Minkowski-Choffard disease is a common hereditary disease belonging to the group of anemias. Its other names are hereditary spherocytosis and microspherocytic hemolytic anemia. The pathology was first described at the end of the 19th century by the Polish physiologist Minkowski, then supplemented by the French doctor Chauffard.
People of all ethnic groups are susceptible to the disease, but it is more often diagnosed in European territory, especially its northern part. It can appear for the first time at any age.
About the disease
All patients with microspherocytosis have a lack of spectrin proteins in the erythrocyte membrane, and in some, a loss of their functional properties. The symptoms of the disease and the severity of its course directly depend on the degree of spectrin deficiency. There are cases where the carrier of the spherocytosis gene had no symptoms.
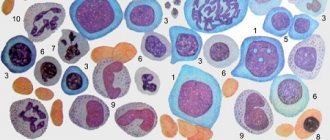
The disease is characterized by a qualitative change in red blood cells, which take on a spherical shape, that is, they become spherocytes. This occurs due to a genetically determined abnormality of a cell membrane protein. As a result, the permeability of the membrane increases, too many sodium ions enter the red cells and excess water accumulates. Spherical red blood cells, unlike normal, biconcave disc-shaped ones, are not able to deform in narrow areas of the blood flow, so their movement slows down, part of their surface is split off, they turn into microspherocytes and gradually die.
Macrophages of the spleen absorb destroyed spherocytes. The constant breakdown of red blood cells in the spleen leads to an increase in its size. In addition, with increased destruction of red blood cells in the blood, the amount of free bilirubin increases, which is released in the feces in the form of a special bile pigment. In people with hereditary spherocytosis, its level in the feces can significantly exceed the norm - 10 and even 20 times. Due to the increased release of bilirubin pigment into bile in the bile ducts and bladder, the formation of stones begins.
Anemia - what is it?
Anemia is characterized by a decrease in the level of red blood cells and hemoglobin in the blood. In some cases, not only the numerical composition of red blood cells falls below normal, but their shape also changes. As the disease progresses, the red blood cells are unable to perform their functions.
Anemia develops against the background of other disorders in the body; it does not manifest itself. Therefore, to get rid of the disease, it is necessary to establish the cause that led to the change in the composition of the blood.
Signs
For the first time, symptoms of spherocytosis can make themselves felt at any age, starting from neonatal age, but more pronounced signs are more often detected in late preschool and early school age. In young children, microspherocytic anemia is often diagnosed during examination for another reason. If the pathology begins to manifest itself in infancy, it can be assumed that its course will be severe.
The clinical picture depends on the severity of hemolysis (destruction of red blood cells). Outside of exacerbation, symptoms may be absent. During an exacerbation, patients complain of increased body temperature, dizziness, weakness, decreased appetite, headache, and increased fatigue.
Symptoms of Minkowski–Choffard disease include the following:
- Jaundice. This is the main clinical sign, which is often the only manifestation of the disease for a long time. Its severity depends on the intensity of the breakdown of red cells and the ability of the liver to combine bilirubin and glucuronic acid.
- Feces have an intense dark brown color due to the high content of the pigment stercobilin.
- Attacks of cholelithiasis, as well as signs of cholecystitis, which is associated with the formation of stones in the gall bladder. If the bile duct is blocked by a stone, obstructive jaundice develops with characteristic signs: a sharp increase in direct bilirubin and bile pigment entering the urine, which becomes dark; discoloration of stool; skin itching; increase in temperature; pain in the right hypochondrium.
- Enlargement of the spleen (it protrudes from under the ribs by about two to three centimeters) and heaviness in the left hypochondrium.
- With a prolonged course of the disease, the liver may increase in size (with an uncomplicated form of spherocytosis, it remains normal in size).
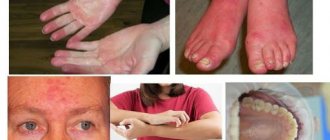
- With early symptoms in children, there is a disturbance in the development of the skull and facial skeleton (a square-shaped tower skull, a saddle nose, narrow eye sockets, abnormal development of teeth and other signs).
- Middle-aged and older people may develop poorly healing trophic ulcers of the legs, which is caused by the gluing of red blood cells in the small capillaries of the legs.
- The severity of anemia can vary, sometimes there are no signs. Most often it manifests itself as a moderate decrease in hemoglobin levels. Severe anemia is usually observed during an exacerbation of the disease.
- Due to anemia, signs of disturbances in the functioning of the heart and blood vessels may appear.
Hereditary spherocytosis is characterized by a wave-like course, that is, periods without exacerbation are replaced by hemolytic crises, during which the symptoms intensify:
- due to the constant destruction of red blood cells, an increase in body temperature occurs;
- jaundice becomes intense;
- bowel movements become more frequent, abdominal pain, nausea and vomiting occur;
- The appearance of convulsions is considered a serious symptom.
The frequency of crises is different for all patients, and for some they never occur at all. A hemolytic crisis can be triggered by infections that join the underlying disease, hypothermia, and in women, pregnancy.
In some patients, the only symptom is jaundice, with which they consult a doctor.
Sometimes anemia is so well compensated that the patient learns about his illness only after a full examination.
Periods of remission can last from several weeks and months to several years.
Hereditary microspherocytosis (Minkowski-Choffard disease)
The article provides a review of the literature concerning clinical and laboratory manifestations, diagnosis, treatment and prognosis of hereditary microspherocytic anemia. The results of our own observations of two patients undergoing treatment in the oncohematology department of the Yoshkar-Ola Children's City Hospital are described.
Hereditary microspherocytosis (Minkowsky - Shauffard disease)
The paper presents a literature review of clinical and laboratory manifestations, diagnosis, treatment and prognosis of hereditary microspherocytic anemia. It is describes the results of his own observations of two patients treated in oncohematological office in Yoshkar-Ola City Children's Hospital.
Hereditary microspherocytosis was first described in 1900 by Minkowski, and later in more detail by Shoffar. The prevalence of this disease is 1:5000 of the population, most often found in residents of Northern Europe [1, 2].
Pathogenesis. Congenital microspherocytic hemolytic anemia is a familial disease inherited in an autosomal dominant manner. The disease is based on a genetic defect in the protein of the erythrocyte membrane, as a result of which its permeability to sodium ions increases, which leads to swelling of erythrocytes, disruption of the ability of erythrocytes to deform, detachment of part of their surface in the spleen, shortening of their life span and destruction by spleen macrophages [3, 4 ]. The pathology of erythrocytes is manifested by a morphological abnormality - microspherocytosis. The duration of stay of microspherocytes in the circulating blood is sharply reduced; the average period of their stay in the bloodstream can be 12-14 days (instead of the normal 120-125 days) [5].
Clinical picture. The central place in the clinical picture belongs to the hemolytic syndrome, which is manifested by three cardinal signs: jaundice, splenomegaly and anemia [5]. Signs of delayed development may be observed, as well as disturbances of the facial skeleton in the form of a “tower skull”, saddle nose, high palate, disturbance of the arrangement of teeth, and narrow eye sockets [6]. The severity of anemic syndrome varies. A moderate decrease in hemoglobin is often observed. Some patients have no anemia at all. The most dramatic anemization is observed during the hemolytic crisis. Microspherocytic hemolytic anemia has a chronic course and is accompanied by periodic crises and remissions. Hemolytic crisis occurs under the influence of provoking factors (infection, hypothermia, overwork, pregnancy and others) and is manifested by a sharp increase in symptoms against the background of continuously ongoing hemolysis [6]. At the same time, the temperature rises due to the massive breakdown of red blood cells, the intensity of jaundice increases, hepatomegaly is pronounced, the spleen is dense and smooth, often painful as a result of tension of the capsule during blood filling or perisplenitis. Hemolytic disease is often complicated by attacks of hepatic colic, due to the formation of pigment stones in the gall bladder and bile ducts. Due to attacks of hepatic colic and stagnation of bile in the liver, patients may experience symptoms of angiocholecystitis and parenchymal hepatitis with the appearance of direct bilirubin in the blood. With exacerbation of the disease, there is a tendency to nosebleeds [3, 7]. Despite its congenital nature, the disease only rarely manifests itself in the first days after birth; symptoms usually appear in childhood, usually at 3-10 years of age, or adulthood [8].
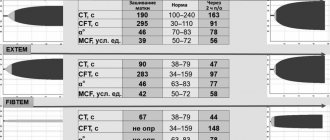
Laboratory diagnostics. Microscopic examination of peripheral blood preparations reveals spherocytes, characterized by a decrease in the average diameter (less than 7.2-7.0 µm) against the background of a normal average MCV volume and an increased MCHC value. The red blood cell size distribution curve (Price-Jones curve) on the graph is shifted to the left. Anemia is normochromic in nature. The RDW value exceeds 12% (anisocytosis). Reticulocytosis - 15%-30% [1, 10]. The white blood cell count is usually normal. In hemolytic crises, neutrophilic leukocytosis with a shift to the left is noted. The platelet count varies within normal limits. The content of indirect bilirubin in the blood is moderately increased and, as a rule, does not exceed 50-70 µmol/l [6, 7]. Autoantibodies are not detected on the erythrocyte membrane; the direct Coombs test is negative. A characteristic laboratory sign is a decrease in the osmotic resistance of red blood cells to hypotonic sodium chloride solutions. The beginning of hemolysis in hereditary microspherocytosis corresponds to 0.6-0.7%, and the end - 0.4% instead of 0.48-0.22% normally [1, 9]. A decrease in osmotic resistance indicates the predominance of spherical erythrocytes in the blood - spherocytes, which are less resistant to osmotic hemolysis than normal macroplanocytes (Fig. 1).
Figure 1. Morphology of microspherocytes in peripheral blood smears of a patient (left), healthy (right)
Differential diagnosis of hereditary microspherocytosis comes down to the diagnosis of hemolytic anemia in general. Many patients are mistakenly diagnosed with Gilbert's syndrome, chronic hepatitis or even cirrhosis of the liver, and anemia is considered a consequence of these diseases. In all cases of jaundice, a thorough examination of the patient is necessary, regardless of hemoglobin content. A predominant increase in the content of indirect bilirubin, reticulocytes, and microspherocytosis detected when viewing a smear provide the basis for the correct diagnosis. A differential diagnosis of autoimmune hemolytic anemia and microspherocytosis is then necessary, since autoimmune hemolytic anemias often produce symptomatic microspherocytosis [3]. In this situation, a thorough ascertainment of the medical history, duration of the disease, the presence of a similar disease in relatives, identification of skeletal changes, and testing to detect autoantibodies (direct Coombs test) help.
Treatment and prognosis. Treatment during a hemolytic crisis is aimed at eliminating anemia, hypoxia, and hyperbilirubinemia. The method of choice for the treatment of hereditary spherocytosis is splenectomy, optimal at the age of 4-5 years. Splenectomy provides practical recovery, despite the preservation of spherocytosis. Indications for splenectomy in microspherocytosis are persistent anemia or that occurs in the form of crises, significant hyperbilirubinemia, even without anemia, the appearance of pain in the right hypochondrium, and developmental delays in children [3]. Blood (packed red blood cell) transfusions are indicated in cases of severe hemolytic crisis [5]. The prognosis for hereditary microspherocytosis is favorable. The probability of developing the disease in children if one of the spouses has microspherocytosis is slightly lower than 50% [9].
Clinical case No. 1. Patient S., 1 year 10 months, from 06/01/09 to 06/09/09 was undergoing an inpatient examination in the oncohematology department of the Yoshkar-Ola Children's City Hospital. From the anamnesis it is known that the child from the first pregnancy was born premature, weighing 900 grams. He was nursed in the department of the second stage of nursing premature babies. Heredity is unknown, since he was adopted at the age of four months. From birth, a decrease in the level of hemoglobin in the blood test and icterus of the skin were noted. Since December 2008, he has been under observation by a hematologist for anemia of prematurity and has been taking iron supplements. Upon receipt of a complaint of lethargy, weakness, fever to febrile levels, pallor and icteric discoloration of the skin. On palpation, the liver protrudes from under the edge of the costal arch by 2.5 cm, the spleen by 2 cm, is soft and elastic.
General blood test dated 06/01/09: HGB - 106 g/l, RBC - 3.9×10¹²/l, WBC - 11.6×109/l, Ht - 26%, MCV - 68.8 fl, MCH — 25.8 pg, MSHC — 375 g/l, RDW — 20.5%, PLT — 434×109/l, ESR — 6 mm/h, in the leukocyte formula: eosinophils — 10%, neutrophils — 34 %, lymphocytes - 47%, monocytes - 9%.
Anemia is normochromic in nature. In blood smears, microspherocytes predominate, characterized by the absence of central clearing characteristic of normal erythrocytes, which is confirmed graphically on the Price-Jones curve, reflecting the quantitative ratios of erythrocytes of different diameters. The apex of the Price-Jones curve is stretched and shifted to the left towards microcytes. In addition, normocytes and single macrocytes are visible in blood smears, this confirms an increase in the RDW index - 20.5%. In many red blood cells, basophilic punctation and polychromatophilia are detected. Reticulocytosis - 27%. Osmotic resistance of erythrocytes: min - 0.52%, max - 0.34% (with a norm of 0.48-0.22%) - a decrease in resistance to hypotonic solutions of table salt is determined. Biochemical analysis dated 06/02/09: concentration of indirect bilirubin - 29.9 µmol/l, ALT - 15 U/l, AST - 26 U/l, al. phosphatase - 415 U/l, LDH - 273 U/l, indicators of iron metabolism are within normal limits: serum Fe - 14.4 µm/l, THC - 69.9 µm/l. A negative result of the direct Coombs test allowed us to exclude autoimmune hemolytic anemia.
In hereditary microspherocytosis, the blood picture is characterized by a pathological triad on the part of erythrocytes: 1) microspherocytosis; 2) reticulocytosis; 3) reduced osmatic resistance. In our case, all three factors are well expressed. The patient was diagnosed with hereditary microspherocytic hemolytic anemia.
The child was discharged in stable condition on 06/09/09 with recommendations: observation by a hematologist and pediatrician, general and biochemical blood tests once every 3 months.
On June 24, 2009, patient S. was re-admitted to the oncohematology department. Upon admission, the condition was serious, complaints of lethargy, lack of appetite, poor sleep, fever up to 39.6°C, pallor, yellowness of the skin, dark urine. The pharynx is hyperemic and loose. The lymph nodes of the cervical group are enlarged. The abdomen is enlarged in volume, the liver protrudes from under the edge of the costal arch by 3.5 cm, the spleen by 5 cm.
General blood test dated June 24, 2009: HGB - 89 g/l, RBC - 3.4×10¹²/l, WBC - 27.7×109/l, Ht - 21%, MCV - 68.6 fl, MCH -25.5 pg, MSHC - 381 g/l, RDW - 20.8%, PLT - 260×109/l, ESR - 12 mm/h, in the formula: eosinophils - 4%, basophils - 1%, p/ I neutrophils - 4%, s/I neutrophils - 15%, lymphocytes - 20%, monocytes - 1%, atypical mononuclear cells - 55%. Reticulocytosis - 46.7%. Blood smears contain up to 50% microspherocytes. Indirect bilirubin - 41 µmol/l, ALT - 19 U/l, AST - 45 U/l, al. phosphatase - 316 U/l, LDH - 295 U/l. An increased content of stercobilin in the feces is determined. In the urine, urobilinuria is noted, the release of urates and uric acid crystals, explained by the increased breakdown of red blood cells. ELISA for Epstein-Barr virus (infectious mononucleosis) - positive.
Taking into account the child's intoxication, fever, enlarged cervical lymph nodes, high leukocytosis in the blood, mononuclear cells up to 55%, positive ELISA, infectious mononucleosis was diagnosed, which in turn was the trigger for the development of a hemolytic crisis in the boy.
Treatment was carried out: diet, bed rest, infusion and antiviral therapy. As a result of therapy, the fever stopped, the size of the liver, spleen and lymph nodes decreased. General blood test dated 07/06/09: HGB - 104g/l, RBC - 3.9×10¹²/l, WBC - 10.7×109/l, Ht - 28%, MCV - 73.3 fl, MCH - 26.4 pg, MSHC - 360 g/l, RDW - 21.8%, PLT - 452×109/l, ESR - 2 mm/h, in the leukocyte formula: eosinophils - 5%, p/n neutrophils - 5% , neutrophils - 25%, lymphocytes - 47%, monocytes - 8%, atypical mononuclear cells - 10%. Reticulocytosis - 9.3%. The number of microspherocytes decreased to 23%. Indirect bilirubin - 18.8 µmol/l. Appetite and sleep normalized. In a stable condition, on July 09, 2009, the child was discharged home with recommendations: observation by a hematologist once every 3 months, a general blood test with reticulocytes and a biochemical blood test once every 3 months, monitoring by ultrasound of the abdominal cavity once a year.
Clinical case No. 2. Patient G., 12 years old, on September 22, 2009, was transferred to the oncohematology department of the Yoshkar-Ola Children's City Hospital from the infectious diseases hospital. Upon receipt of a complaint of nasal congestion, sore throat, general weakness, enlarged cervical and occipital lymph nodes, fever up to 39.2°C, icteric skin, hepatosplenomegaly with pronounced density of parenchymal organs. From the anamnesis it is known that the girl’s father suffers from Gilbert’s syndrome and since birth the girl has developed yellowness of the skin and sclera due to fever and ARVI.
General blood test dated September 23, 2009: HGB - 61g/l, RBC - 1.8×10¹²/l, WBC - 10.7×109/l, Ht - 16%, MCV - 85 fl, MCH - 32, 5 pg, MSHC - 382g/l, RDW - 20.3%, PLT - 162×109/l, ESR - 48 mm/h, in the leukocyte formula: myelocytes - 1%, metamyelocytes - 2%, p/n neutrophils - 29%, neutrophils - 9%, lymphocytes - 24%, monocytes - 4%, atypical mononuclear cells - 31%. Reticulocytosis - 82.5%. Blood smears contain a significant number of microspherocytes. Osmotic resistance of erythrocytes: min - 0.72%, max - 0.42%, a sharp decrease in resistance to hypotonic solutions of table salt is determined. Increased levels of urobilin in urine and stercobilin in feces. Biochemical blood test dated September 23, 2009: ALT - 71 U/l, AST - 78 U/l, LDH 1234 U/l, direct bilirubin - 5.68 µmol/l, indirect - 54.8 µmol/l. Direct Coombs test is negative.
Severe normochromic anemia, the detection of a significant number of microspherocytes in blood products, a pronounced reticulocyte crisis, a sharp decrease in the osmotic resistance of erythrocytes, hyperbilirubinemia, as well as the presence of similar symptoms in the father gave grounds to diagnose the girl with a hemolytic crisis of hereditary microspherocytic anemia, provoked by infectious mononucleosis (Epstein virus ELISA - Barr - positive). An examination by an infectious disease specialist confirms the diagnosis of infectious mononucleosis.
The patient was prescribed infusion, antibacterial and antiviral therapy, and vitamins. During treatment, the patient's condition stabilized. General blood test dated 10/12/09: HGB - 106g/l, RBC - 3.5×10¹²/l, WBC - 3.9×109/l, Ht - 29.3%, MCV - 83.8 fl, MCH - 30.4 pg, MCHC - 362 g/l, RDW - 217.8%, PLT - 170×109/l, ESR - 8 mm/h, in the formula: basophils - 1%, p/n neutrophils - 4 %, neutrophils - 62%, lymphocytes - 20%, monocytes - 4%, atypical mononuclear cells - 9%. Reticulocytosis - 13.1%. Biochemical blood test: ALT - 41 U/l, AST - 32 U/l, direct bilirubin - 2.75 µmol/l, indirect - 24.35 µmol/l. According to abdominal ultrasound, positive dynamics are noted in the form of a decrease in the size of the liver and spleen. The girl was discharged home on October 13, 2009 with recommendations: observation by a hematologist, infectious disease specialist and pediatrician, complete blood count with reticulocytes once every 3 months, monitoring by ultrasound of the abdominal cavity.
Both patients are being monitored by a hematologist.
The diagnosis of congenital microspherocytosis sometimes presents certain difficulties. The most typical manifestations of hemolysis - yellowness of the sclera and skin - are not always correctly assessed by the doctor. The clinical cases we described are direct confirmation of this. In both patients, icterus of the sclera and skin was determined from birth, but a detailed examination was not carried out on the patients before the development of a hemolytic crisis. The presence of similar symptoms in the girl’s father (Gilbert’s syndrome?) suggests that patient G. inherited the disease from her father. The heredity of patient S. is unknown.
In both cases, the provoking factor in the development of the hemolytic crisis was the Epstein-Barr virus. Children have suffered from viral infections before, but they did not cause the development of a hemolytic crisis. Perhaps not every, but only a certain group of viruses can cause crises. This question remains open, since based on the example of two cases it cannot be argued that the Epstein-Barr virus always provokes the development of a hemolytic crisis in patients with hereditary microspherocytosis.
E.V. Shirdanina, Z.S. Gordeeva
Yoshkar-Ola Children's City Hospital, Yoshkar-Ola
Shirdanina Ekaterina Valerievna – doctor of the clinical diagnostic laboratory
Literature:
1. Pogorelov V.M., Kozinets G.I., Kovaleva L.G. Laboratory and clinical diagnosis of anemia. - Moscow. Medical Information Agency, 2004. - pp. 136-137.
2. Kokolina V.F., Rumyantsev A.G. Practical guide to childhood diseases. Pediatric Hematology/Oncology. - Volume 4. - M.: Medpraktika-M, 2004. - P. 169-172.
3. Vorobyov A.I. Guide to Hematology. - Volume 2. - M.: Medicine, 1985. - P. 46-54.
4. Kishkun A.A. Guide to laboratory diagnostic methods. M.: GEOTAR-Media, 2007. - pp. 468-680.
5. Kassirsky I.A., Alekseev G.A. Clinical hematology. - M.: Medicine, 1970. - P. 235-242.
6. Lugovskaya S.A., Pochtar M.E. Hematological atlas. - M.: Triada, 2004. - 227 p.
7. Kolokolov G.R., Gerasina E.V., Ananyev O.L. et al. Analyzes. Complete reference book. - M.: Eksmo, 2008. - P. 497-499.
8. Maksimov V.A., Damidovich K.K., Fedorchuk A.N. and others. Rare diseases, clinical syndromes and symptoms of diseases of the digestive system. - M.: Adamant, 2007. - P. 128-129.
9. Rukavitsin O.A., Skvortsov S.V., Zenina M.N. Hematology. Atlas-directory. St. Petersburg: Detstvo-Press, 2009. - pp. 219-220.
10. Pogorelov V.M., Kozinets G.I., Dyagileva O.A. and others. Color atlas of cells of the blood system. - M.: Practical Medicine, 2007. - P. 98-99.
Diagnostics
The diagnosis of hereditary spherocytosis is made on the basis of laboratory tests, clinical manifestations and conversation with the patient to determine the hereditary predisposition to the disease.
The diagnosis is confirmed if hemolytic anemia, morphological features of red blood cells and their reduced osmotic stability are detected.
With hereditary spherocytosis, red blood cells have a spherical shape, their diameter is reduced and their thickness is increased, while the average cell volume remains normal. Hemoglobin levels are usually close to normal. Changes in the number of leukocytes (the leukocyte formula is shifted to the left, the level of neutrophils is increased) and ESR (increased) are observed only during a crisis, while the platelet level is normal.
A characteristic sign of the disease is a decrease in the osmotic resistance (stability) of red blood cells. The diagnosis can be confirmed only if it decreases significantly. It happens that with obvious spherocytosis, osmotic resistance remains normal. In this case, additional laboratory testing is required after daily incubation of red blood cells.
In the case of hereditary spherocytosis, differential diagnosis is required. The disease should be distinguished from other types of anemia. As a rule, this is hemolytic disease of newborns, autoimmune hemolytic anemia, viral hepatitis.
Prevention measures
Minkowski-Choffard anemia is a disease that is inherited, so it is impossible to prevent its development. People suffering from this pathology need to be registered with a hematologist.
The probability of a child being born with hemolytic anemia in a sick mother is equal to 50%. Therefore, such children should be thoroughly examined from birth.
Author of the article:
Shutov Maxim Evgenievich |
Hematologist Education: Graduated from Kursk State Medical University in 2013 and received a diploma in General Medicine. After 2 years, he completed his residency in the specialty “Oncology”. In 2021, she completed postgraduate studies at the National Medical and Surgical Center named after N.I. Pirogov. Our authors
Treatment
Treatment of this disease depends on the frequency of crises, clinical picture and age.
During a hemolytic crisis, conservative treatment with hospitalization is indicated. Therapy is aimed at combating hypoxia, hyperbilirubinemia, cerebral edema, hyperglycemic and other disorders that develop during a crisis. Treatment usually occurs according to the following scheme:
- For severe anemia, red blood cell transfusions are prescribed.
- After the crisis is over, choleretic drugs are prescribed, the diet and daily routine are expanded.
The only effective method of combating spherocytosis is removal of the spleen (splenectomy)
The operation is indicated for frequent crises, severe anemia, hepatic colic, and splenic infarction. Sometimes, along with this operation, removal of the gallbladder (cholecystectomy) is also performed. Before surgery, in case of severe anemia, red blood cell transfusion is performed.
As a rule, after surgery, recovery occurs in 100% of cases. At the same time, the red blood cells remain pathologically altered, that is, they have a spherical shape and low resistance to sodium chloride solutions. The cessation of the breakdown of red cells is due to the fact that there is no organ in which this destruction occurs.
In some cases, surgery must be performed immediately; even pregnancy is not a contraindication. Otherwise, cholelithiasis, liver cirrhosis, and hepatitis may develop.
The prognosis for hereditary spherocytosis is relatively good and people can live with this disease into old age. In severe cases of the disease, if help is not provided on time, death may occur.
If one of the parents is sick, the probability of developing pathology in children is less than 50%.
For microspherocytic anemia, a special diet is indicated. The patient's diet should contain foods containing large amounts of folic acid. Recommended to eat:
- porridge: buckwheat, millet, oatmeal;
- mushrooms;
- products made from wholemeal flour,
- beef liver;
- cheese;
- beans, soy;
- cottage cheese;
- green onions, cauliflower, chopped carrots.