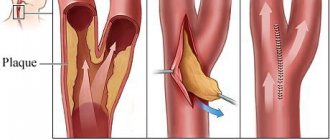
Action potential (AP) is a short-term amplitude change in the resting membrane potential (RMP) that occurs when a living cell is excited. Essentially, this is an electrical discharge - a rapid, short-term change in potential in a small area of the membrane of an excitable cell (neuron or muscle fiber), as a result of which the outer surface of this area becomes negatively charged in relation to neighboring areas of the membrane, while its inner surface becomes positively charged in relation to to adjacent areas of the membrane. The action potential is the physical basis of a nerve or muscle impulse that plays a signaling (regulatory) role.
Action potential phases. Physiology
All cells of the nervous system are polarized, that is, they have different electrical charges inside and outside a special membrane.
A nerve cell always has its own lipoprotein membrane, which has the function of a bioelectrical insulator. Thanks to the membranes, a resting potential is created in the cell, which is necessary for subsequent activation. The resting potential is maintained by ion transport. The release of potassium ions and the entry of chlorine increases the resting membrane potential.
The action potential accumulates in the depolarization phase, that is, the rise in electrical charge.
So, depolarization in physiology is a decrease in membrane potential. Depolarization is the basis for the occurrence of excitability, that is, the action potential for a nerve cell. When a critical level of depolarization is reached, no stimulus, even a strong one, is capable of causing reactions in nerve cells. There is a lot of sodium inside the axon.
This stage is immediately followed by a phase of relative excitability. A response is already possible, but only to a strong stimulus signal. Relative excitability slowly moves into the exaltation phase. What is exaltation? This is the peak of tissue excitability.
All this time, sodium activation channels are closed. And their opening will occur only when the nerve fiber is discharged. Repolarization is needed to restore the negative charge inside the fiber.
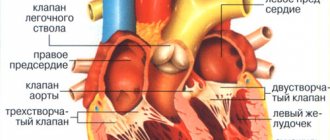
Cardiac contraction cycles are also associated with electrical depolarization of conduction pathways. The contraction signal always comes from SA cells located in the right atrium and spreads along the Hiss pathway to the bundle of Thorel and Bachmann to the left atrium. The right and left branches of the Hiss bundle transmit the signal to the ventricles of the heart.
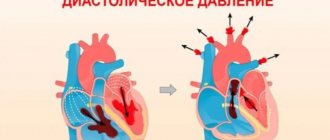
Nerve cells depolarize and transmit signals more quickly due to the presence of the myelin sheath, but muscle tissue also gradually depolarizes. That is, their charge turns from negative to positive. This phase of the cardiac cycle is called diastole. All cells here are interconnected and act as one complex, since the work of the heart must be coordinated as much as possible.
When a critical level of depolarization of the walls of the right and left ventricles occurs, a release of energy is generated - the heart contracts. Then all cells repolarize and prepare for a new contraction.
The process of depolarization depends entirely on the intrinsic electrical nature of most cells. When a cell is at rest, the cell maintains what is known as a resting potential. The resting potential generated in almost all cells results in the inside of the cell having a negative charge compared to the outside of the cell.
To maintain this electrical imbalance, microscopic positively and negatively charged particles called ions are transported across the cell's plasma membrane. The transport of ions across the plasma membrane is accomplished through several different types of transmembrane proteins embedded in the cell's plasma membrane that function as pathways for ions both in and out of the cell, such as ion channels, sodium potassium pumps, and voltage ion channels.
Resting potential
The resting potential must be established within the cell before the cell can be depolarized. There are many mechanisms by which a cell can generate a resting potential, however there is a typical pattern of generating this resting potential that many cells follow. The cell uses ion channels, ion pumps, and voltage-gated ion channels to generate a negative resting potential within the cell.
However, the process of generating a resting potential inside the cell also creates an environment outside the cell that promotes depolarization. The sodium potassium pump is largely responsible for optimizing conditions on both the inside and outside of the cell for depolarization. By pumping three positively charged sodium ions (Na) out of the cell for every two positively charged potassium ions (K) pumped into the cell, not only is the cell's resting potential set, but an unfavorable concentration gradient is created by increasing the sodium concentration outside the cell and increasing the potassium concentration within the cell .
Although there is an excess amount of potassium in the cell and sodium outside the cell, the generated resting potential keeps the voltage-gated ion channels in the plasma membrane closed, preventing ions that have been pumped through the plasma membrane from diffusing into an area of lower concentration. Additionally, despite the high concentration of positively charged potassium ions, most cells contain internal components (negative charge) that accumulate to create a negative internal charge.
depolarization
Gated sodium channel voltage. Open channel
(up)
carries out an influx of Na
ions, which leads to depolarization. As the channel becomes closed/inactivated
(at the bottom)
, depolarization ends.
Once a cell has established a resting potential, the cell has the ability to undergo depolarization. During depolarization, the membrane potential quickly changes from negative to positive. For this rapid change to occur in the cell's interior, some events must occur along the cell's plasma membrane.
While the sodium-potassium pump continues to operate, the voltage-gated sodium and calcium channels that were closed while the cell was at resting potential open in response to the initial voltage change. As sodium ions rush back into the cell, they add a positive charge inside the cell, and the membrane potential changes from negative to positive. Once the inside of the cell becomes more positively charged, cell depolarization is complete, and the channels close again.
repolarization
After a cell has been depolarized, it undergoes one final change in internal charge. Following depolarization, sodium voltage-ion channels that were open while the cell was undergoing depolarization close again. The increased positive charge inside the cell now causes potassium channels to open.
Potassium ions (K) begin to move down the electrochemical gradient (in favor of the concentration gradient and the newly created electrical gradient). As potassium leaves the cell the potential inside the cells decreases and approaches its resting potential once again. The sodium potassium pump operates continuously throughout this process.
Depolarization occurs in four chambers of the heart: the Atria first, and then both ventricles.
- The sinus (SA) node on the wall of the right atrium initiates depolarization in the right and left atria, causing compression, which is symbolized by P waves on the ECG.
- Knot S.A. sends a depolarizing wave to the atrioventricular (AV) node, which—with about a 100 ms delay to allow the atria to complete—causes contraction in both ventricles, seen in the QRS wave. At the same time, the atria re-polarize and relax.
- The ventricles are re-polarized and relaxed on the T wave.
This process continues regularly unless there is a problem in the heart.
general characteristics
Action potentials can differ in their parameters depending on the type of cell and even on different parts of the membrane of the same cell. The most typical example of differences is the action potential of the heart muscle and the action potential of most neurons. Nevertheless, the basis of any action potential is the following phenomena:
- “The membrane of a living cell is polarized” - its inner surface is charged negatively in relation to the outer one due to the fact that in the solution at its outer surface there is a larger number of positively charged particles (cations), and at the inner surface there is a larger number of negatively charged particles (anions).
- “A membrane has selective permeability” - its permeability to different particles (atoms or molecules) depends on their size, electrical charge and chemical properties.
- “The membrane of an excitable cell is capable of quickly changing its permeability for a certain type of cations, causing a transition of positive charge from the outside to the inside
The first two properties are characteristic of all living cells. The third is a feature of excitable tissue cells and the reason why their membranes are able to generate and conduct action potentials.
The main mathematical model describing the generation and transmission of action potentials is the Hodgkin-Huxley model.
What does a violation of repolarization processes on the ECG mean in adults?
Every person knows: his health and life expectancy largely depend on how well his heart works. Therefore, if in an adult’s ECG transcript, instead of the word “normal,” the entry “impaired repolarization processes” appears, then he begins to feel anxious. What does such a verdict mean and how dangerous is it?
What does a violation of repolarization processes on an ECG indicate? In adults, this can be either a normal option that does not require medical attention or a warning about a serious pathology. Let's try to describe the essence of the phenomenon in simple language.
The heart's activity is based on the alternation of excitation (depolarization) and relaxation (repolarization). The functioning of this organ is controlled by the brain. He sends him electrical impulses. They are picked up by nerve cells and transmitted to the corresponding receptors.
When such a signal passes, the structure of the cell membrane changes at the molecular level - without this, sodium ions simply will not be able to move freely through it. Repolarization is the process of restoring the electrical charge of a cardiomyocyte (muscle cell of the heart) after a nerve impulse has passed through it, causing its excitation.
It occurs in those short moments when the heart rests before the next contraction.
Repolarization disruption can be caused by a number of factors. This phenomenon is often caused by increased physical activity - intense training or simply quickly climbing the stairs. A deviation in the ECG may also appear because the person drank cold water before the test or was nervous.
Signs of repolarization are often found in women during pregnancy and menopause. But heart pathologies (ischemic heart disease, cardiosclerosis), disorders of the nervous system, kidney disease, and hormonal imbalance can also cause such a disorder. Similar deviations sometimes occur while taking adrenergic agonists.
During repolarization, the heart muscle is in a state of complete rest. On the cardiogram this is reflected in the QT segment. Its duration, if everything is normal in a person, is 0.3-0.4 s.
A decrease or increase in the duration of this interval indicates that the repolarization process is disrupted.
However, the diagnostician takes into account not only this indicator - he also evaluates the shape and size of the teeth, the presence of additional waves.
An increase in QT often accompanies congenital pathologies that are associated with gene disorders. An extended QT interval appears on the cardiogram, the T wave changes. Such a deviation can be manifested by the following symptoms:
- sudden rapid heartbeat due to strong emotions or physical overload;
- fainting.
If the QT is short, it is usually due to potassium channel dysfunction. In this case, you can see on the graph that the value of the interval is less than or equal to 0.33-0.35 s. How might the patient feel? Sometimes this does not manifest itself by external signs, but the following warning symptoms may be observed:
- slow heart rate at any time of the day;
- acceleration of the pulse in the form of an attack of atrial fibrillation or tachycardia;
- loss of consciousness.
There is an increased content of calcium and potassium in the blood. In addition, an increase in the acidity of the pH of the internal environment of the body is detected.
Another variant of the disorder is early repolarization.
The main signs of such a deviation, which are visible on the graph, are additional notches and waves on the descending part of the R wave (it is called “pseudo-wave R”), a change in the ST segment, which is expressed in its oblique or horizontal increase above the isoline (this creates a bend, directed downward).
If repolarization affects the entire myocardium, then this negatively affects general well-being, which manifests itself as follows:
- change in heart rate towards increase or decrease;
- stabbing, aching or cutting pain in the heart;
- decreased performance;
- dizziness;
- fainting;
- “quick tears”, irritability.
If a certain area of the heart muscle is affected, then one symptom prevails. So, if a patient’s pulse often jumps, then we can assume that this is a violation of the repolarization processes in the myocardium of the left ventricle.
If there is a disturbance in the repolarization processes in the lower wall of the left ventricle, this is manifested by the fact that when performing work that requires physical effort, the person begins to feel dizzy, spots float before the eyes, and blood pressure rises. Then shortness of breath develops and swelling appears in the legs.
Disruption of repolarization processes in the anteroseptal region, caused by hyperactivity of nerve fibers passing in the anterior wall of the myocardium and the interventricular septum, is often recorded with VSD.
Treatment will depend on how serious the cause of the pathology is. If it cannot be found, then the following treatment regimen is used:
- multivitamin and mineral complexes;
- corticotropic hormones;
- cocarboxylase hydrochloride (restores carbohydrate metabolism, normalizes the functioning of the cardiovascular system);
- in the most severe cases - beta blockers Anaprilin, Panangin.
No one will argue that it is best to have an ideal cardiogram. But if you have discovered violations of the repolarization processes, then you should not make hasty conclusions.
Get an ultrasound of the heart to make sure there is no coronary artery disease, do a stress test. If, according to the results of such a diagnosis, everything is normal, then the worst thing can be ruled out.
Most often, to normalize the condition, it is enough to review your regimen and take vitamins.
So, excitability, in physiology, is the ability of a cell or tissue to respond to a stimulus and generate some kind of impulse. As we found out, cells need a certain charge - polarization - to work. The increase in charge from minus to plus is called depolarization.
After depolarization there is always repolarization. The charge inside after the excitation phase must again become negative so that the cell can prepare for the next reaction.
When the voltmeter readings are fixed at 80, this is the rest phase. It occurs after the end of repolarization, and if the device shows a positive value (greater than 0), it means that the phase reverse to repolarization is approaching the maximum level - the critical level of depolarization.
How are impulses transmitted from nerve cells to muscles?
Electrical impulses generated when the membrane is excited are transmitted along nerve fibers at high speed. The speed of the signal is explained by the structure of the axon. The axon is partially enveloped by a sheath. And between the areas with myelin there are nodes of Ranvier.
Thanks to this arrangement of the nerve fiber, a positive charge alternates with a negative one, and the depolarizing current spreads almost simultaneously along the entire length of the axon. The contraction signal reaches the muscle in a split second. An indicator such as the critical level of membrane depolarization means the point at which the peak action potential is achieved. After muscle contraction along the entire axon, repolarization begins.
Spreading
Spread into unmyelinated fibers
In unmyelinated (without pulp) nerve fibers, the AP spreads from point to point, since excitation can be registered as one that gradually “runs” along the entire fiber from its point of origin. Sodium ions entering the excited area serve as a source of electric current for the occurrence of AP in adjacent areas. In this case, the impulse occurs between the depolarized section of the membrane and its non-excited section. The potential difference here is many times higher than necessary for membrane depolarization to reach the maximum level. The speed of pulse propagation in such fibers is 0.5-2 m/s
Spread in myelinated fibers
The nerve processes of most somatic nerves are myelinated.
Only very small areas of them, the so-called node interception (interception of Ranvier), are covered with a normal cell membrane. Such nerve fibers are characterized by the fact that voltage-dependent ion channels are located on the membrane only at the interceptions. In addition, this shell increases the electrical resistance of the membrane. Therefore, when the membrane potential shifts, the current passes through the membrane of the intercepting area, that is, by jumping (saltatory) from one interception to another, which allows you to increase the speed of the nerve impulse, which ranges from 5 to 120 m/s. Moreover, the action potential that arose in one of the nodes of Ranvier causes action potentials in neighboring nodes due to the emergence of an electric field, which causes an initial depolarization in these nodes. The parameters of the EMF field and the distance of its effective action depend on the cable properties of the axon. Types of nerve fibers, impulse conduction speed, depending on myelination
| Type | Diameter (µm) | Myelination | Conduction speed (m/s) | Functional purpose |
| A alpha | 12-20 | strong | 70-120 | Mobile fibers of the somatic NS; proprioceptor sensory fibers |
| A beta | 5-12 | strong | 30-70 | Sensory fibers of skin receptors |
| A gamma | 3-16 | strong | 15-30 | Sensory fibers of proprioceptors |
| A delta | 2-5 | strong | 12-30 | Sensitive fibers of thermoreceptors, nociceptors |
| IN | 1-3 | weak | 3-15 | Preganglionic fibers of the sympathetic nervous system |
| WITH | 0,3-1,3 | absent | 0,5-2,3 | Postganglionic fibers of the sympathetic nervous system; sensory fibers of thermoreceptors, nociceptors of some mechanoreceptors |
Neurons
Depolarizations are important for the functions of many cells in the human body, which has been demonstrated by the transmission of stimuli both within a neuron and between two neurons. The reception of stimuli, the neural integration of that stimulus, and the neuron's response to the stimulus all rely on the ability of neurons to use depolarization to transmit stimuli either within a neuron or between neurons.
Stimuli to neurons can be physical, electrical, chemical stimuli, which can either inhibit or excite the neuron being stimulated. The inhibitory stimulus is transmitted to the dendrite of the neuron, causing hyperpolarization of the neuron. Hyperpolarization following an inhibitory stimulus causes a further decrease in voltage within the neuron below the resting potential.
By hyperpolarizing a neuron, inhibitory stimulation results in a greater negative charge that must be overcome for depolarization to occur. Exciting stimuli, on the other hand, increase the voltage in the neuron, resulting in a neuron that is more easily depolarized than the same neuron at rest. Regardless of excitatory or inhibitory, stimuli move down the neuron's dendrites toward the cell body for integration.
Incentive Integration
Once the stimuli have reached the cell body, the nerve must integrate the various stimuli before the nerve can respond. Stimuli that have traveled down the dendrites converge at the axon hillock, where they are summed to determine the neuronal response. If the sum of the stimuli reaches a certain voltage, known as the threshold potential, depolarization continues from the colliculus axon down the axon.
response
A wave of depolarization traveling from axon-to-axon is known as an action potential. Action potentials reach the axon, where the action potential causes the release of neurotransmitters from the neuron. Transmitters that are released from the axon continue to stimulate other cells such as other neurons or muscle cells.
After an action potential travels down the neuron's axon, the axon's resting membrane potential must be restored before another action potential can travel the axon. This is known as the neuron's recovery period, during which the neuron is unable to transmit another action potential.
Rod eyes
The importance and universality of depolarization in cells can be seen in the relationships between rod cells in the eyes and their associated neurons. When rod cells are in the dark, they are depolarized. In rod cells, this depolarization is maintained by ion channels that remain open due to the higher voltage of the rod cell in the depolarized state.
Ion channels allow calcium and sodium to pass freely into the chamber, maintaining a depolarized state. The rods in a depolarized state continually release neurotransmitters which in turn stimulate the nerves associated with the rod cells. This cycle is disrupted when the rod cells are exposed to light;
The absorption of light by the rod cell causes the channels that facilitate the entry of sodium and calcium into the rod cell to close. When these channels are closed, the rod produces less neurotransmitter, which is perceived by the brain as light. In the case of rod cells and neurons, depolarization actually prevents the signal from reaching the brain, as opposed to stimulating signal transmission.
Story
The main provisions of the membrane theory of excitation were formulated by the German neurophysiologist Yu. Bernstein
In 1902, Julius Bernstein put forward a hypothesis according to which the cell membrane allows K+ ions into the cell, and they accumulate in the cytoplasm. The calculation of the resting potential value using the Nernst equation for the potassium electrode satisfactorily coincided with the measured potential between the muscle sarcoplasm and the environment, which was about -70 mV. According to the theory of Yu. Bernstein, when a cell is excited, its membrane is damaged, and K + ions leave the cell along a concentration gradient until the membrane potential becomes zero. The membrane then restores its integrity and the potential returns to the resting potential level.
This model was developed in their 1952 work by Alan Lloyd Hodgkin and Andrew Huxley, in which they described the electrical mechanisms responsible for the generation and transmission of a nerve signal in the squid giant axon. For this, the authors of the model received the Nobel Prize in Physiology or Medicine for 1963. The model is called the Hodgkin-Huxley model
In 2005, Thomas Heimburg and Andrew D. Jackson proposed the soliton model, based on the assumption that the signal propagates through neurons in the form of solitons - stable waves propagating along the cell membrane.
What happens during depolarization?
What does such an indicator as the critical level of depolarization mean? In physiology, this means that the nerve cells are already ready to work. The proper functioning of the entire organ depends on the normal, timely change of phases of the action potential.
The critical level (CLL) is approximately 40–50 MV. At this time, the electric field around the membrane decreases. The degree of polarization directly depends on how many sodium channels in the cell are open. The cell at this time is not yet ready to respond, but collects electrical potential. This period is called absolute refractoriness. The phase lasts only 0.004 s in nerve cells, and in cardiomyocytes - 0.004 s.
After passing a critical level of depolarization, superexcitability occurs. Nerve cells can respond even to the action of a subthreshold stimulus, that is, a relatively weak influence of the environment.
Vascular endothelium
The endothelium is a thin layer of simple squamous epithelial cells lining the interior of blood and lymph vessels. The endothelium, that line of blood vessels, is known as the vascular endothelium, which is subject to and must withstand the forces of blood flow and blood pressure from the cardiovascular system.
In order to withstand these cardiovascular forces, endothelial cells must simultaneously have a structure capable of withstanding circulatory forces while maintaining a certain level of plasticity in the strength of their structure. This plasticity in the structural strength of the vascular endothelium is important for the overall function of the cardiovascular system.
Endothelial cells in blood vessels can change the strength of their structures to maintain the vascular tone of the blood vessel they line, prevent vascular stiffness, and even help regulate blood pressure in the cardiovascular system. Endothelial cells accomplish these feats by using depolarization to change their structural strength.
When endothelial cells undergo depolarization, the result is a marked reduction in the rigidity and structural strength of the cell, altering the network of fibers that provide these cells with their structural support. Depolarization of the vascular endothelium is important not only for the structural integrity of endothelial cells, but also for the ability of the vascular endothelium to help regulate vascular tone, prevent vascular stiffness, and regulate blood pressure.
The structure of heart cells
Using electron microscopy, it became possible to study the structure of heart cells. Myofibrils—protein fibers of two types—were identified: thick fibrils turned out to be myosin, and thin ones turned out to be actin.
During the contraction process, thin fibers slide over thick ones, actin and myosin combine to form a new protein complex (actomyosin), muscle tissue shortens and tenses. When you relax, everything returns to normal. Between them there are bridges through which chemicals are transferred from one cell to another.
Functions of sodium and potassium channels
So, an important participant in the processes of depolarization and repolarization is the protein ion channel. Let's figure out what this concept means. Ion channels are protein macromolecules located inside the plasma membrane. When they are open, inorganic ions can pass through them. Protein channels have a filter. Only sodium passes through the sodium duct, and only this element passes through the potassium duct.
These electrically controlled channels have two gates: one is activation and has the property of allowing ions to pass through, the other is inactivation. At a time when the resting membrane potential is -90 mV, the gate is closed, but when depolarization begins, sodium channels slowly open. An increase in potential leads to a sharp closure of the duct valves.
A factor that influences the activation of channels is the excitability of the cell membrane. Under the influence of electrical excitability, 2 types of ion receptors are triggered:
- the action of ligand receptors is triggered - for chemo-dependent channels;
- an electrical signal is supplied for electrically controlled channels.
When a critical level of depolarization of the cell membrane is reached, the receptors give a signal that all sodium channels need to be closed, and potassium channels begin to open.
The processes of transfer of excitation impulses occur everywhere due to electrical polarization, carried out due to the movement of sodium and potassium ions. The movement of elements occurs based on the principle of active transport of ions - 3 Na inward and 2 K outward. This metabolic mechanism is called the sodium-potassium pump.
Links[edit]
- Zuckerman, Marvin (1991-05-31). Psychobiology of Personality
. Cambridge University Press. ISBN 9780521359429. - Gorsuch, Joseph W. (1993-01-01). Environmental Toxicology and Risk Assessment: Volume 2
. ASTM International. ISBN 9780803114852. - Lodish, H; Burke, A; Kaiser, C; Krieger, M; Bretcher, A; Ploegh, H; Amon, A (2000). Molecular Cell Biology
(7th ed.). New York, NY: W. H. Freeman and Company. pp. one thousand twenty-one -1022, 1025, 1045. - Salters-Nuffield Advanced Biology for Edexcel A2 Biology. Pearson Education, Angela Hall, 2009, ISBN 9781408205914
- Lodish, H; Burke, A; Kaiser, C; Krieger, M; Bretcher, A; Ploegh, H; Amon, A (2000). Molecular Cell Biology
(7th ed.). New York, NY: W. H. Freeman and Company. P. 695. - Callies, C; Fels, J; Lyashkovich, I; Kliche, K; Jiggle, P; Kushe-Vikhrog, K; Oberleitner, H (1 June 2011). "Membrane potential depolarization reduces vascular endothelial cell stiffness". Journal of Cell Science
.
124
(11): 1936–1942. DOI: 10.1242/jcs.084657. PMID 21558418. - Marieb, E. N., & Heng, K. (2014). Human anatomy and physiology.
San Francisco, CA: Pearson Education Inc. - Jump up
↑ Rang, H. P. (2003).
Pharmacology
. Edinburgh: Churchill Livingstone. ISBN 978-0-443-07145-4. Page Reshebnika 149
Depression Verigo
In 1889, a phenomenon in physiology called Verigo's Catholic depression was described. The critical level of depolarization is the level of depolarization at which all sodium channels are already inactivated, and potassium channels work instead. If the degree of current increases even more, then the excitability of the nerve fiber decreases significantly. And the critical level of depolarization under the influence of stimuli goes off scale.
During Verigo's depression, the rate of conduction of excitation decreases and, finally, completely subsides. The cell begins to adapt by changing its functional characteristics.
Adaptation mechanism
It happens that under certain conditions the depolarizing current does not switch for a long time. This is characteristic of sensory fibers. A gradual, long-term increase in such current above the norm of 50 mV leads to an increase in the frequency of electronic pulses.
In response to such signals, the conductance of the potassium membrane increases. Slower channels are activated. As a result, the nervous tissue becomes capable of repeated responses. This is called nerve fiber adaptation.
During adaptation, instead of a large number of short signals, cells begin to accumulate and release a single strong potential. And the intervals between two reactions increase.