Medical editor: Strokina O.A. - therapist, functional diagnostics doctor. September, 2021.
ICD-10 code: I35.0, I06.8, I35.8.
Aortic insufficiency is a malfunction of the aortic valve with the formation of reverse blood flow from the aorta into the cavity of the left ventricle. Symptoms directly depend on the amount of blood entering the ventricle. Diagnosis of pathology is possible only with the help of echocardiographic examination (ultrasound of the heart). Treatment also depends on the degree of deficiency and may involve both conservative and surgical techniques.
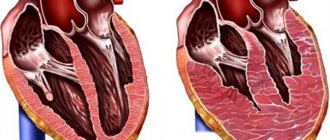
Aortic insufficiency is a heart defect that is characterized by the development of regurgitation (backflow of blood) on the aortic valve during the relaxation phase of the heart muscle. As a result, blood from the aorta flows back into the ventricle. There is an overflow of its volume, which in the future may threaten the expansion of the cavity of the left chambers of the heart with the development of heart failure.
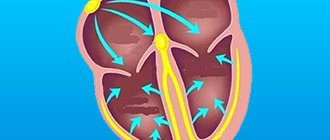
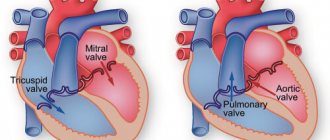
During the contraction phase, the left ventricle ejects blood into the aorta, after which the ventricular relaxation phase begins. At this moment, the blood tends to return back. Her path is blocked by 3 leaflets of the aortic valve, which are “sacs”. As they fill with blood, the valves straighten, close with each other and completely close the aortic opening.
On the mitral and tricuspid valves, a small discharge of blood in the opposite direction is normally allowed, and the term “insufficiency” is not used here. In the case of the aortic valve, even minimal regurgitation is a pathology that needs to be monitored.
Causes and risk factors
Aortic valve insufficiency develops due to loose closure of the valves during the relaxation phase of the ventricles, which can develop for a number of reasons:
- idiopathic (unknown cause) dilation of the aorta;
- congenital defects of the aortic valve (usually bicuspid aortic valve);
- sclerosis of the valves (due to atherosclerosis);
- rheumatism;
- infective endocarditis;
- arterial hypertension;
- myxomatous degeneration (disorder of connective tissue development);
- dissection of the ascending aorta;
- Marfan syndrome;
- aortic valve injuries;
- ankylosing spondylitis;
- syphilitic aortitis;
- rheumatoid arthritis.
Most of these causes lead to chronic aortic insufficiency, which can be asymptomatic for a long time. Others, in particular infective endocarditis, aortic dissection, and trauma are often accompanied by a sudden development of severe aortic insufficiency. This leads to serious hemodynamic disturbances. As a result, much less blood reaches vital organs: the brain, kidneys, liver, heart.
Causes
The pathology develops during the period of intrauterine development, therefore it is a congenital heart defect. This happens between 6 and 8 weeks. During this period of time, the active process of formation of internal organs in children begins. The two doors are fused together, forming only two moving parts. They take on the functions of the missing component of the heart muscle. In a child, this pathology develops under the influence of a number of provoking factors. The main reasons for the formation of a bicuspid aortic valve include:
- suffered by the mother during the period of gestation of various infectious diseases;
- the influence of radiation, exposure caused by X-ray examination;
- harmful effects of the environment due to poor ecology;
- severe stress, anxiety, psycho-emotional disorders and shocks;
- abuse of alcohol and tobacco products while carrying a baby;
- a predisposition that is inherited if the baby’s parents have cardiovascular system defects;
- genetic predisposition to pathologies associated with connective tissues.
If parents have a bicuspid aortic valve as a congenital pathology, when planning conception and during gestation, it is necessary to carefully monitor all processes, carry out comprehensive diagnostics and take timely measures to ensure the normal functioning of the baby’s cardiovascular system.
Symptoms
In a child, a congenital pathology may not manifest itself in any way for a long time. Symptoms can intensify as he grows up and gradually increases the load on his body, which is in the stage of active development and formation. There are cases when a congenital valve defect was discovered when the child reached adolescence and adulthood. With such congenital heart disease as a bicuspid aortic valve, symptoms manifest themselves in the form of the following characteristic signs:
- Strong pulsation, which is felt in the neck, head or projection of the heart muscle. The pulsation intensifies when the patient assumes a supine position. Such symptoms manifest themselves due to high myocardial emissions and elevated pulse rates.
- Sinus type tachycardia. Its manifestations are characterized by rapid heartbeat. It can occur for no apparent reason, that is, in a state of rest.
- Fainting, dizziness. They are observed quite often in patients. They can develop with moderate physical activity, or when a person suddenly changes the position of his body. This happens if the patient stands up suddenly. These symptoms are caused by a lack of blood circulation in the brain. Such signs are relevant when serious changes occur in the structure of the valves.
- Dyspnea. It first appears if the patient has undergone significant physical exertion. But over time, symptoms are observed even in a state of complete rest. If the systolic function assigned to the left ventricle decreases, the person exhibits signs of orthopnea. As the disease progresses, myocardial asthma and pulmonary edema gradually develop. Because of them, the patient faces dangerous attacks of suffocation.
- Increased and excessive fatigue with minimal activity, a feeling of general weakness throughout the body.
- A person’s vision noticeably decreases, even if it was initially 100%.
- In the region of the myocardium, pain is felt behind the chest, which has no emotional or physical provoking factors. Can be observed at rest. If the aortic valve is severely damaged, the pain becomes pressing and squeezing, lasts for a long time and does not go away after taking potent drugs. The most difficult pain for the patient is the attacks of pain that occur at night. At the same time, sweat is actively released. This condition is caused by hypertrophy of the affected ventricles.
Only hardware diagnostics can detect a bicuspid aortic valve in a person as a congenital pathology of the cardiovascular system. Therefore, at the first signs, seek advice from a cardiologist. If symptoms are detected in time, the diagnosis is confirmed and treatment is started, the functional cardiovascular system will suffer minimally. It is often possible to return patients to normal life and restore blood flow. Prediction is made based on the results of the examination and the therapeutic methods prescribed for treatment.
Types and stages
For aortic regurgitation, doctors use a classification based on the severity of regurgitation (backflow of blood). the degree is determined only by a functional diagnostics doctor based on the results of echocardiography:
- Soft (light);
- Moderate;
- Expressed.
There are many ways to define it. But all of them are possible thanks to Doppler research. All echocardiography devices have this function. It allows you to detect pathological flows inside the heart using an ultrasound sensor. One way or another, this is the most objective assessment of the severity of aortic insufficiency.
Acute and chronic deficiency are also distinguished. This is largely a clinical classification based on symptoms and how quickly they develop.
Diagnostic methods
The bicuspid aortic valve can only be examined using instrumental diagnostic methods. But first, a cardiologist conducts a visual examination if such a pathology is suspected. With BAV, patients experience paleness of the skin or some areas with bluish tints. External signs also include increased pulsation. There are several basic instrumental diagnostic methods that can confirm the fact of a congenital defect and assess the patient’s current condition.
- Using ultrasound or echocardiography, it is possible to determine the degree of damage to the affected valves, as well as confirm or refute the development of an aortic aneurysm.
- The method of vascular Dopplerography or ultrasound dopplerography is used to determine the current state of the valve structure, the speed of blood flow through them and cavities.
- To identify symptoms of blood stagnation, the fact of an enlarged ventricle and aorta, an x-ray method is used. The x-ray scans the chest and provides the doctor with relevant information.
- If it is necessary to check the heart muscle for pathological noise, resort to phonocardiography.
The most effective and useful examination method for such pathology is considered to be ultrasound. Based on the results of the instrumental study, the doctor forms an individual tactic for treating the disorder.
Symptoms of aortic insufficiency
The rate at which symptoms develop depends on the cause of aortic insufficiency.
Acute form
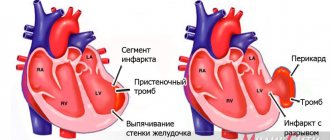
Acute aortic insufficiency develops abruptly, literally in a few minutes to hours, less often during the day. The cause is often some acute pathology, such as valve injury or aortic dissection. As a result, severe aortic insufficiency develops with large backflow of blood into the left ventricle. It's overflowing with volume. Mitral valve insufficiency develops quickly and blood stagnates in the pulmonary circulation, which can lead to pulmonary edema. If emergency measures are taken, the heart can recover almost completely.
In the acute form, acute heart failure with pulmonary edema and cardiogenic shock develops:
- severe shortness of breath,
- difficulty breathing,
- cough,
- forced position of the patient sitting,
- wheezing in the lungs,
- the appearance of foamy pink sputum,
- decreased blood pressure until loss of consciousness.
Even with intensive drug therapy, death often occurs from ventricular arrhythmias, cardiogenic shock, and pulmonary edema.
Chronic form
Chronic develops slowly. The clinic has not developed for years. A person may not be aware of the pathology and deficiency often becomes an accidental finding. Or the patient comes in with some symptoms, when the heart is greatly changed. Treatment in this case is mainly symptomatic, although in some cases surgical correction is possible.
Chronic aortic insufficiency can be asymptomatic for years. The first symptoms may be lethargy, decreased performance, fainting and presyncope. The clinical picture of heart failure is gradually developing due to volume overload of the left ventricle:
- shortness of breath during exercise, then over time at rest;
- attacks of suffocation;
- pain in the heart area of a pressing, squeezing nature, which goes away after taking nitroglycerin;
- swelling of the lower extremities, and subsequently, in the absence of adequate treatment, fluid appears in the abdominal, pleural and even pericardial cavities;
- heart rhythm disturbances - from atrial fibrillation to ventricular tachycardia. life-threatening.
Bicuspid aortic valve
Bicuspid aortic valve (BAV) is the most common congenital heart defect (CHD), occurring in 1–2% of people.
Anatomy
In a healthy human heart, the aortic valve normally has three leaflets. They ensure normal blood flow to the heart.
When the fetal heart develops, around the eighth month of pregnancy, two valves may join into one, and instead of three valves there will be two. Therefore, during the first trimester, women are advised to overload and expose themselves to less stress.
Pathogenesis and genetics.
BAV is the result of a violation of the formation of aortic valves during valvulogenesis. Adjacent valves fuse and form one large valve; it is larger than the opposite one, but less than 2 normal ones. BAV is a consequence of a complex defect in the developmental process. The genetic theory of the occurrence of BAV is more substantiated, therefore this defect is often associated with congenital pathologies of the aorta - patent ductus arteriosus and coarctation of the aorta. And subsequently, the disease is often associated with dilatation, aneurysm or aortic dissection. Also, a lack of fibrillin protein at the stage of valvulogenesis leads to the formation of a bicuspid aortic valve.
Clinic
One of the characteristic symptoms of BAV is disruption of blood flow. Accordingly, the main manifestations of the disease include:
- sensation of pulsation in the head;
- sinus tachycardia;
- dizziness, even fainting;
- dyspnea;
- vision problems;
- short-term pain in the heart area, which appears due to ventricular hypertrophy.
Prognosis and complications
When the right coronary and non-coronary leaflets fuse, stenosis can form if there is no excess leaflet tissue, and AV insufficiency if there is prolapse or excess leaflet tissue. Aortic stenosis develops in 15−71%, aortic regurgitation in 1.5−3%, infective endocarditis in 9.5−40%, dilatation and dissecting aneurysm in 5%. Aortic stenosis is more common in middle age, infective endocarditis in young people.
Aortic stenosis
In many cases, developed aortic stenosis requires implantation of a prosthesis. Patients with BAV are prone to premature fibrosis, increased leaflet stiffness and calcium deposition. Echo studies have shown that sclerosis of the BAV begins in the 2nd decade of life, and calcification in the 4th decade, with the valvular gradient increasing by approximately 18 mmHg every 10 years. Also, the development of stenosis can be influenced by hypercholesterolemia and smoking, which contribute to faster wear of the leaflets.
Aortic regurgitation
The etiology of aortic regurgitation in patients with BAV is more complex than in aortic stenosis. Aortic regurgitation can be isolated and occurs when a larger, eccentrically located leaflet prolapses or when the aortic root expands due to the fact that the elastic properties of the valve ring are lost. Because of the association with associated lesions, patients with BAV and aortic regurgitation have a worse prognosis than patients with aortic stenosis.
Infective endocarditis (IE)
IE may develop in 10–30% of patients with BAV. More often at a young age, so tetralogy of Fallot, ventricular septal defect, BAV and mitral valve prolapse are the substrate for 80−90% of cases of the disease in young people.
Conclusions:
To a patient with BAV and aortic dilatation, it is necessary to explain:
- BAV is the most common congenital heart defect;
- An echocardiography study in relatives is necessary, since BAV is a genetically determined disease;
- BAV is often accompanied by aortic root pathology and is prone to valvular and aortic complications that require surgical intervention
- Infectious endocarditis is a common complication of the disease, so prophylaxis with antibiotics is required;
- Early referral to cardiac surgery can facilitate surveillance and prevent life-threatening complications;
- The patient should undergo an annual examination by a cardiologist with echocardiography to identify the progression of aortic dilatation and prevent dissection. Hypercholesterolemia and hypertension should be carefully monitored. Smoking should be avoided.
Be healthy!
Sign up for functional diagnostics
Functional diagnostics doctor - Andrienko Olga Leonidovna
You can make an appointment by calling (391) 205−00−48 or through your personal account
Surveys
Examination of a patient always begins with examining and listening to the heart. Already at this stage, the doctor is able to suspect a valve defect by the presence of a heart murmur. It is also possible to decrease diastolic (lower limit) blood pressure and, as a result, increase pulse pressure (the difference between the upper and lower limits of blood pressure). However, such signs of aortic insufficiency are detected only in chronic pathology, when the clinical picture of heart failure described above is already clearly manifested.
Instrumental methods are the only way to detect regurgitation on the aortic valve.
Echocardiography (ultrasound of the heart)
First line diagnostic method. It detects backflow of blood through the aortic valve. The doctor determines the degree of its severity and the possible cause - for example, trauma, vegetations on the valve (accumulations of bacteria on the valves - a sign of infective endocarditis), volumes of the left chambers of the heart, left ventricular ejection fraction and many other morpho-functional indicators.
Magnetic resonance imaging (MRI)
Used only if echocardiography is not available or visualization of the heart and its structures is extremely difficult.
Multislice computed tomography angiography and magnetic resonance imaging angiography (both contrast-enhanced procedures) are used in patients with a bicuspid aortic valve to evaluate the initial aorta to its arch, but only if echocardiographic findings are unsatisfactory.
X-ray of the chest organs
Used to assess the size of the heart and ascending aorta.
Electrocardiogram (ECG)
Allows you to identify rhythm disturbances, conduction disorders in the heart and signs of myocardial hypertrophy.
The methods listed are more than enough to make an accurate diagnosis of aortic insufficiency and begin treatment on time.
Connective tissue dysplasia syndrome of the heart in children
In recent years, there has been an increase in the number of congenital malformations and hereditary diseases, as well as an increase in the prevalence of various variants of connective tissue dysplasia due to the deterioration of the environmental situation. According to modern concepts, connective tissue dysplasia syndrome is defined as an independent syndrome of a polygenic-multifactorial nature, manifested by external phenotypic signs in combination with dysplastic changes in connective tissue and clinically significant dysfunction of one or more internal organs (V. A. Gavrilova, 2002).
The term “cardiac connective tissue dysplasia” (CDTS) refers to an anomaly of tissue structure, which is based on a genetically determined defect in collagen synthesis. DSTS syndrome was identified as an independent nosological form at a symposium in Omsk (1990), dedicated to the problem of congenital connective tissue dysplasia. The problem of DSTS syndrome attracts attention due to the high risk of developing complications such as cardiac rhythm and conduction disturbances, infective endocarditis, thromboembolism of various vessels and sudden cardiac death.
The high frequency of DSTS syndrome in various diseases indicates the systemic nature of the lesion, which is associated with the “omnipresence” of connective tissue, which makes up the stroma of all organs and tissues.
Dysplastic heart is a combination of constitutional, topographical, anatomical and functional features of the heart in a person with connective tissue dysplasia (CTD). In Western literature, the term “myxoid heart disease” is used (Morales AB, Romanelli BEA, 1992), but this formulation is used mainly by foreign authors.
The incidence of dysplastic heart is 86% among individuals with primary undifferentiated DST (G. N. Vereshchagina, 2008).
According to modern concepts, DSTS syndrome includes prolapses of the heart valves, aneurysms of the interatrial septum and sinuses of Valsalva, ectopically attached chords of the mitral valve and many others.
The pathology is based on the inferiority of the extracellular matrix and its collagen structures.
A dysplastic heart is formed by:
I. Constitutional features - “drip”, “hanging” heart, its rotation around the sagittal and longitudinal axis.
II. Bone-vertebral dysplasia and deformities with compression, rotation, displacement of the heart and torsion of large vessels: according to Urmonas V.K. et al. (1983). Deformations of the chest and spine lead to the development of thoraco-phrenic syndrome, which limits the functioning of all organs of the chest.
III. Features of the structure of the heart and blood vessels:
- redundancy of tissue of the mitral, tricuspid and aortic valves;
- prolapse of the mitral valve leaflets (MVP) with regurgitation;
- myxomatous degeneration of leaflets, chords, valve ring;
- valvular-ventricular dissociation;
- bicuspid aortic valve;
- elongation, excessive mobility of the chordae;
- ectopically attached chordae;
- increased trabecularity of the left ventricle (LV);
- open oval window;
- aneurysm of the interatrial septum (small);
- dilatation of the sinuses of Valsalva;
- ventriculoseptal features of the LV: transient systolic ridge of the upper third of the interventricular septum (IVS), S-shaped bend of the IVS;
- tortuosity, hypoplasia, aplasia, fibromuscular dysplasia of the coronary arteries;
- coronary artery aneurysms;
- myocardial bridges;
- anomalies of the conduction system;
- expansion of the proximal part of the aorta, pulmonary trunk;
- aortic hypoplasia, borderline narrow aortic root, pulmonary trunk hypoplasia;
- systemic failure of the venous wall - varicose veins of the upper and lower extremities, pelvis, vulva, varicocele.
IV. Pathology of the respiratory system with a decrease in vital capacity of the lungs:
- diffuse and bullous emphysema;
- multiple fistulas;
- repeated spontaneous pneumothorax;
- bronchiectasis;
- cystic hypoplasia of the lungs.
Myxomatous degeneration of valves, chords, subvalvular structures is a genetically determined process of destruction and loss of the architectonics of collagen and elastic structures of connective tissue with the accumulation of acidic mucopolysaccharides in the loose fibrous layer. In this case, there are no signs of inflammation. It is based on a defect in the synthesis of type III collagen, which leads to thinning of the fibrous layer, the valves are enlarged, loose, redundant, the edges are curled, and sometimes fringe is detected. The primary locus of autosomal dominant myxomatosis in MVP is localized on chromosome 16. Morales AB (1992) identifies myxoid heart disease.
In population studies, the phenomenon of MVP was detected in 22.5% of children under the age of 12 years. In children with CTD, MVP is found much more often - in 45–68%.
Clinical manifestations of MVP in children vary from minimal to significant and are determined by the degree of connective tissue dysplasia of the heart, autonomic and neuropsychiatric abnormalities.
Most older children complain of short-term chest pain, palpitations, shortness of breath, a feeling of interruptions in the heart, dizziness, weakness, and headaches. Children characterize heart pain as stabbing, pressing, aching and feel it in the left half of the chest without any irradiation. They arise in connection with emotional stress and are usually accompanied by autonomic disorders: unstable mood, cold extremities, palpitations, sweating, and disappear spontaneously or after taking sedatives. The absence in most cases of ischemic changes in the myocardium according to a comprehensive examination allows us to regard cardialgia as a manifestation of sympathalgia associated with the psycho-emotional characteristics of children with MVP. Cardialgia with MVP may be associated with regional ischemia of the papillary muscles when they are excessively tense. Neurovegetative disorders are also associated with palpitations, a feeling of “interruptions” in the work of the heart, “tingling”, and “fading” of the heart. Headaches often occur during overwork, anxiety, in the morning before school starts and are combined with irritability, sleep disturbance, anxiety, and dizziness.
On auscultation, characteristic signs of mitral valve prolapse are isolated clicks (clicks), a combination of clicks with late systolic murmur, isolated late systolic murmur, holosystolic murmur.
The origin of the noise is associated with turbulent blood flow associated with bulging of the valves and vibration of the tense chords. Late systolic murmur is heard better in the left lateral decubitus position and intensifies during the Valsalva maneuver. The nature of the noise may change with deep breathing. As you exhale, the noise intensifies and sometimes takes on a musical tone. Often, the combination of systolic clicks and late murmur is most clearly detected in an upright position after exercise. Sometimes, when systolic clicks are combined with a late murmur in a vertical position, a holosystolic murmur may be recorded.
Holosystolic murmur with primary mitral valve prolapse is rare and indicates the presence of mitral regurgitation. This noise occupies the entire systole and practically does not change in intensity when changing body position, is carried out in the axillary region, and intensifies during the Valsalva maneuver.
The main methods for diagnosing MVP are two-dimensional Echo-CG and Dopplerography. MVP is diagnosed when the maximum systolic displacement of the mitral valve leaflets beyond the line of the mitral valve ring in the parasternal longitudinal position is 3 mm or more. The presence of isolated displacement of the anterior leaflet beyond the line of the mitral valve annulus in the four-chamber apical position is not enough to diagnose MVP; this is the main reason for its overdiagnosis.
Echo-CG classification of myxomatous degeneration (MD) (G. I. Storozhakov, 2004):
- MD 0 - no signs.
- MD I - minimally expressed: thickening of the leaflets 3–5 mm, arched deformation of the mitral orifice within 1–2 segments. The closure of the valves is preserved.
- MD II - moderately expressed: thickening of the leaflets 5–8 mm, elongation of the leaflets, deformation of the contour of the mitral orifice, its stretching, impaired closure of the leaflets. Mitral regurgitation.
- MD III - pronounced: thickening of the leaflets is more than 8 mm, the leaflets are elongated, multiple ruptures of the chords, significant expansion of the mitral ring, there is no closure of the leaflets. Multivalve lesion. Dilatation of the aortic root. Mitral regurgitation.
The degree of regurgitation in MVP depends on the presence and severity of myxomatous degeneration, the number of prolapsed leaflets and the depth of prolapse.
Degrees of regurgitation:
- 0—regurgitation is not recorded.
- I - minimal - the regurgitant jet penetrates into the cavity of the left atrium no more than one third of the atrium.
- II - medium - the regurgitation jet reaches the middle of the atrium.
- III - severe - regurgitation throughout the left atrium.
At rest, mitral regurgitation (MR) of the first degree is diagnosed in 16–20%, the second degree in 7–10% and the third degree in 3–5% of children with MVP.
The prognosis of a patient with MVP determines the degree of mitral regurgitation. Moreover, any degree of prolapse leads to changes in myocardial perfusion, changes most often in the area of the anterior wall of the LV and the interventricular septum (Nechaeva G.I., Viktorova I.A., 2007)).
Severe complications from MVP in children are rare. They are: life-threatening arrhythmias, infective endocarditis, thromboembolism, acute or chronic mitral regurgitation, and even sudden death.
Acute mitral regurgitation occurs due to the separation of the tendon threads from the cusps of the mitral valve (loppy mitral valve syndrome); it is rarely observed in childhood and is mainly associated with chest trauma in patients with myxomatous chordae degeneration. The main pathogenetic mechanism of acute mitral regurgitation is pulmonary venous hypertension, which occurs due to a large volume of regurgitation into an insufficiently distensible left atrium. Clinical symptoms are manifested by the sudden development of pulmonary edema.
In children, mitral regurgitation with MVP is most often asymptomatic and is diagnosed by Doppler echocardiography. Subsequently, as regurgitation progresses, complaints of shortness of breath during physical activity, decreased physical performance, weakness, and retarded physical development appear.
Risk factors for the development of “pure” (non-inflammatory) mitral regurgitation in prolapse syndrome according to two-dimensional echocardiography are:
- Dilatation of the left atrioventricular orifice.
- Prolapse of predominantly the posterior mitral leaflet.
- Thickening of the posterior mitral leaflet.
MVP is a high risk factor for infective endocarditis. The absolute risk of the disease is 4.4 times higher than in the population.
Diagnosis of infective endocarditis in MVP presents certain difficulties. Since the valves during prolapse are excessively scalloped, this does not allow us to detect the beginning of the formation of bacterial vegetations according to echocardiography. Therefore, the main importance in the diagnosis of endocarditis is played by: 1) clinical symptoms of the infectious process (fever, chills, rash, and other symptoms), 2) the appearance of the noise of mitral regurgitation and the fact of detection of the pathogen during repeated blood cultures.
The incidence of sudden death in MVP syndrome depends on many factors, the main ones being electrical instability of the myocardium in the presence of long QT interval syndrome, ventricular arrhythmias, concomitant mitral regurgitation, and neurohumoral imbalance.
The risk of sudden death in the absence of mitral regurgitation is low and does not exceed 2:10,000 per year, while with concomitant mitral regurgitation it increases 50–100 times.
In most cases, sudden death in patients with MVP is of arrhythmogenic origin and is caused by the sudden onset of idiopathic ventricular tachycardia (fibrillation) or against the background of long QT interval syndrome.
In rare cases, sudden cardiac death in patients with MVP may be due to a congenital anomaly of the coronary arteries (abnormal origin of the right or left coronary artery), leading to acute myocardial ischemia and necrosis.
Thus, the main risk factors for sudden death in children with MVP syndrome are: ventricular arrhythmias of grade III–V according to Lown; prolongation of the corrected QT interval more than 440 ms; the appearance of ischemic changes on the ECG during physical activity; history of cardiogenic syncope.
DSTS are one of the unfavorable factors predisposing to the development of arrhythmic complications in childhood and adolescence, including hemodynamically significant ones. In the structure of rhythm disturbances in children with DSTS, supraventricular extrasystole in pathological quantities and ventricular extrasystole, interrelated with the degree of cardiac dysplasia, are more often detected (Gnusaev S. F., co-authors, 2006).
Morphological manifestations of DSTS syndrome in children with concomitant kidney pathology, according to Domnitskaya T. M., Gavrilova V. A. (2000), are: spherical or triangular shape of the heart, rounding of the apex of the heart, an increase in heart weight by 1.4–2. 5 times, thickening and shortening of the mitral valve chords, fan-shaped discharge of the chords, hypertrophy of the papillary muscles, funnel-shaped mitral valve, open oval window. Myxomatous degeneration of the atrioventricular valve leaflets was observed in the majority of patients with DSTS syndrome and diseases of the urinary system (its frequency ranged from 66.7% to 77%). Endocardial fibroelastosis was detected in 10 children of the analyzed group.
In the population of children, the most frequently detected displacement of the septal leaflet of the tricuspid valve into the ventricular cavity within 10 mm, impaired distribution of chords of the anterior leaflet of the mitral valve, dilatation of the sinuses of Valsalva, enlarged eustachian valve more than 1 cm, dilatation of the pulmonary artery trunk, MVP, diagonally located trabeculae in the cavity left ventricle.
The management tactics for children with primary MVP vary depending on the severity of leaflet prolapse and the nature of autonomic and cardiovascular changes. The main principles of treatment are: 1) complexity; 2) duration; 3) taking into account the direction of functioning of the autonomic nervous system.
It is mandatory to normalize work, rest, daily routine, adherence to the correct regime with sufficient sleep.
The issue of physical education and sports is decided individually after the doctor evaluates the indicators of physical performance and adaptability to physical activity. Most children, in the absence of mitral regurgitation, severe disturbances in the repolarization process and ventricular arrhythmias, tolerate physical activity satisfactorily. If they have medical supervision, they can lead an active lifestyle without any restrictions on physical activity. Children can be recommended swimming, skiing, skating, and cycling. Sports activities associated with jerky movements (jumping, karate wrestling, etc.) are not recommended. The detection of mitral regurgitation, ventricular arrhythmias, changes in metabolic processes in the myocardium, and prolongation of the QT interval in a child dictates the need to limit physical activity and sports. These children are allowed to engage in physical therapy under the supervision of a doctor.
Treatment is based on the principle of restorative and vegetotropic therapy. The entire complex of therapeutic measures should be built taking into account the individual characteristics of the patient and the functional state of the autonomic nervous system.
An important part of the complex treatment of children with DSTS is non-drug therapy: psychotherapy, auto-training, physiotherapy (electrophoresis with magnesium, bromine in the upper cervical spine), water procedures, acupuncture, spinal massage. The doctor's attention should be directed to the sanitation of chronic foci of infection; tonsillectomy is performed according to indications.
Drug therapy should be aimed at: 1) treatment of vegetative-vascular dystonia; 2) prevention of the occurrence of myocardial neurodystrophy; 3) psychotherapy; 4) antibacterial prophylaxis of infective endocarditis.
For moderate manifestations of sympathicotonia, herbal medicine with sedative herbs, tincture of valerian, motherwort, herbal collection (sage, wild rosemary, St. John's wort, motherwort, valerian, hawthorn), which simultaneously has a slight dehydration effect, is prescribed. If there are changes in the repolarization process on the ECG, or rhythm disturbances, courses of treatment are carried out with drugs that improve metabolic processes in the myocardium (panangin, carnitine, Kudesan, vitamins). Carnitine is prescribed at a dose of 50 mg/kg per day for 2–3 months. Carnitine plays a central role in lipid and energy metabolism.
As a cofactor for beta-oxidation of fatty acids, it transports acyl compounds (fatty acids) across mitochondrial membranes, prevents the development of myocardial neurodystrophy, and improves its energy metabolism. In our studies, 35 children with extrasystole (more than 15 per minute) included carnitine in complex therapy. At the end of treatment, extrasystole decreased significantly in 25 children, and was not detected in 10 children.
A beneficial effect has been noted from the use of the drug Coenzyme Q10®, which significantly improves bioenergetic processes in the myocardium and is especially effective in secondary mitochondrial failure.
Early diagnosis of CTD in children allows for appropriate rehabilitation therapy and prevention of disease progression. One of the most striking therapeutic results is the effective treatment of children with DST (mainly with MVP) using the magnesium-containing drug magnesium orotate - Magnerot®. The choice of the drug was due to the known properties of the magnesium ion, observed in class I and IV antiarrhythmic drugs (membrane stabilizing and calcium antagonists), as well as the absence of side effects that may appear when using traditional antiarrhythmic therapy. It was also taken into account that the active ingredient of the drug is magnesium orotate, which, by inducing protein synthesis and participating in the exchange of phospholipids, which are an integral part of cell membranes, is necessary for the fixation of intracellular magnesium (O. A. Gromova, 2007).
The drug Magnerot® was used as monotherapy at a dose of 40 mg/kg per day during the first 7 days of administration, then 20 mg/kg per day for 6 months. The result of treatment was a decrease in the depth of prolapse of the mitral valve leaflets by 20–25% and a decrease in the degree of regurgitation by 15–17%. Treatment with Magnerot® did not affect the size of the left chambers of the heart and myocardial contractility, the values of which were within normal limits before treatment.
Studies conducted by E. N. Basargina (2008) revealed the antiarrhythmic effect of the drug Magnerot®. During daily ECG monitoring in children of groups 2 and 3, a decrease in the number of ventricular complexes by 50% or more was noted in 18 (27.7%) patients. Moreover, in 6 children, the disappearance of ventricular arrhythmia or a decrease in the number of ventricular complexes to 30–312 per day was noted. In 14 (21.5%) children, the number of ventricular complexes decreased by at least 30%. In two patients, an increase in the number of ventricular extrasystoles was noted up to 30% of the initial level. Thus, the antiarrhythmic effectiveness of Magnerot® was 27.7%. Similar results were previously obtained in other studies (Domnitskaya T. M. et al., 2005).
At the same time, rare supraventricular and ventricular extrasystoles, if not combined with long QT interval syndrome, as a rule, do not require the prescription of any antiarrhythmic drugs.
Thus, children with DSTS syndrome require timely diagnosis using Doppler echocardiography, electrocardiography, and in some cases 24-hour ECG monitoring, individual therapy, and monitoring by a pediatric cardiologist.
Therapy with Magnerot® in children with DSTS syndrome leads to a decrease in signs of valve prolapse, the frequency of detection of mitral regurgitation, a decrease in the severity of clinical manifestations of autonomic dysfunction, the frequency of ventricular arrhythmias, and is accompanied by an increase in the level of intraerythrocyte magnesium.
Literature
- Zemtsovsky E. V. Dysplastic syndromes and phenotypes. Dysplastic heart. St. Petersburg: "Olga". 2007. 80 p.
- Gavrilova V. A. Cardiac connective tissue dysplasia syndrome in children with diseases of the urinary system. Author's abstract. diss. Doctor of Medical Sciences M., 2002.
- Morales AB, Romanelli B., Boucek RJ et al. Myxoid heart disease: an assessment of extravalvular cardiac pathology in severe mitrae valve prolapse // Hum.Pathol. 1992, v. 23, no. 2, p. 129–137.
- Vereshchagina G. N. Systemic connective tissue dysplasia. Clinical syndromes, diagnosis, treatment approaches. Methodological manual for doctors. Novosibirsk, 2008, 37 p.
- Urmonas V.K., Kondrashin N.I. Funnel chest. Vilnius: Mokslas, 1983, 115 p.
- Gnusaev S. F. The significance of minor cardiac anomalies in healthy children and in cardiovascular pathology. Author's abstract. diss. Doctor of Medical Sciences, M., 1996.
- Belozerov Yu. M., Gnusaev S. F. Mitral valve prolapse in children. M.: Martis, 1995. 120 p.
- Storozhakov G.I., Vereshchagina G.S., Malysheva N.V. Assessment of individual prognosis for mitral valve prolapse // Cardiology, 2004, 4, p. 14–18.
- Nechaeva G. I., Viktorova I. A. Connective tissue dysplasia: terminology, diagnosis, patient management tactics. Omsk: Publishing house "Typography Blankom", 2007. 188 p.
- Gnusaev S. F., Belozerov Yu. M., Vinogradov A. F. Clinical significance of minor cardiac anomalies in children // Russian Bulletin of Perinatology and Pediatrics. 2006, no. 4. pp. 20–24.
- Domnitskaya T. M., Gavrilova V. A. Cardiac connective tissue dysplasia syndrome in children with diseases of the urinary system / Materials of the Second Congress of Pediatric Nephrologists of Russia. M., 2000. P. 159.
- Gromova O. A, Gogoleva I. V. The use of magnesium in the mirror of evidence-based medicine and fundamental research in therapy // Farmateka. 2007, v. 146, no. 12, p. 3–6.
- Basargina E. N. Cardiac connective tissue dysplasia syndrome in children // Issues of modern pediatrics. 2008, vol. 7, no. 1, 129–133.
- Domnitskaya T. M., Dyachenko A. V., Kupriyanova O. O., Domnitsky M. V. Clinical evaluation of the use of magnesium orotate in young people with cardiac connective tissue dysplasia syndrome // Cardiology. 2005; 45 (3): 76–81.
S. F. Gnusaev, Doctor of Medical Sciences, Professor
State Educational Institution of Higher Professional Education Tver State Medical Academy of Roszdrav , Tver
Contact information about the author for correspondence
Treatment
Aortic insufficiency is treated by a cardiologist and cardiovascular surgeon.
Drug treatment
Drug treatment is prescribed depending on the cause of aortic insufficiency. It is considered as an option in preparation for surgery or to reduce the symptoms of heart failure and alleviate the condition of patients who have contraindications to surgical treatment.
For rheumatism and infective endocarditis, a course of antibacterial therapy is prescribed. In the first case, penicillin antibiotics (benzathine benzylpenicillin) are used to prevent exacerbation of the disease. For infective endocarditis, amoxicillin, ceftriaxone, gentamicin, and, less commonly, vancomycin are usually prescribed for a long course of 2 weeks. Drugs are prescribed based on the clinic and the resistance of bacteria to them. Can be used as a single drug or in combination.
Treatment of arterial hypertension consists of prescribing ACE inhibitors (perindopril, enalapril, lisinopril) or sartans (valsartan, losartan) and dihydropyridine calcium antagonists (nifedipine, amlodipine).
Beta blockers (bisoprolol, metoprolol, nebivolol) are added to treatment in the case of reduced cardiac ejection fraction as determined by echocardiography.
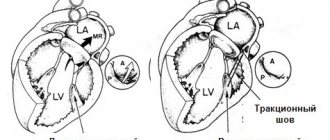
Surgery
Aortic valve replacement is the only possible method of valve correction.
It is recommended for patients with:
- severe aortic regurgitation and the presence of symptoms regardless of ejection fraction;
- asymptomatic chronic severe aortic regurgitation and ejection fraction less than 50%;
- chronic aortic regurgitation in parallel with coronary artery bypass surgery or surgery on the aorta and other heart valves;
- asymptomatic severe aortic regurgitation with normal ejection fraction. on significant expansion of the left ventricle (EDV - more than 75 mm).
The duration of treatment for patients with aortic insufficiency varies greatly depending on the severity of the condition and the cause of the pathology. After the operation, it is recommended to undergo a course of rehabilitation in a cardiological sanatorium or rehabilitation center. The recovery period is at least 3 months, after which the patient can resume work with the doctor’s permission.
Publications in the media
Aortic valve insufficiency is a pathological condition characterized by retrograde blood flow from the aorta into the left ventricular cavity through a defective aortic valve.
Frequency. Among those who died from various heart pathologies, aortic valve insufficiency was detected in 14% of cases, of which in 3.7% - in isolated form, and in 10.3% - in combination with other defects. Since 1999, more than 80% of heart valve surgeries have involved aortic valve replacement.
Etiology • Rheumatism, including cases of secondary infective endocarditis (46.5%) • Medionecrosis (18%) • Primary infective endocarditis (12.8%) • Congenital anomalies (flapping cusp syndrome, isolated aortic valve insufficiency, annuloaortic ectasia , abnormalities in the number of aortic valve cusps), including cases of secondary infective endocarditis - 13.5% • Syphilis (2.1%) • Aortitis in Reiter's disease, ankylosing spondylitis, rheumatoid arthritis (2.1%) • Connective tissue diseases, such as syndrome Marfana et al. (1.9%) • Traumatic and spontaneous rupture of aortic valves (1.3%) • Atherosclerosis (0.9%) • Arterial hypertension (0.9%).
Pathophysiology • Hemodynamic disturbances are caused by regurgitation of blood from the aorta into the left ventricle during diastole. The volume of regurgitation depends on the area of the defect, the magnitude of the diastolic pressure gradient between the aorta and the left ventricle, the duration of diastole • An increase in the diastolic volume of the left ventricle leads to its tonogenic dilatation • According to the Frank-Starling law, the left ventricle ejects an increased volume of blood into the aorta, which, together with regurgitation, leads to a decrease in diastolic blood pressure, an increase in systolic and pulse blood pressure • As myogenic dilatation develops, the end-diastolic volume and end-diastolic pressure of the left ventricle increase, which leads to stagnation of blood in the pulmonary circulation • Congestion in the pulmonary circulation increases with the development of relative insufficiency mitral valve due to dilatation of the left ventricle • Compensation mechanisms: decreased peripheral vascular resistance, tachycardia, left ventricular hypertrophy.
Clinical picture and diagnosis • Complaints •• No complaints - 4.5% •• Episodes of angina - 20.1% •• Syncope episodes - 1.0% •• Dyspnea on exertion - 31.4% •• Orthopnea - 2.8 % •• Symptoms of systemic venous hypertension - 25.6% •• Paroxysmal nocturnal dyspnea (cardiac asthma) or pulmonary edema - 32.4% •• Combination of dyspnea with syncope and episodes of angina - 0.8% •• Other symptoms - 1 .3%.
• Peripheral symptoms are caused by low diastolic and high pulse blood pressure. It should be borne in mind that all peripheral symptoms are nonspecific and are possible with neuroses, anemia, thyrotoxicosis, arteriovenous malformations, etc. •• Corrigen's sign (carotid dance) - pronounced pulsation of the carotid arteries •• High and fast pulse •• Musset's sign - shaking of the head with each pulse wave •• Müller's sign - pulsating uvula •• Pulsation of retinal arterioles •• Quincke's sign - pulsating color change lips or nail bed, synchronous with pulse; determined by pressing on them with a glass slide •• Hill's symptom - the difference between blood pressure in the arms and legs is more than 20 mm Hg •• Double Traube tone - listening to loud (similar to a pistol shot) tones on the femoral arteries •• Durosier's symptom - systolic murmur on the femoral artery when it is clamped proximal to the site of auscultation and diastolic murmur when the femoral artery is clamped distal to the site of auscultation •• Listening to heart sounds on the palmar surface of the hand •• Listening to heart sounds on the palmar surface of the hand when raising the hand up •• Listening to the pulse above the superficial palmar arch .
• Valve symptoms •• Soft (flowing) diastolic decreasing murmur, following immediately after the aortic component of the second sound (best heard in the second intercostal space to the right of the sternum during exhalation when the patient’s torso is tilted forward), carried out to Botkin’s point •• Rough musical murmur (murmur “cooing pigeon”) occurs when the valve flaps or perforates the valve •• With decompensation and a pronounced increase in end-diastolic pressure of the left ventricle, the intensity of the murmur of aortic regurgitation weakens •• Austin Flint murmur is a mesodiastolic low-frequency murmur of relative mitral stenosis, arising in connection with the covering of the anterolateral mitral leaflet valve with a stream of regurgitation in severe aortic insufficiency. The degree of attenuation of the first tone reflects the severity of decompensation of the defect for the same reason. •• Systolic murmur over the aorta, caused by an increase in the volume of ejection from the left ventricle, can be heard in the absence of aortic stenosis.
• Left ventricular symptoms are caused by hypertrophy, dilatation and insufficiency of the pumping and contractile functions of the left ventricle •• Diffuse, prolonged apical impulse, shifted to the left and down •• Palpation detectable III tone •• Increase in the area of relative dullness of the heart to the left •• Auscultatory signs of congestion in the lungs - diffuse moist rales of various sizes, best heard in the basal regions.
• Symptoms of an underlying disease , such as Marfan syndrome, aortic aneurysm, syphilis, infective endocarditis.
Special studies • ECG: signs of hypertrophy and overload of the left parts of the heart, primarily the left ventricle. • X-ray examination of the chest organs: bulging of the left ventricular arch and aorta, enrichment of the pulmonary pattern in pulmonary hypertension.
• EchoCG •• Dilation of the cavity and hypertrophy of the left ventricular myocardium •• Violation of local and global systolic, as well as diastolic functions of the left ventricle •• Dilation of the ascending aorta •• Damage to the aortic valve leaflets (defects, vegetations, abnormalities in the number of leaflets, expansion of the fibrous ring, leaflet prolapse) •• In Doppler mode - pathological flow from the aorta to the left ventricle during diastole, the volume of which (recorded in color mapping mode) corresponds to the severity of the defect •• Increased systolic pressure of the right ventricle with stagnation of blood in the pulmonary circulation •• Signs lesions of other heart valves with combined defects •• In order to determine the size of the prosthesis, it is necessary to measure the diameter of the aorta at the level of the fibrous ring, sinuses and ascending limb ••
Transesophageal echocardiography is performed for the purpose of detailed diagnosis of the condition of the thoracic aorta, more accurate identification of vegetations on the valves, thrombosis of the left atrium in the presence of atrial fibrillation, as well as for patients in whom transthoracic visualization turned out to be difficult (due to obesity, emphysema, etc.).
• Catheterization of the left and right ventricles and aorta •• In case of left ventricular failure - increase in end-diastolic pressure of the left ventricle •• In case of congestion in the pulmonary circle - increase in pressure in the right ventricle, pulmonary artery wedge pressure •• Tests are performed to determine the prognosis for pulmonary hypertension with aminophylline and oxygen inhalation •• Depending on the volume of regurgitation as a percentage of the stroke volume of blood, four degrees of aortic valve insufficiency are distinguished ••• I degree - 15% ••• II degree - 15–30% ••• III degree - 30–50%, ••• IV degree - more than 50%.
• Left ventriculography , ascending aortography •• The presence and degree of regurgitation is determined by the number of contractions required to completely expel the contrast agent from the cavity of the left ventricle •• The presence of zones of hypo- and akinesia of the left ventricle indicates myocardial ischemia •• Combined valvular lesions are also diagnosed.
• Coronary angiography •• Performed in the presence of episodes of angina and positive results of stress testing, as well as in all women over 45 years of age, men over 40 years of age and all candidates for aortic valve replacement to exclude concomitant coronary artery disease.
TREATMENT • Drug therapy •• For asymptomatic mild aortic insufficiency, only annual dynamic monitoring (examination, echocardiography, ECG) and limitation of isometric physical activity are indicated (an increase in aortic regurgitation and damage to the aortic root is possible) •• For moderate asymptomatic aortic insufficiency, ACE inhibitors and conduct a clinical examination every 6 months •• In case of severe asymptomatic aortic insufficiency, constant use of peripheral vasodilators and clinical examination every 6 months (or immediately in case of decompensation) are necessary •• Treatment of decompensated aortic insufficiency is carried out according to the general principles of treatment of circulatory failure (vasodilators, cardiac glycosides, diuretics ).
• Surgical treatment •• Indications: aortic regurgitation grade III–IV or grade II in the presence of at least one of the following conditions: heart failure functional class III–IV (according to the New York Heart Association classification), angina pectoris, syncope, acute left ventricular failure (cardiac asthma or pulmonary edema), end-diastolic pressure in the left ventricle above 15 mm Hg •• Contraindications: severe concomitant pathology that threatens the patient’s life; terminal stage of circulatory failure •• Methods of surgical treatment ••• Aortic valve replacement with a mechanical artificial heart valve under artificial circulation ••• Biological prostheses are used in children ••• In the presence of an aneurysm of the ascending aorta - simultaneous replacement of the aortic valve and ascending aorta with a valve-containing conduit according Bentall or Cabrol method.
Specific postoperative complications • Thromboembolism • Secondary infective endocarditis of prostheses • Degenerative changes in biological valves and the need for re-prosthesis • Aneurysms of the ascending aorta when using disc prostheses with a small opening angle.
Prognosis • In the natural course, the 5-year survival rate does not exceed 45%, and the 10-year survival rate does not exceed 38% • After identifying symptoms of the disease, the average life expectancy is 2–5 years • After identifying left ventricular dilatation, the 10-year survival rate does not exceed 56% • With acute development of the defect (usually infective endocarditis), the average life expectancy is 7 months •• With surgical treatment, hospital mortality is 1–3%, 12-year survival exceeds 70% (with initial heart failure of functional class III according to the New York Heart Association classification ). Synonym. Aortic valve insufficiency
ICD-10 • I06.1 Rheumatic aortic valve insufficiency • I06.2 Rheumatic aortic stenosis with insufficiency • I35.1 Aortic (valvular) insufficiency • I35.2 Aortic (valvular) stenosis with insufficiency • Q23.1 Congenital aortic valve insufficiency
Complications
Complications of aortic insufficiency develop as its severity increases. At the beginning, normal syncope (fainting) may appear due to insufficient blood supply to the brain. Conduction disorders in the form of blocks of the left bundle branch and atrioventricular conduction of impulses can also often occur in patients with aortic insufficiency.
Coronary insufficiency can accompany aortic insufficiency, but more often in combination with aortic valve stenosis. The patient develops attacks of angina (pain in the heart of a pressing, squeezing nature) and even myocardial infarction.
Heart failure is the most common complication of already severe aortic regurgitation. The patient complains of shortness of breath during physical exertion, asthma attacks, and swelling of the lower extremities.
Sudden death often develops as a result of life-threatening arrhythmias - paroxysmal ventricular tachycardia, ventricular fibrillation. In this case, the person does not even have time to call an ambulance - the arrhythmia develops so quickly.
What is the treatment for patients with bicuspid aortic valve?
If the patient is not bothered by anything, he is able to perform the physical activities he needs, and according to ultrasound there are no signs of valve dysfunction and heart overload, then there is no need for treatment. The patient should be regularly monitored by a cardiologist.
If the patient has characteristic complaints and cardiac ultrasound reveals a significant dysfunction of the valve (for example, high-grade insufficiency or critical stenosis) and signs of heart overload, then the doctor may decide to replace the aortic valve.
Make an appointment through the application or by calling +7 +7 We work every day:
- Monday—Friday: 8.00—20.00
- Saturday: 8.00–18.00
- Sunday is a day off
The nearest metro and MCC stations to the clinic:
- Highway of Enthusiasts or Perovo
- Partisan
- Enthusiast Highway
Driving directions
Forecast
The prognosis for aortic insufficiency is better, the lower the degree of regurgitation and the earlier treatment is started. Preventive examination by a general practitioner, cardiologist, full antibiotic therapy in case of infective endocarditis and rheumatic fever - all this helps to improve the prognosis. It is very important to listen to yourself, your body, so as not to miss the first symptoms (lethargy, decreased performance, fainting, shortness of breath during physical activity).
Usually, with severe clinical heart failure, the prognosis worsens, since irreversible changes occur in the structure of the heart.