical syndromes:
1. Anemia: weakness, drowsiness, fatigue, shortness of breath, palpitations, functional systolic murmur, floaters floating before the eyes.
2. Sideropenic syndrome:
· dryness, fragility, hair loss, early gray hair;
· flattening and brittleness of nails;
· dry skin, hyperkeratosis;
· painful, non-healing cracks in the corners of the mouth, tongue, fingers, toes, heels;
· perversion of taste and olfactory addictions;
· frequent infections;
· impaired swallowing, muscle weakness, sphincter weakness (urinary incontinence when laughing, coughing)
The diagnostic algorithm for IDA, in addition to a detailed clinical study to identify hidden bleeding, includes the mandatory determination of basic indicators of iron metabolism. The diagnosis of IDA is confirmed by the following values of these indicators:
- serum iron below 12.5 µmol/l
- total iron binding capacity (TIBC) above 64.4 µmol/l
- serum ferritin below 12 µg/l.
Correction of IDA is carried out by long-term oral treatment with iron preparations (6-8 weeks after hemoglobin restoration).
It is necessary to carry out a differential diagnosis between IDA and anemia in chronic inflammation.
Anemia during chronic inflammation in terms of the morphological composition of the blood is no different from IDA and is also accompanied by a decrease in serum iron content. However, its development is not based on exogenous iron deficiency, but on the impossibility of its utilization. Iron treatment for such anemia is contraindicated. Differential diagnosis is based on the study of iron metabolism indicators and is presented in Table 2.
Table 2. Differential diagnosis between IDA and anemia in chronic inflammation.
| Indicators | ZhDA | Anemia due to chronic inflammation |
| Serum iron | Reduced | Reduced |
| OZhSS | Increased | Normal or reduced |
| Serum ferritin | Reduced | Normal or increased |
To summarize the brief analysis of the clinical significance of the morphological parameters of red blood, we should dwell separately on reticulocytes and normoblasts.
A morphological sign indicating the hemolytic nature of the decrease in hemoglobin is an increase in the number of reticulocytes. With normal hemoglobin, the number of reticulocytes does not exceed 0.5-1.5%. The expected reticulocyte response to hemolytic anemia with intact hematopoiesis is presented in Table 3.
Table 3. Expected reticulocyte response to hemolytic anemia.
| Hematocrit, % | 45 | 40 | 35 | 30 | 25 | 20 | 15 |
| Hemoglobin, g/l | 130 | 120 | 115 | 100 | 83 | 66 | 50 |
| Reticulocytes, % | 0,5 | 1,5 | 5 | 10 | 15 | 20 | 30 |
Dynamic monitoring of reticulocyte levels is also necessary to assess the expected effectiveness of treatment for IDA and pernicious anemia. An increase in reticulocytes is naturally observed on days 5-8 of treatment with iron for IDA and is especially pronounced (up to 60%) on days 5-8 of treatment with vitamin B-12 for pernicious anemia. Such a hematopoietic response to the therapy of these diseases can also be considered as confirmation of the corresponding diagnosis of exjuvantibus.
Normoblastosis in peripheral blood is rare and always indicates a serious pathology. Its appearance is naturally observed in severe forms of hemolytic anemia and in patients who have undergone splenectomy. The detection of normoblasts in the blood of patients who do not suffer from this pathology should be a reason to search for oncological pathology.
about erythrocytosis when the following blood parameters are present: red blood cells above 5.7x10*12/l in men and 5.2x10*12/l in women, hemoglobin above 177 g/l and 172 g/l, respectively, hematocrit above 52% and 48 % respectively.
Primary erythrocytosis is considered to be rare genetically determined familial erythrocytosis and erythremia.
Secondary erythrocytosis, caused by increased formation of erythropoietin in response to arterial hypoxia or in certain tumors, is much more common.
Secondary erythrocytoses can be divided into the following groups:
1. Arterial hypoxia
- Altitude sickness
- Chronic pulmonary failure
- "Blue" heart defects
2. Tumors that produce erythropoietin
- Kidney tumors, hypernephroma
- Adrenal tumor
- Cerebellar hemangioma
- Ovarian cancer
3. Local renal ischemia
- Cyst
- Hydronephrosis
- Renal artery stenosis
4. Harmful production
- Cobalt poisoning
Treatment of secondary erythrocytosis requires elimination of their cause, but can also be symptomatic due to the threat of thrombosis. Symptomatic treatment of erythrocytosis is bloodletting.
White blood counts.
An increase or decrease in the number of different types of peripheral blood leukocytes can be judged only by changes in the absolute number of these formed elements.
Neutrophilia is an increase in the number of neutrophils more than 6x10*9/l.
Less commonly, neutrophilia is a manifestation of chronic myeloid leukemia, accompanied by clinical and hematological features specific to it (enlarged spleen, lymph nodes, blood rejuvenation, anemia, hyperthrombocytosis, bone marrow hyperplasia, the presence of a Ph chromosome and the chimeric c-abl-bcr gene).
Much more often, neutrophilia is a blood reaction to inflammation, the result of exposure to bacterial endotoxin and the release of inflammatory cytokines and chemokines by tissues. Neutrophilic leukocytosis can accompany any inflammation, bacterial, fungal and parasitic infections, necrotic tissue changes, hypoxemia, intoxication and tumors of various locations. With prolonged exposure to factors that induce neutrophilia, the bone marrow granulocyte reserve is depleted and young cells of the neutrophil series (band cells, metamyelocytes and myelocytes) begin to enter the blood. This blood condition is called leukemoid reaction of the neutrophil series. Sometimes it becomes necessary to make a differential diagnosis between such a reaction and the initial form of chronic myeloid leukemia. The absence of anemia, hyperthrombocytosis and a high level of alkaline phosphatase in neutrophils is characteristic of the leukemoid reaction.
Neutropenia is a decrease in the number of neutrophils less than 1.8 x 10*9/l.
Agranulocytosis - a decrease in the number of neutrophils less than 0.5 x 10*9/l.
Neutropenia can be primary (congenital and acquired), associated with blood diseases (acute leukemia, aplasia of hematopoiesis, cyclic neutropenia), and secondary, accompanying diseases, during which destruction and increased consumption of neutrophils occurs.
Secondary, reactive neutropenia includes immune and neutropenia in severe infections. Neutropenia in sepsis is usually accompanied by a rejuvenation of the leukocyte count and is a poor prognostic symptom, indicating depletion of hematopoiesis.
It is necessary to dwell on constitutional, so-called harmless neutropenias. About 4% of people have normal blood composition with a low content of neutrophils. This feature is associated with the genetically determined rapid movement of neutrophils into tissues, where they carry out their inherent protective functions. People with this blood composition are usually less susceptible to intercurrent infections and recover from them faster. However, often such patients, unfortunately, are the subject of close attention of doctors, are subjected to many unnecessary invasive studies, and they develop iatrogenic pathology. Thus, neutropenia, not accompanied by other blood changes and any clinical symptoms, does not require immediate intervention. Such patients require dynamic monitoring.
Separately, I would like to touch upon redistributive neutrophilia and neutropenia. The circulation of neutrophils has its own characteristics: half of the cells circulate with the blood (these cells are to be counted), while the other half is in the “marginal position” at the walls of the vessels. Irritation of the sympathetic system and vasospasm increase the number of circulating cells, while irritation of the parasympathetic system, on the contrary, reduces their number. Hence, stressful conditions contribute to transient neutrophilia (for example, neutrophilia in small children when screaming), and vagotonia - neutropenia.
Eosinophilia – an increase in the number of eosinophils above 0.4 x 10*9/l.
An increased release of eosinophils into the blood occurs under the influence of IL-4 and IL-5, which are formed in increased quantities during immunological tissue damage. Recently, the killer effect of eosinophils has been proven in some helminthiases and parasitic infections. Eosinophilia is a characteristic feature of collagenosis, allergies, many helminthic and parasitic infestations, immunodeficiency, especially hyper-IG-E syndrome, and some tumors.
Monocytosis – the number of monocytes is higher than 0.8 x 10*9/l.
Conditions often, but not always, associated with monocytosis include:
- Infections (especially tuberculosis, endocarditis, syphilis).
- Fever of unknown origin
- Various forms of neoplasia and myeloproliferative diseases.
- Chronic inflammation (especially cholecystitis and rheumatoid polyarthritis)
- Condition after splenectomy.
Lymphocytosis – an increase in the number of lymphocytes more than 4.0 x 10*9/l
Among malignant lymphoproliferative diseases with high lymphocytosis, the most common is chronic lymphocytic leukemia, a disease of people over 45 years of age. The distinctive feature of this lymphocytosis is its monoclonal nature and B-cell origin.
Secondary, reactive lymphocytosis , which is polyclonal in nature, accompanies many viral infections and some inflammatory and immune complex diseases. These include:
1. Lymphotropic viral diseases:
— infectious mononucleosis (atypical mononuclear cells, characteristic clinical picture);
- infectious lymphocytosis (asymptomatic epidemic form in young children - up to 20-30 thousand)
2. Cytomegalovirus infection (atypical mononuclear cells, characteristic clinical picture).
3. Childhood infections: whooping cough, chickenpox, scarlet fever prodrome.
4. Other viral infections: rubella, hepatitis, some respiratory adenoviral infections in the convalescent stage.
5. Inflammatory and immune complex diseases: thyrotoxicosis, ulcerative colitis, Crohn's disease, vasculitis.
Lymphocytopenia - a decrease in the number of lymphocytes below 1.2 x 10*9/l.
It is observed relatively rarely, most often with corticosteroid therapy. May also accompany AIDS, lymphogranulomatosis and various chronic infections (for example, tuberculosis, disseminated lupus erythematosus, sarcoidosis).
Hyperthrombocytosis is considered to be an increase in the number of platelets more than 400.0 x 10*9/l.
Primary hyperthrombocytosis accompanies myeloproliferative diseases and is a consequence of tumor transformation of the megakaryocytic lineage of the bone marrow.
Secondary reactive hyperthrombocytosis is observed:
- After surgical interventions (about 2 weeks).
- After splenectomy (up to 1 year).
- For malignant tumors
- For acute posthemorrhagic and hemolytic anemia.
- For certain inflammations (tuberculosis, acute rheumatism, ulcerative colitis, osteomyelitis).
Thrombocytopenia - a decrease in the number of platelets below 100.0 x 10*9/l most often occurs with tumor diseases of the blood, aplastic anemia and immune thrombocytopenic purpura. Thrombocytopenia is an obligatory component of the hypersplenism syndrome with splenomegaly. It should be borne in mind that a serious threat of bleeding occurs when the platelet count drops below 20.0 x 10*9/l.
Reactive thrombocytopenia is rare and can accompany any immune pathology and disseminated intravascular coagulation.
ESR - erythrocyte sedimentation rate is a nonspecific reaction. Normally, it is 2-15 mm per hour in men under 50 years old, and 2-20 mm per hour in women under 50 years old. After 50 years, men have up to 20 mm per hour, and women – up to 30 mm per hour.
The rate of erythrocyte aggregation depends on their number (it accelerates when their number decreases) and the amount of coarse proteins (inflammatory proteins, fibrinogen, antibodies, gamma globulin, etc.) adsorbed on erythrocytes and accelerating their sedimentation. Based on this, it is clear that there is a wide range of pathologies in which ESR acceleration can be detected.
Thus, analysis of the morphological composition of blood is of great clinical importance, and sometimes is a leading sign in the diagnosis and choice of therapy for many human diseases.
However, it should be remembered that the most important link in such an analysis is the integral assessment of all blood parameters and the mandatory correlation of changes in the blood with the history and clinical manifestations of the disease.
Literature:
1. Guide to Hematology, ed. A.I. Vorobyov, Moscow, 1985
2. Hematology, ed. by WSBeck, London, 1991
3. Manual of Clinical Hematology, ed.by J. Mazza, NY, 1995
Tags: Laboratory, Blood
« Back to article list Share on